User login
Early pregnancy loss: Pretreat with mifepristone?
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
PRACTICE CHANGER
Pretreat patients with oral mifepristone prior to using vaginal misoprostol to increase the efficacy of medical management of early pregnancy loss over that with misoprostol alone.
STRENGTH OF RECOMMENDATION
B: Based on a single, well-executed, randomized controlled trial.1
Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
Deliver or Wait with Late Preterm Membrane Rupture?
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.

A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.

A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
Deliver or wait with late preterm membrane rupture?
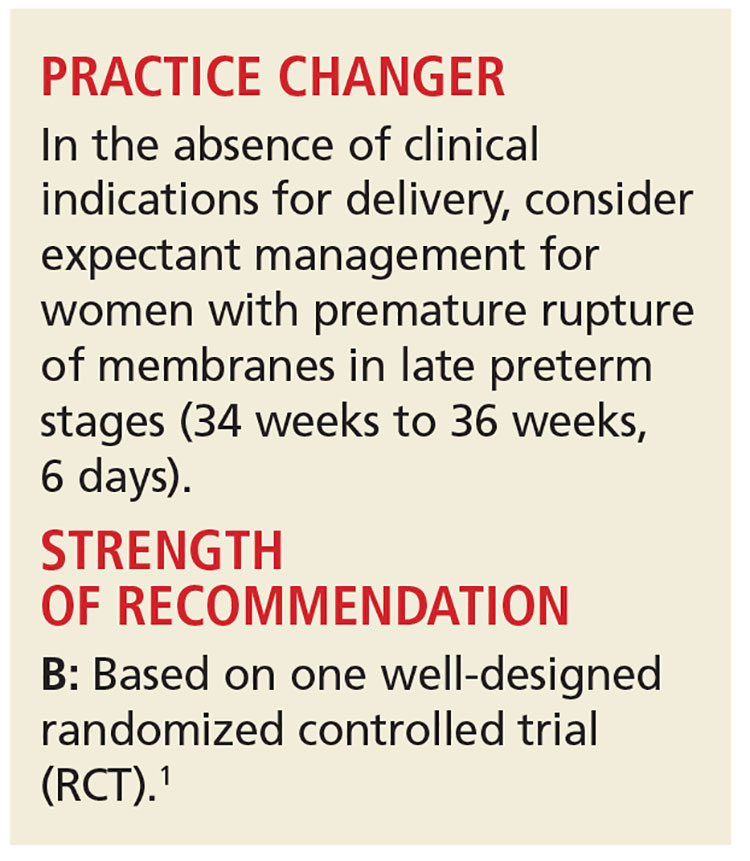
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Skip the Compression Stockings Following DVT
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
Skip the compression stockings following DVT
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Corticosteroids for a Sore Throat?
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat; this has been found to provide quick pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.1
ILLUSTRATIVE CASE
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past two days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A β-hemolytic Streptococcus infections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?
Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk for adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may be seeking primarily pain relief.8 Thus, a treatment that is more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
STUDY SUMMARY
Steroids bring speedier pain relief
This meta-analysis included eight RCTs (the same eight trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (n = 413); the other three studied children (n = 393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A β-hemolytic Streptococcus.
In all eight RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia (either acetaminophen or NSAIDs). Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied between studies. Four of the eight RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in five of the eight studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were three times more likely to report complete resolution of symptoms at 24 hours (relative risk = 3.2) and had a reduced mean time to onset of pain relief of about six hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was < 4.
Adverse events were reported in only one of the trials (n = 125): Five patients (three in the steroid group and two receiving placebo) were hospitalized for fluid rehydration, and three patients (one in the steroid group and two receiving placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and four trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
WHAT’S NEW
Steroids haven’t been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk for harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary use of antibiotics.
CAVEATS
Questions about effects on antibiotic use, heterogeneity
The studies in this meta-analysis did not assess whether the use of corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all subgroups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk for exacerbation of an existing infection. That did not occur in these studies. Nor has it occurred when short courses of corticosteroids are used for other illnesses, such as croup, infectious mononucleosis, asthma, contact dermatitis, and COPD.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
CHALLENGES TO
IMPLEMENTATION
Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
REFERENCES
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med. 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines. J Eval Clin Pract. 2011;17:1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12): CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(7):372-374.
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat; this has been found to provide quick pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.1
ILLUSTRATIVE CASE
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past two days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A β-hemolytic Streptococcus infections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?
Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk for adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may be seeking primarily pain relief.8 Thus, a treatment that is more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
STUDY SUMMARY
Steroids bring speedier pain relief
This meta-analysis included eight RCTs (the same eight trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (n = 413); the other three studied children (n = 393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A β-hemolytic Streptococcus.
In all eight RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia (either acetaminophen or NSAIDs). Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied between studies. Four of the eight RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in five of the eight studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were three times more likely to report complete resolution of symptoms at 24 hours (relative risk = 3.2) and had a reduced mean time to onset of pain relief of about six hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was < 4.
Adverse events were reported in only one of the trials (n = 125): Five patients (three in the steroid group and two receiving placebo) were hospitalized for fluid rehydration, and three patients (one in the steroid group and two receiving placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and four trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
WHAT’S NEW
Steroids haven’t been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk for harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary use of antibiotics.
CAVEATS
Questions about effects on antibiotic use, heterogeneity
The studies in this meta-analysis did not assess whether the use of corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all subgroups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk for exacerbation of an existing infection. That did not occur in these studies. Nor has it occurred when short courses of corticosteroids are used for other illnesses, such as croup, infectious mononucleosis, asthma, contact dermatitis, and COPD.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
CHALLENGES TO
IMPLEMENTATION
Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
REFERENCES
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med. 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines. J Eval Clin Pract. 2011;17:1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12): CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(7):372-374.
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat; this has been found to provide quick pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.1
ILLUSTRATIVE CASE
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past two days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A β-hemolytic Streptococcus infections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?
Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk for adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may be seeking primarily pain relief.8 Thus, a treatment that is more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
STUDY SUMMARY
Steroids bring speedier pain relief
This meta-analysis included eight RCTs (the same eight trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (n = 413); the other three studied children (n = 393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A β-hemolytic Streptococcus.
In all eight RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia (either acetaminophen or NSAIDs). Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied between studies. Four of the eight RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in five of the eight studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were three times more likely to report complete resolution of symptoms at 24 hours (relative risk = 3.2) and had a reduced mean time to onset of pain relief of about six hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was < 4.
Adverse events were reported in only one of the trials (n = 125): Five patients (three in the steroid group and two receiving placebo) were hospitalized for fluid rehydration, and three patients (one in the steroid group and two receiving placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and four trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
WHAT’S NEW
Steroids haven’t been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk for harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary use of antibiotics.
CAVEATS
Questions about effects on antibiotic use, heterogeneity
The studies in this meta-analysis did not assess whether the use of corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all subgroups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk for exacerbation of an existing infection. That did not occur in these studies. Nor has it occurred when short courses of corticosteroids are used for other illnesses, such as croup, infectious mononucleosis, asthma, contact dermatitis, and COPD.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
CHALLENGES TO
IMPLEMENTATION
Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
REFERENCES
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med. 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines. J Eval Clin Pract. 2011;17:1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12): CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(7):372-374.
Corticosteroids for a sore throat?
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat, which has been found to bring quicker pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.
Hayward G, Thompson M, Perera R, et al. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
Illustrative case
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past 2 days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A B-hemolytic Streptococcusinfections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk of adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may primarily be seeking pain relief.8 Thus, a treatment that’s more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
Study summary: Steroids bring speedier pain relief
This meta-analysis included 8 RCTs (the same 8 trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (N=413); the other 3 studied children (N=393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A B-hemolytic Streptococcus.
In all 8 RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia— either acetaminophen or nonsteroidal anti-inflammatory drugs. Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied among the studies. Four of the 8 RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in 5 of the 8 studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were 3 times more likely to report complete resolution of symptoms at 24 hours (relative risk=3.2; 95% confidence interval, 2.0-5.1; P<.001) and had a reduced mean time to onset of pain relief of about 6 hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was <4.
Adverse events were reported in only one of the trials (N=125): Five patients (3 in the steroid group and 2 on placebo) were hospitalized for fluid rehydration, and 3 patients (one in the steroid group and 2 on placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and 4 trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
What's new: Steroids haven't been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk of harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary antibiotic use.
Caveats: Questions about effects on antibiotic use, heterogeneity remain
The studies in this meta-analysis did not assess whether the use corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all sub-groups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk of exacerbating an existing infection. That did not occur in these studies. Nor has it occurred when used for short courses in other illnesses such as croup, infectious mononucleosis, asthma, contact dermatitis, and chronic obstructive pulmonary disease.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
Challenges to implementation: Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat, and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant number ul1rr024999 from the national center for research resources, a clinical Translational Science award to the university of chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the national center for research resources or the national institutes of health.
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane
Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med . 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines.
J Eval Clin Pract. 2011;17: 1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12):CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat, which has been found to bring quicker pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.
Hayward G, Thompson M, Perera R, et al. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
Illustrative case
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past 2 days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A B-hemolytic Streptococcusinfections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk of adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may primarily be seeking pain relief.8 Thus, a treatment that’s more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
Study summary: Steroids bring speedier pain relief
This meta-analysis included 8 RCTs (the same 8 trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (N=413); the other 3 studied children (N=393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A B-hemolytic Streptococcus.
In all 8 RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia— either acetaminophen or nonsteroidal anti-inflammatory drugs. Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied among the studies. Four of the 8 RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in 5 of the 8 studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were 3 times more likely to report complete resolution of symptoms at 24 hours (relative risk=3.2; 95% confidence interval, 2.0-5.1; P<.001) and had a reduced mean time to onset of pain relief of about 6 hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was <4.
Adverse events were reported in only one of the trials (N=125): Five patients (3 in the steroid group and 2 on placebo) were hospitalized for fluid rehydration, and 3 patients (one in the steroid group and 2 on placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and 4 trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
What's new: Steroids haven't been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk of harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary antibiotic use.
Caveats: Questions about effects on antibiotic use, heterogeneity remain
The studies in this meta-analysis did not assess whether the use corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all sub-groups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk of exacerbating an existing infection. That did not occur in these studies. Nor has it occurred when used for short courses in other illnesses such as croup, infectious mononucleosis, asthma, contact dermatitis, and chronic obstructive pulmonary disease.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
Challenges to implementation: Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat, and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant number ul1rr024999 from the national center for research resources, a clinical Translational Science award to the university of chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the national center for research resources or the national institutes of health.
PRACTICE CHANGER
Consider prescribing a single dose of corticosteroids for patients with sore throat, which has been found to bring quicker pain relief and resolution of symptoms.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) in ambulatory care settings.
Hayward G, Thompson M, Perera R, et al. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CDC008268.
Illustrative case
A 28-year-old woman comes to your clinic because she’s had a severe sore throat and low-grade fever for the past 2 days. She has no associated cough. Examination reveals erythematous posterior oropharynx with exudate. A rapid strep test is negative. The patient says the sore throat is very painful and asks for medication to make it better. What should you prescribe?
Most sore throats—particularly in adults—are viral and self-limiting.2,3 Group A B-hemolytic Streptococcusinfections account for just 10% of sore throats in adults and 15% to 30% in children.4 Yet US physicians have been found to prescribe antibiotics for more than half of patients who present with sore throat.5-7
Do patients want antibiotics, or simply pain relief?Antibiotics produce only a modest reduction in symptoms of pharyngitis (fever and throat soreness), presumably in patients with bacterial infections, and increase the risk of adverse events.5,6 Research suggests that patients who request antibiotics for sore throat may primarily be seeking pain relief.8 Thus, a treatment that’s more effective in alleviating symptoms of a sore throat would likely contribute to a decrease in unnecessary use of antibiotics.
A short course of corticosteroids has been used successfully and shown to be safe for conditions such as acute sinusitis, croup, and asthma.9-11 Could the anti-inflammatory effects of corticosteroids reduce pain in patients with sore throat, as well? A 2010 systematic review suggested that was the case.12 Cochrane reviewers recently took another look.1
Study summary: Steroids bring speedier pain relief
This meta-analysis included 8 RCTs (the same 8 trials used in the systematic review9) that compared corticosteroids with placebo for the symptomatic treatment of exudative or severe sore throat.1 Sore throat was defined as clinical evidence of pharyngitis and/or tonsillitis or the clinical syndrome of painful throat and odynophagia.
Five studies were conducted in the United States, and one each in Canada, Turkey, and Israel. Five studies focused on adults (N=413); the other 3 studied children (N=393). Overall, 47% of participants had exudative sore throat, and 44% were positive for group A B-hemolytic Streptococcus.
In all 8 RCTs, antibiotics were given to those in both the treatment and placebo groups. In addition, all participants were allowed to use traditional analgesia— either acetaminophen or nonsteroidal anti-inflammatory drugs. Corticosteroids (oral dexamethasone, oral prednisone, or intramuscular [IM] dexamethasone) were used as an adjunctive treatment in all the RCTs.
Primary outcomes varied among the studies. Four of the 8 RCTs included the proportion of patients with improvement or complete resolution of symptoms within 24 to 48 hours. Mean time to onset of pain relief was the primary outcome in 5 of the 8 studies. Some of the secondary outcomes in the individual trials included relapse rates, adverse events, and days missed from school or work.
Overall, patients who received corticosteroids were 3 times more likely to report complete resolution of symptoms at 24 hours (relative risk=3.2; 95% confidence interval, 2.0-5.1; P<.001) and had a reduced mean time to onset of pain relief of about 6 hours. The number needed to treat to prevent one patient from experiencing pain at 24 hours was <4.
Adverse events were reported in only one of the trials (N=125): Five patients (3 in the steroid group and 2 on placebo) were hospitalized for fluid rehydration, and 3 patients (one in the steroid group and 2 on placebo) developed peritonsillar abscess.12 Three RCTs did not find any significant difference in days missed from school or work, and 4 trials reported no difference in recurrence of symptoms. One of the trials found that 16% of the patients in the placebo group returned to seek additional care, while none in the steroid group did.13
What's new: Steroids haven't been tested as standalone treatment
Steroids are not currently recommended for routine use to treat symptoms of sore throat. This Cochrane review found that patients with severe or exudative sore throat benefit from pain reduction with corticosteroids, used as an adjunct to antibiotics and other analgesics without increased risk of harm. Nonetheless, the use of steroids in this patient population would address a practical concern of those seeking symptom relief and has the potential to decrease unnecessary antibiotic use.
Caveats: Questions about effects on antibiotic use, heterogeneity remain
The studies in this meta-analysis did not assess whether the use corticosteroids would reduce unnecessary use of antibiotics, so we cannot conclude that this would be the case. Because the effect was similar in all sub-groups analyzed, however, it is reasonable to expect that reduced antibiotic use could be a positive effect. The main documented benefit was resolution of pain, an important patient-centered outcome that justifies consideration of treating painful pharyngitis with corticosteroids.
Corticosteroids have an immunosuppressant effect and carry the theoretical risk of exacerbating an existing infection. That did not occur in these studies. Nor has it occurred when used for short courses in other illnesses such as croup, infectious mononucleosis, asthma, contact dermatitis, and chronic obstructive pulmonary disease.14 Thus, this theoretical risk is not a barrier to implementation.
It is important to note that single and multiple doses of corticosteroids and oral and IM routes were effective, with only minimal differences in results.
Challenges to implementation: Determining the severity
Acetaminophen and NSAIDs are used for pain relief in sore throat, and have been shown to be effective—but may be inadequate for severe pain.15 There are no head-to-head trials that have compared steroids to NSAIDs or acetaminophen in this clinical scenario. So the challenge for clinicians will be to decide when pharyngitis is severe enough to justify the use of corticosteroids, rather than simple analgesics alone.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant number ul1rr024999 from the national center for research resources, a clinical Translational Science award to the university of chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the national center for research resources or the national institutes of health.
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane
Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med . 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines.
J Eval Clin Pract. 2011;17: 1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12):CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
1. Hayward G, Thompson M, Perera R, et al. Corticosteroids as stand-alone or add-on treatment for sore throat. Cochrane
Database Syst Rev. 2012;(10):CDC008268.
2. Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;328:1-32.
3. Bisno AL. Acute pharyngitis. N Engl J Med . 2001;344:205-211.
4. Del Mar CB, Glasziou PP, Sprinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023.
5. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989-1999. JAMA. 2001;286:1181-1186.
6. Linder JA, Bates DW, Lee GM, et al. Antibiotic treatment of children with sore throat. JAMA. 2005;294:2315-2322.
7. Hong SY, Taur Y, Jordan MR. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines.
J Eval Clin Pract. 2011;17: 1176-1183.
8. van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4:494-499.
9. Venekamp RP, Thompson MJ, Hayward G, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011;(12):CD008115.
10. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.
11. Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178.
12. Korb K, Scherer M, Cenot J. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med. 2010;8:58-63.
13. Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159:278-282.
14. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975-994.
15. Wei JL, Kasperbauer JL, Weaver AL, et al. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112:87-93.
Copyright © 2013. The Family Physicians Inquiries Network. All Rights Reserved.

