User login
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
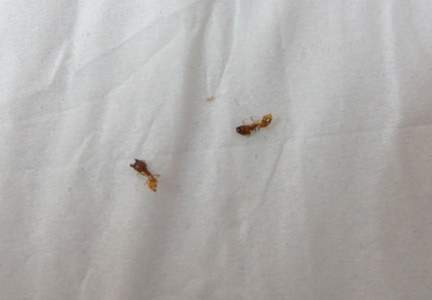
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.
What Is Your Diagnosis? Idiopathic Guttate Hypomelanosis
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.
Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.


Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.


Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
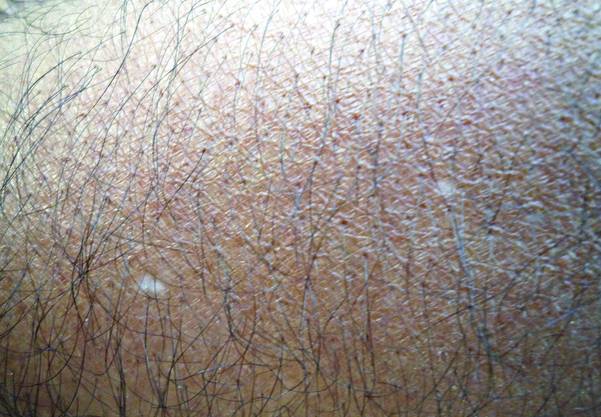
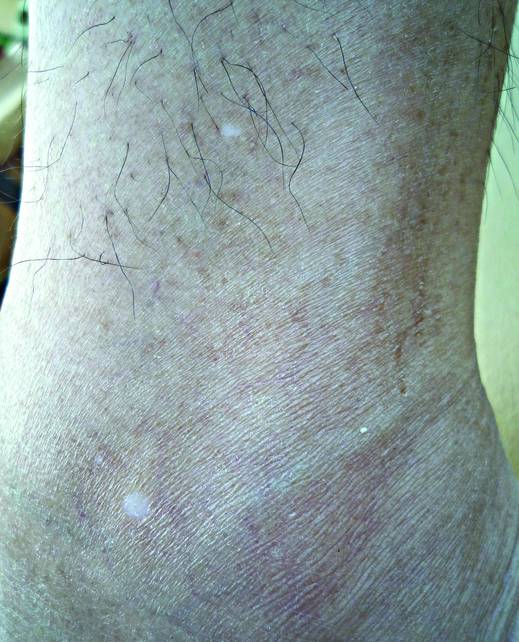
A 58-year-old man presented with disseminated, hypopigmented, asymptomatic lesions on the right arm (top) and left leg (bottom) that had been present for approximately 6 years. The patient reported that the lesions had become more visible and greater in number within the last year. Multiple circular hypopigmented macules of various sizes ranging from 1 to 3 mm in diameter were identified. No scaling was seen. Physical examination was otherwise unremarkable.
Indurated Erythematous Papules and Plaques on the Forearm
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
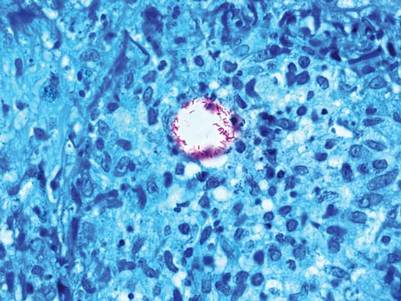
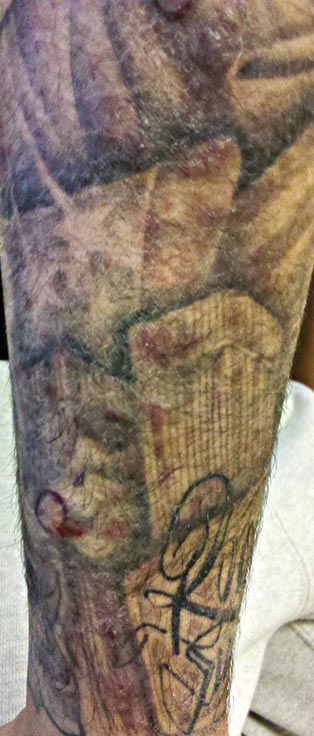
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
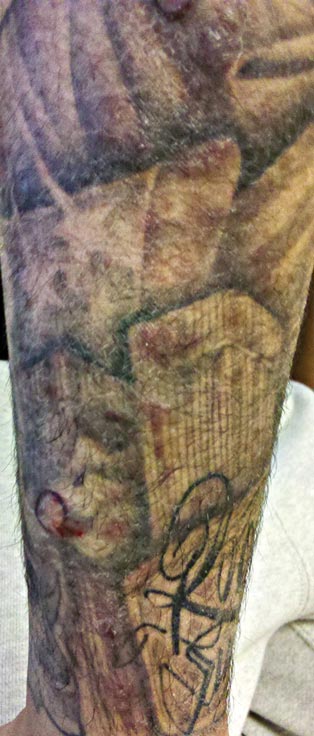
A 21-year-old man presented with growing, mildly pruritic, cutaneous papules and plaques on the right extensor forearm of 3 weeks’ duration. The lesions appeared 1 week after receiving a tattoo on the arm. One year prior the patient had a similar tattoo placed on another section of the right arm without any complications. The patient was afebrile and denied a history of sarcoidosis. Physical examination revealed indurated erythematous papules and plaques on the right extensor forearm that were most prominent in the gray-colored areas of the tattoo.