User login
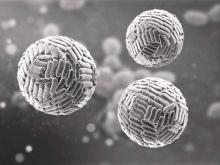
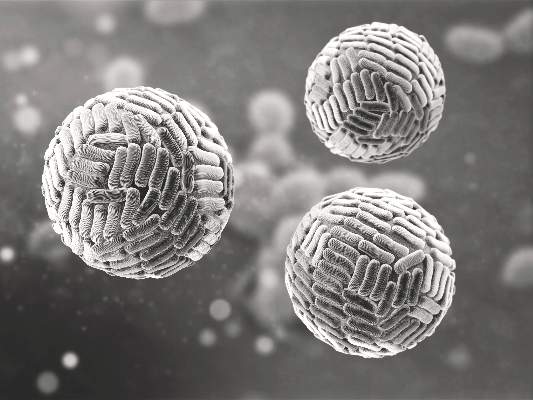
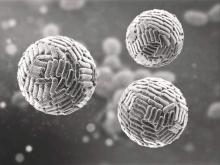
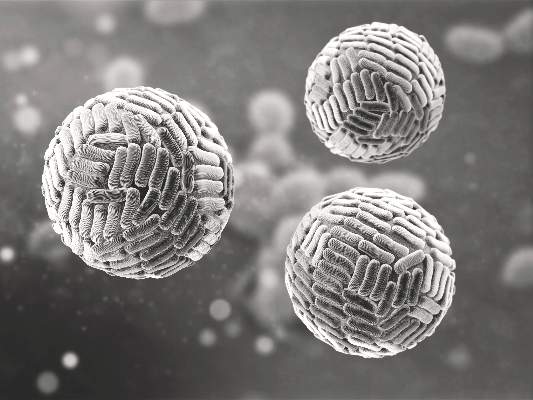
Zika Vaccine Development to Get Underway
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
Zika vaccine development to get underway
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has announced that they will provide up to $21.9 million to help develop a vaccine for the Zika virus.
Emergent BioSolutions’ Center for Innovation in Advanced Development and Manufacturing will conduct the early stages of vaccine development and will submit any candidate vaccines for an investigational drug request to the Food and Drug Administration to begin clinical studies.
At any stage in development, the technology could be transferred to other vaccine manufacturers to produce and market any vaccine developed.
“The threat posed by Zika presents an urgent need for vaccines and diagnostics,” Richard J. Hatchett, MD, acting director of ASPR’s Biomedical Advanced Research and Development Authority, said in a statement. “To meet that need as quickly as possible, we need to leverage the infrastructure, experience, and expertise available within BARDA, other federal agencies, industry, and academia.”
Rising expenses for hospital stays pressure patients
Total patient out-of-pocket cost sharing – per inpatient hospitalization – is on the rise, driven by a number of insurance benefit design changes in recent years, according to a new study.
Researchers at the University of Michigan, Ann Arbor, performed a retrospective analysis of medical claims for 7.3 million hospitalizations using data from Aetna, UnitedHealthcare and Humana, and found that between 2009 and 2013, total cost sharing per inpatient hospitalization for patients aged 18-64 years increased by 37% – to $1,013 in 2013 from $738 in 2009 – after adjusting for inflation and case-mix differences.
“We found that the growth in cost sharing was driven primarily by increases in the amount applied to patients’ deductibles, which rose by 86%, and by increases in coinsurance, which grew by 33% during the study period, rather than by copayments,” wrote Emily Adrion, Ph.D., of the University of Michigan, Ann Arbor, and colleagues in a study published online in JAMA Internal Medicine (JAMA Intern Med. 2016 Jun 27. doi:10.1001/jamainternmed.2016.3663).
“Our findings indicate a trend toward fewer plans requiring copayments at the point of service and more plans requiring higher coinsurance and deductibles after care is delivered,” the authors said.
The investigators called these trends “notable,” as research cited in the article suggests “that most Americans lack a basic understanding of the different forms of cost sharing associated with medical care.”
Dr. Adrion and colleagues also point out a large part of the variation in cost sharing for inpatient hospitalizations is driven by the type of insurance product. Those enrolled in individual market and consumer-directed health plans were associated with significantly higher cost sharing for inpatient hospitalizations during the study period.
“Increasing cost sharing may also reflect a recent elevation in insurer and provider consolidation, which may limit competition and increase costs for beneficiaries,” the authors noted, adding that future research should “assess the market dynamics” underlying patient cost sharing.
“With an estimated 85% of all commercial health insurance benefit packages requiring coinsurance for inpatient hospitalizations in addition to meeting an annual deductible, cost sharing for inpatient hospitalization remains an important, if often overlooked, area for policy reform,” Dr. Adrion and colleagues concluded.
The report’s authors claimed no financial conflicts of interest.
As plans begin requiring more and more cost sharing, the role of insurance moves further away from what it is designed to do.
“Even our language suggests that we have forgotten that the core purpose of health insurance is to protect people when an unexpected problem develops,” Dr. Mitchell Katz said in an editor’s note published June 27 online in JAMA Internal Medicine. “When we speak of coinsurance, we mean the portion of the bill that the person has to pay: there is no ‘coinsurer.’ ”
Dr. Katz is advocating for no out-of-pocket expenses for unavoidable emergency hospitalizations.
“To require consumers to pay large amounts of out-of-pocket expenses for health care may lead to delay or foregoing of needed care or to financial ruin, the latter of which insurance is supposed to protect you against,” Dr. Katz wrote. “There are no easy answers for how to deal with the rising cost of medical care, but increasing out-of-pocket spending for unavoidable, necessary care is counter to the goals of a health insurance system.”
Dr. Mitchell Katz of Los Angeles is deputy editor of JAMA Internal Medicine. The comments above were adapted from his Editor’s Note accompanying the study. He reported no conflicts of interest.
As plans begin requiring more and more cost sharing, the role of insurance moves further away from what it is designed to do.
“Even our language suggests that we have forgotten that the core purpose of health insurance is to protect people when an unexpected problem develops,” Dr. Mitchell Katz said in an editor’s note published June 27 online in JAMA Internal Medicine. “When we speak of coinsurance, we mean the portion of the bill that the person has to pay: there is no ‘coinsurer.’ ”
Dr. Katz is advocating for no out-of-pocket expenses for unavoidable emergency hospitalizations.
“To require consumers to pay large amounts of out-of-pocket expenses for health care may lead to delay or foregoing of needed care or to financial ruin, the latter of which insurance is supposed to protect you against,” Dr. Katz wrote. “There are no easy answers for how to deal with the rising cost of medical care, but increasing out-of-pocket spending for unavoidable, necessary care is counter to the goals of a health insurance system.”
Dr. Mitchell Katz of Los Angeles is deputy editor of JAMA Internal Medicine. The comments above were adapted from his Editor’s Note accompanying the study. He reported no conflicts of interest.
As plans begin requiring more and more cost sharing, the role of insurance moves further away from what it is designed to do.
“Even our language suggests that we have forgotten that the core purpose of health insurance is to protect people when an unexpected problem develops,” Dr. Mitchell Katz said in an editor’s note published June 27 online in JAMA Internal Medicine. “When we speak of coinsurance, we mean the portion of the bill that the person has to pay: there is no ‘coinsurer.’ ”
Dr. Katz is advocating for no out-of-pocket expenses for unavoidable emergency hospitalizations.
“To require consumers to pay large amounts of out-of-pocket expenses for health care may lead to delay or foregoing of needed care or to financial ruin, the latter of which insurance is supposed to protect you against,” Dr. Katz wrote. “There are no easy answers for how to deal with the rising cost of medical care, but increasing out-of-pocket spending for unavoidable, necessary care is counter to the goals of a health insurance system.”
Dr. Mitchell Katz of Los Angeles is deputy editor of JAMA Internal Medicine. The comments above were adapted from his Editor’s Note accompanying the study. He reported no conflicts of interest.
Total patient out-of-pocket cost sharing – per inpatient hospitalization – is on the rise, driven by a number of insurance benefit design changes in recent years, according to a new study.
Researchers at the University of Michigan, Ann Arbor, performed a retrospective analysis of medical claims for 7.3 million hospitalizations using data from Aetna, UnitedHealthcare and Humana, and found that between 2009 and 2013, total cost sharing per inpatient hospitalization for patients aged 18-64 years increased by 37% – to $1,013 in 2013 from $738 in 2009 – after adjusting for inflation and case-mix differences.
“We found that the growth in cost sharing was driven primarily by increases in the amount applied to patients’ deductibles, which rose by 86%, and by increases in coinsurance, which grew by 33% during the study period, rather than by copayments,” wrote Emily Adrion, Ph.D., of the University of Michigan, Ann Arbor, and colleagues in a study published online in JAMA Internal Medicine (JAMA Intern Med. 2016 Jun 27. doi:10.1001/jamainternmed.2016.3663).
“Our findings indicate a trend toward fewer plans requiring copayments at the point of service and more plans requiring higher coinsurance and deductibles after care is delivered,” the authors said.
The investigators called these trends “notable,” as research cited in the article suggests “that most Americans lack a basic understanding of the different forms of cost sharing associated with medical care.”
Dr. Adrion and colleagues also point out a large part of the variation in cost sharing for inpatient hospitalizations is driven by the type of insurance product. Those enrolled in individual market and consumer-directed health plans were associated with significantly higher cost sharing for inpatient hospitalizations during the study period.
“Increasing cost sharing may also reflect a recent elevation in insurer and provider consolidation, which may limit competition and increase costs for beneficiaries,” the authors noted, adding that future research should “assess the market dynamics” underlying patient cost sharing.
“With an estimated 85% of all commercial health insurance benefit packages requiring coinsurance for inpatient hospitalizations in addition to meeting an annual deductible, cost sharing for inpatient hospitalization remains an important, if often overlooked, area for policy reform,” Dr. Adrion and colleagues concluded.
The report’s authors claimed no financial conflicts of interest.
Total patient out-of-pocket cost sharing – per inpatient hospitalization – is on the rise, driven by a number of insurance benefit design changes in recent years, according to a new study.
Researchers at the University of Michigan, Ann Arbor, performed a retrospective analysis of medical claims for 7.3 million hospitalizations using data from Aetna, UnitedHealthcare and Humana, and found that between 2009 and 2013, total cost sharing per inpatient hospitalization for patients aged 18-64 years increased by 37% – to $1,013 in 2013 from $738 in 2009 – after adjusting for inflation and case-mix differences.
“We found that the growth in cost sharing was driven primarily by increases in the amount applied to patients’ deductibles, which rose by 86%, and by increases in coinsurance, which grew by 33% during the study period, rather than by copayments,” wrote Emily Adrion, Ph.D., of the University of Michigan, Ann Arbor, and colleagues in a study published online in JAMA Internal Medicine (JAMA Intern Med. 2016 Jun 27. doi:10.1001/jamainternmed.2016.3663).
“Our findings indicate a trend toward fewer plans requiring copayments at the point of service and more plans requiring higher coinsurance and deductibles after care is delivered,” the authors said.
The investigators called these trends “notable,” as research cited in the article suggests “that most Americans lack a basic understanding of the different forms of cost sharing associated with medical care.”
Dr. Adrion and colleagues also point out a large part of the variation in cost sharing for inpatient hospitalizations is driven by the type of insurance product. Those enrolled in individual market and consumer-directed health plans were associated with significantly higher cost sharing for inpatient hospitalizations during the study period.
“Increasing cost sharing may also reflect a recent elevation in insurer and provider consolidation, which may limit competition and increase costs for beneficiaries,” the authors noted, adding that future research should “assess the market dynamics” underlying patient cost sharing.
“With an estimated 85% of all commercial health insurance benefit packages requiring coinsurance for inpatient hospitalizations in addition to meeting an annual deductible, cost sharing for inpatient hospitalization remains an important, if often overlooked, area for policy reform,” Dr. Adrion and colleagues concluded.
The report’s authors claimed no financial conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Medicare Trust Fund projected to run dry in 2028
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
AAP promotes streamlined tools to help screen for pediatric substance use
While the overall American Academy of Pediatrics policy objectives on screening for pediatric substance use have not changed, the academy is highlighting streamlined screening tools in a new clinical report.
The AAP remains focused on pushing for abstinence. That being said, the organization recommends “that pediatricians screen kids for substance use and talk about substance use,” lead author Dr. Sharon Levy said in an interview. “The big picture, bottom line, [is that] nothing has changed. It’s just an opportunity to reemphasize that we think talking about substance use is an important part of pediatric care.”
What has changed are the tools highlighted in the clinical report, published online June 20 (Pediatrics. 2016 Jul. doi: 10.1542/peds.2016-1211).
“We simplified the algorithm quite a bit,” said Dr. Levy, director of the adolescent substance abuse program at Boston Children’s Hospital. “We demonstrated use of more screening tools that are a bit simpler to use, a bit shorter, and lead more directly to intervention. For people who are using the older tools, that’s fine if that’s really been established in their practice and [they] are doing a good job with [those tools].”
However, Dr. Levy noted that many of the established screening tools were not being used, or were being shortened or modified in others ways to render them less effective.
“We recommend … new tools that we call frequency-based screening tools,” she explained. Pediatric patients are given a risk level based on their answer to a question regarding how many times in the past year they’ve used alcohol, marijuana, or tobacco.
Slightly different interventions are recommended based on the child’s risk level, including counseling about substance use and some strategies, especially with kids with heavier use, for conducting the conversation in a way that will make kids more likely to consider behavior change, she sa
The clinical report suggests that when no substance use is disclosed, doctors should offer positive reinforcement about smart decision making. For patients disclosing one or two uses per year with a low likelihood of having a substance use disorder, doctors should be promoting abstaining from use to support a healthy lifestyle, as well as discussions on the negative health effects of substance use. As the response to frequency of use increases and the greater likelihood of substance abuse disorder grows, so does the level of questions, including understanding the types of substances being used, coupled with more counseling on quitting use, and, in severe cases, referring for treatment.
The common theme in all the advice given to patients, though, is the importance of abstinence from substance use.
“The American Academy of Pediatrics recommends abstinence as the best health recommendation for adolescents,” Dr. Levy said. “That sometimes gets lost in the shuffle. That really is something that stands out in the policy, and that substance use is such an important health risk behavior that it should be addressed as part of routine health care for adolescents.”
The authors disclosed no relevant financial conflicts.
While the overall American Academy of Pediatrics policy objectives on screening for pediatric substance use have not changed, the academy is highlighting streamlined screening tools in a new clinical report.
The AAP remains focused on pushing for abstinence. That being said, the organization recommends “that pediatricians screen kids for substance use and talk about substance use,” lead author Dr. Sharon Levy said in an interview. “The big picture, bottom line, [is that] nothing has changed. It’s just an opportunity to reemphasize that we think talking about substance use is an important part of pediatric care.”
What has changed are the tools highlighted in the clinical report, published online June 20 (Pediatrics. 2016 Jul. doi: 10.1542/peds.2016-1211).
“We simplified the algorithm quite a bit,” said Dr. Levy, director of the adolescent substance abuse program at Boston Children’s Hospital. “We demonstrated use of more screening tools that are a bit simpler to use, a bit shorter, and lead more directly to intervention. For people who are using the older tools, that’s fine if that’s really been established in their practice and [they] are doing a good job with [those tools].”
However, Dr. Levy noted that many of the established screening tools were not being used, or were being shortened or modified in others ways to render them less effective.
“We recommend … new tools that we call frequency-based screening tools,” she explained. Pediatric patients are given a risk level based on their answer to a question regarding how many times in the past year they’ve used alcohol, marijuana, or tobacco.
Slightly different interventions are recommended based on the child’s risk level, including counseling about substance use and some strategies, especially with kids with heavier use, for conducting the conversation in a way that will make kids more likely to consider behavior change, she sa
The clinical report suggests that when no substance use is disclosed, doctors should offer positive reinforcement about smart decision making. For patients disclosing one or two uses per year with a low likelihood of having a substance use disorder, doctors should be promoting abstaining from use to support a healthy lifestyle, as well as discussions on the negative health effects of substance use. As the response to frequency of use increases and the greater likelihood of substance abuse disorder grows, so does the level of questions, including understanding the types of substances being used, coupled with more counseling on quitting use, and, in severe cases, referring for treatment.
The common theme in all the advice given to patients, though, is the importance of abstinence from substance use.
“The American Academy of Pediatrics recommends abstinence as the best health recommendation for adolescents,” Dr. Levy said. “That sometimes gets lost in the shuffle. That really is something that stands out in the policy, and that substance use is such an important health risk behavior that it should be addressed as part of routine health care for adolescents.”
The authors disclosed no relevant financial conflicts.
While the overall American Academy of Pediatrics policy objectives on screening for pediatric substance use have not changed, the academy is highlighting streamlined screening tools in a new clinical report.
The AAP remains focused on pushing for abstinence. That being said, the organization recommends “that pediatricians screen kids for substance use and talk about substance use,” lead author Dr. Sharon Levy said in an interview. “The big picture, bottom line, [is that] nothing has changed. It’s just an opportunity to reemphasize that we think talking about substance use is an important part of pediatric care.”
What has changed are the tools highlighted in the clinical report, published online June 20 (Pediatrics. 2016 Jul. doi: 10.1542/peds.2016-1211).
“We simplified the algorithm quite a bit,” said Dr. Levy, director of the adolescent substance abuse program at Boston Children’s Hospital. “We demonstrated use of more screening tools that are a bit simpler to use, a bit shorter, and lead more directly to intervention. For people who are using the older tools, that’s fine if that’s really been established in their practice and [they] are doing a good job with [those tools].”
However, Dr. Levy noted that many of the established screening tools were not being used, or were being shortened or modified in others ways to render them less effective.
“We recommend … new tools that we call frequency-based screening tools,” she explained. Pediatric patients are given a risk level based on their answer to a question regarding how many times in the past year they’ve used alcohol, marijuana, or tobacco.
Slightly different interventions are recommended based on the child’s risk level, including counseling about substance use and some strategies, especially with kids with heavier use, for conducting the conversation in a way that will make kids more likely to consider behavior change, she sa
The clinical report suggests that when no substance use is disclosed, doctors should offer positive reinforcement about smart decision making. For patients disclosing one or two uses per year with a low likelihood of having a substance use disorder, doctors should be promoting abstaining from use to support a healthy lifestyle, as well as discussions on the negative health effects of substance use. As the response to frequency of use increases and the greater likelihood of substance abuse disorder grows, so does the level of questions, including understanding the types of substances being used, coupled with more counseling on quitting use, and, in severe cases, referring for treatment.
The common theme in all the advice given to patients, though, is the importance of abstinence from substance use.
“The American Academy of Pediatrics recommends abstinence as the best health recommendation for adolescents,” Dr. Levy said. “That sometimes gets lost in the shuffle. That really is something that stands out in the policy, and that substance use is such an important health risk behavior that it should be addressed as part of routine health care for adolescents.”
The authors disclosed no relevant financial conflicts.
FROM PEDIATRICS
Gastroenterologists call for collaborative approach to Part B drug payments
Gastroenterologists are calling on the Centers for Medicare & Medicaid Services to withdraw a proposed Part B drug payment demonstration and collaborate with physicians and patient groups to find a more equitable solution.
In March, the CMS proposed changing the current payment scheme by which physicians are reimbursed for administration of certain drugs at average sales price (ASP) plus 6%; instead, the agency proposes to test reimbursing physicians at ASP plus 2.5% and provide them with an additional flat fee of $16.80. The proposed rule also calls for testing value-based purchasing tools during a second phase of the overall 5-year demonstration project.
Participation in the test would be mandatory for physicians in 49 states (Maryland is excluded as it is part of another ongoing demonstration project), and physician practices would receive either the full current reimbursement or the proposed reduced percentage plus the flat fee.
The American Gastroenterological Association noted in comments with the agency that drug prices “are not set by physicians” and if the CMS “is searching for new methods to control costs, this is not the way to achieve those goals.”
“If you’re going to drop the margin from 6% to 2.5% plus a flat fee of $16.80, your ability to successfully economically provide that service starts to really go away,” Dr. Rajeev Jain, practice councillor at AGA, said in an interview.
He added that it could force patients to get their infusions in a hospital outpatient department. “Those are reimbursed at a much higher rate, and that is a much greater cost to Medicare and the health care system in general. In the office, we’re providing a very cost-efficient, patient-friendly way of providing these medications. … These kind of draconian cuts will make it less and less likely that the private practitioner will provide this additional service to their patients, and it will force them to get more of their care in HOPDs [hospital outpatient departments], which increases the cost plus is less efficient and less patient friendly.”
Dr. Jain also suggested that implementing such a change could put smaller practices out of business or at the very least limit the services they provide.
With “what’s going on now, this sort of evolution in the health care field with the Affordable Care Act and all these other economic pressures, physicians are either flipping and being bought by health care systems or they are merging themselves into consolidated larger practices to have these economies of scale,” he said. “But if you are just a three- or four-person GI practice and your overhead is substantial and because you are small, you’re not getting the best price on the medications. Your margins are probably slim, and this may just tip it enough where it’s not economically viable to give those infusions.”
The AGA joins a chorus of other physician groups who have called upon the CMS to withdraw its proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in its comments.
Likewise, the Coalition of State Rheumatology Organizations (CSRO) called for the withdrawal of the proposal.
The CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according to the coalition’s comments.
The AGA voiced concern that the CMS did not consult with physicians and patient advocates on crafting the proposal before releasing it.
“The AGA urges CMS to withdraw this proposal and instead, bring physicians and patient groups together to work on substantive solutions to the problems CMS hoped to address with these payment models,” the organization said in its comment letter. “We believe that a collaborative effort will benefit everyone involved.”
The lack of collaboration on a solution was a key point at a House Committee on Energy and Commerce health subcommittee hearing to review the CMS’s proposal.
Witnesses called into question one of the CMS’s primary reasons for the proposal – that the current ASP formula is leading doctors to choose medications based on cost.
The “CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher-priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the CMS to rescind the proposed rule.
Gastroenterologists are calling on the Centers for Medicare & Medicaid Services to withdraw a proposed Part B drug payment demonstration and collaborate with physicians and patient groups to find a more equitable solution.
In March, the CMS proposed changing the current payment scheme by which physicians are reimbursed for administration of certain drugs at average sales price (ASP) plus 6%; instead, the agency proposes to test reimbursing physicians at ASP plus 2.5% and provide them with an additional flat fee of $16.80. The proposed rule also calls for testing value-based purchasing tools during a second phase of the overall 5-year demonstration project.
Participation in the test would be mandatory for physicians in 49 states (Maryland is excluded as it is part of another ongoing demonstration project), and physician practices would receive either the full current reimbursement or the proposed reduced percentage plus the flat fee.
The American Gastroenterological Association noted in comments with the agency that drug prices “are not set by physicians” and if the CMS “is searching for new methods to control costs, this is not the way to achieve those goals.”
“If you’re going to drop the margin from 6% to 2.5% plus a flat fee of $16.80, your ability to successfully economically provide that service starts to really go away,” Dr. Rajeev Jain, practice councillor at AGA, said in an interview.
He added that it could force patients to get their infusions in a hospital outpatient department. “Those are reimbursed at a much higher rate, and that is a much greater cost to Medicare and the health care system in general. In the office, we’re providing a very cost-efficient, patient-friendly way of providing these medications. … These kind of draconian cuts will make it less and less likely that the private practitioner will provide this additional service to their patients, and it will force them to get more of their care in HOPDs [hospital outpatient departments], which increases the cost plus is less efficient and less patient friendly.”
Dr. Jain also suggested that implementing such a change could put smaller practices out of business or at the very least limit the services they provide.
With “what’s going on now, this sort of evolution in the health care field with the Affordable Care Act and all these other economic pressures, physicians are either flipping and being bought by health care systems or they are merging themselves into consolidated larger practices to have these economies of scale,” he said. “But if you are just a three- or four-person GI practice and your overhead is substantial and because you are small, you’re not getting the best price on the medications. Your margins are probably slim, and this may just tip it enough where it’s not economically viable to give those infusions.”
The AGA joins a chorus of other physician groups who have called upon the CMS to withdraw its proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in its comments.
Likewise, the Coalition of State Rheumatology Organizations (CSRO) called for the withdrawal of the proposal.
The CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according to the coalition’s comments.
The AGA voiced concern that the CMS did not consult with physicians and patient advocates on crafting the proposal before releasing it.
“The AGA urges CMS to withdraw this proposal and instead, bring physicians and patient groups together to work on substantive solutions to the problems CMS hoped to address with these payment models,” the organization said in its comment letter. “We believe that a collaborative effort will benefit everyone involved.”
The lack of collaboration on a solution was a key point at a House Committee on Energy and Commerce health subcommittee hearing to review the CMS’s proposal.
Witnesses called into question one of the CMS’s primary reasons for the proposal – that the current ASP formula is leading doctors to choose medications based on cost.
The “CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher-priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the CMS to rescind the proposed rule.
Gastroenterologists are calling on the Centers for Medicare & Medicaid Services to withdraw a proposed Part B drug payment demonstration and collaborate with physicians and patient groups to find a more equitable solution.
In March, the CMS proposed changing the current payment scheme by which physicians are reimbursed for administration of certain drugs at average sales price (ASP) plus 6%; instead, the agency proposes to test reimbursing physicians at ASP plus 2.5% and provide them with an additional flat fee of $16.80. The proposed rule also calls for testing value-based purchasing tools during a second phase of the overall 5-year demonstration project.
Participation in the test would be mandatory for physicians in 49 states (Maryland is excluded as it is part of another ongoing demonstration project), and physician practices would receive either the full current reimbursement or the proposed reduced percentage plus the flat fee.
The American Gastroenterological Association noted in comments with the agency that drug prices “are not set by physicians” and if the CMS “is searching for new methods to control costs, this is not the way to achieve those goals.”
“If you’re going to drop the margin from 6% to 2.5% plus a flat fee of $16.80, your ability to successfully economically provide that service starts to really go away,” Dr. Rajeev Jain, practice councillor at AGA, said in an interview.
He added that it could force patients to get their infusions in a hospital outpatient department. “Those are reimbursed at a much higher rate, and that is a much greater cost to Medicare and the health care system in general. In the office, we’re providing a very cost-efficient, patient-friendly way of providing these medications. … These kind of draconian cuts will make it less and less likely that the private practitioner will provide this additional service to their patients, and it will force them to get more of their care in HOPDs [hospital outpatient departments], which increases the cost plus is less efficient and less patient friendly.”
Dr. Jain also suggested that implementing such a change could put smaller practices out of business or at the very least limit the services they provide.
With “what’s going on now, this sort of evolution in the health care field with the Affordable Care Act and all these other economic pressures, physicians are either flipping and being bought by health care systems or they are merging themselves into consolidated larger practices to have these economies of scale,” he said. “But if you are just a three- or four-person GI practice and your overhead is substantial and because you are small, you’re not getting the best price on the medications. Your margins are probably slim, and this may just tip it enough where it’s not economically viable to give those infusions.”
The AGA joins a chorus of other physician groups who have called upon the CMS to withdraw its proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in its comments.
Likewise, the Coalition of State Rheumatology Organizations (CSRO) called for the withdrawal of the proposal.
The CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according to the coalition’s comments.
The AGA voiced concern that the CMS did not consult with physicians and patient advocates on crafting the proposal before releasing it.
“The AGA urges CMS to withdraw this proposal and instead, bring physicians and patient groups together to work on substantive solutions to the problems CMS hoped to address with these payment models,” the organization said in its comment letter. “We believe that a collaborative effort will benefit everyone involved.”
The lack of collaboration on a solution was a key point at a House Committee on Energy and Commerce health subcommittee hearing to review the CMS’s proposal.
Witnesses called into question one of the CMS’s primary reasons for the proposal – that the current ASP formula is leading doctors to choose medications based on cost.
The “CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher-priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the CMS to rescind the proposed rule.
Dermatologists call for delay in proposed Part B drug payment regs
Dermatologists are calling on the Centers for Medicare & Medicaid Services to delay implementation of the proposed regulations that will test new payment methods for Medicare Part B drugs.
CMS’s proposed rule is aimed at addressing the rising cost of drugs by targeting how doctors are reimbursed for the drugs that are administered in the physician office.
The agency argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools.
The American Academy of Dermatology Association (AADA) criticized the proposal in written comments submitted May 9. “While the proposal is meant to control Medicare drug spending, it does not address the main factor behind rising health care costs, the increasing cost of drugs,” according to the comment.
“I think [the proposal] needs an indefinite period of delay until the structure and issues can be worked out,” Dr. Sabra Sullivan, a dermatologist from Jackson, Miss., said in an interview. The main issue is the rising drug costs “and this Part B proposed rule really doesn’t address any of that or the price of drugs and how they are fluctuating. It is not just brand name drugs. Many generics [are having the same issue]. We try to use generics, but in many ways generics are costing as much as a brand name.”
Dr. Sullivan said that the rule “really hasn’t been fleshed out with support structures. There’s going to be a big possibility and probability of having many, many unintended consequences, including cutting off drugs to patients that are stable and denying access to drugs to people that need it.”
The AADA noted that “there is the potential for infusions to increasingly be administered at the hospital or infusion centers as opposed to the physician’s office. [The Medicare Payment Advisory Commission] predicted that small and solo practices may have a hard time obtaining expensive drugs for their patients. If it is not financially feasible to administer the drug in the provider’s office, they may direct patients to hospitals. If this is the case, there may be a higher copay for the patient and CMS will likely have to pay more for the administration of the drug in the hospital or infusion centers.”
Dr. Sullivan also challenged the assertion that there is a profit motive connected to the selection of appropriate drugs to administer to patients.
“People aren’t picking drugs because they cost more money and they make a lot of money,” Dr. Sullivan said.
AADA also noted that the proposed test, which would randomly assign zip codes for testing of the proposals, could affect dermatologists with multiple offices.
“Dermatologists often maintain practices at several different locations in order to provide access to a wider group of patients,” AADA noted in its comments. “Rural practices also often have multiple offices to meet the geographical need of patients. CMS’ proposal would require practices with multiple locations to comply with different components of the models, which would be both administratively complex and burdensome. The AADA recommends that CMS more carefully consider the impact of the testing groups on practices and provide an exemption for practices with multiple locations to ensure that all the branches of the practice test the same payment models.”
Dermatologists are calling on the Centers for Medicare & Medicaid Services to delay implementation of the proposed regulations that will test new payment methods for Medicare Part B drugs.
CMS’s proposed rule is aimed at addressing the rising cost of drugs by targeting how doctors are reimbursed for the drugs that are administered in the physician office.
The agency argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools.
The American Academy of Dermatology Association (AADA) criticized the proposal in written comments submitted May 9. “While the proposal is meant to control Medicare drug spending, it does not address the main factor behind rising health care costs, the increasing cost of drugs,” according to the comment.
“I think [the proposal] needs an indefinite period of delay until the structure and issues can be worked out,” Dr. Sabra Sullivan, a dermatologist from Jackson, Miss., said in an interview. The main issue is the rising drug costs “and this Part B proposed rule really doesn’t address any of that or the price of drugs and how they are fluctuating. It is not just brand name drugs. Many generics [are having the same issue]. We try to use generics, but in many ways generics are costing as much as a brand name.”
Dr. Sullivan said that the rule “really hasn’t been fleshed out with support structures. There’s going to be a big possibility and probability of having many, many unintended consequences, including cutting off drugs to patients that are stable and denying access to drugs to people that need it.”
The AADA noted that “there is the potential for infusions to increasingly be administered at the hospital or infusion centers as opposed to the physician’s office. [The Medicare Payment Advisory Commission] predicted that small and solo practices may have a hard time obtaining expensive drugs for their patients. If it is not financially feasible to administer the drug in the provider’s office, they may direct patients to hospitals. If this is the case, there may be a higher copay for the patient and CMS will likely have to pay more for the administration of the drug in the hospital or infusion centers.”
Dr. Sullivan also challenged the assertion that there is a profit motive connected to the selection of appropriate drugs to administer to patients.
“People aren’t picking drugs because they cost more money and they make a lot of money,” Dr. Sullivan said.
AADA also noted that the proposed test, which would randomly assign zip codes for testing of the proposals, could affect dermatologists with multiple offices.
“Dermatologists often maintain practices at several different locations in order to provide access to a wider group of patients,” AADA noted in its comments. “Rural practices also often have multiple offices to meet the geographical need of patients. CMS’ proposal would require practices with multiple locations to comply with different components of the models, which would be both administratively complex and burdensome. The AADA recommends that CMS more carefully consider the impact of the testing groups on practices and provide an exemption for practices with multiple locations to ensure that all the branches of the practice test the same payment models.”
Dermatologists are calling on the Centers for Medicare & Medicaid Services to delay implementation of the proposed regulations that will test new payment methods for Medicare Part B drugs.
CMS’s proposed rule is aimed at addressing the rising cost of drugs by targeting how doctors are reimbursed for the drugs that are administered in the physician office.
The agency argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools.
The American Academy of Dermatology Association (AADA) criticized the proposal in written comments submitted May 9. “While the proposal is meant to control Medicare drug spending, it does not address the main factor behind rising health care costs, the increasing cost of drugs,” according to the comment.
“I think [the proposal] needs an indefinite period of delay until the structure and issues can be worked out,” Dr. Sabra Sullivan, a dermatologist from Jackson, Miss., said in an interview. The main issue is the rising drug costs “and this Part B proposed rule really doesn’t address any of that or the price of drugs and how they are fluctuating. It is not just brand name drugs. Many generics [are having the same issue]. We try to use generics, but in many ways generics are costing as much as a brand name.”
Dr. Sullivan said that the rule “really hasn’t been fleshed out with support structures. There’s going to be a big possibility and probability of having many, many unintended consequences, including cutting off drugs to patients that are stable and denying access to drugs to people that need it.”
The AADA noted that “there is the potential for infusions to increasingly be administered at the hospital or infusion centers as opposed to the physician’s office. [The Medicare Payment Advisory Commission] predicted that small and solo practices may have a hard time obtaining expensive drugs for their patients. If it is not financially feasible to administer the drug in the provider’s office, they may direct patients to hospitals. If this is the case, there may be a higher copay for the patient and CMS will likely have to pay more for the administration of the drug in the hospital or infusion centers.”
Dr. Sullivan also challenged the assertion that there is a profit motive connected to the selection of appropriate drugs to administer to patients.
“People aren’t picking drugs because they cost more money and they make a lot of money,” Dr. Sullivan said.
AADA also noted that the proposed test, which would randomly assign zip codes for testing of the proposals, could affect dermatologists with multiple offices.
“Dermatologists often maintain practices at several different locations in order to provide access to a wider group of patients,” AADA noted in its comments. “Rural practices also often have multiple offices to meet the geographical need of patients. CMS’ proposal would require practices with multiple locations to comply with different components of the models, which would be both administratively complex and burdensome. The AADA recommends that CMS more carefully consider the impact of the testing groups on practices and provide an exemption for practices with multiple locations to ensure that all the branches of the practice test the same payment models.”
Doctors ask Congress to stop Part B drug payment test
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
WASHINGTON – Physician organizations are calling on Congress to stop a proposed federal regulation that would test how Medicare pays for drugs administered in a physician’s office.
Subcommittee member Rep. Larry Bucshon (R-Ind.), a cardiothoracic surgeon, called the premise of the proposed rule – that physicians are making prescribing decisions based on the cost of drugs – “almost an insult to the medical profession.” He has introduced legislation, H.R. 5122, that would require the Centers for Medicare & Medicaid Services to rescind the proposed rule.
CMS argues in the proposed rule that the current payment formula – average sales price (ASP) plus 6% – incentivizes the use of expensive drugs over lower-cost alternatives. Therefore, the agency seeks to run a test – half of doctors would continue to receive ASP plus 6%, while others would receive ASP plus 2.5% and a flat fee of $16.80. The proposed rule also would test other value-based tools. At the May 17 hearing, physicians presented their concerns regarding the proposed rule.
“CMS has yet to produce any evidence indicating that physician prescribing patterns show any correlation to that of choosing higher priced drugs as opposed to appropriate therapeutic treatment for patients,” said Dr. Debra A. Patt, vice president of Texas Oncology. Dr. Patt testified on behalf of the American Society of Clinical Oncology, the Community Oncology Alliance, and the U.S. Oncology Network. “Additionally, there is no evidence that the payment changes contemplated by CMS’s model will improve the quality of care, or for that matter, ensure patients have access to the same level of care they are currently receiving.”Dr. Michael Schweitz, national advocacy chair of the Coalition of State Rheumatology Organizations (CSRO), cited CMS’s admission that this rule will likely have no impact on ASP.
“While we appreciate CMS’s attention to the topic of drug costs, we feel that this proposal is misguided,” Dr. Schweitz testified. “As CMS acknowledges in the rule, the proposed approach ‘does not directly address the manufacturer’s ASP, which is a more significant driver of drug expenditures than the add-on payment amount for Part B drugs.’ Given that a slash to the ASP add-on is unlikely to actually lower costs for patients ... and may jeopardize access, we have requested that CMS withdraw the model and we urge the committee to do the same.”
Dr. Patt also questioned ASP, noting that it is simply an average, and the prices actually paid by rural and small group providers could be higher than larger group and hospital practices – and that difference puts the smaller and rural practices at a potentially significant financial disadvantage.
“Average sales price is by its very nature an average,” Dr. Patt testified. “Some people will pay higher amounts for procurement and some people will pay lower amounts. Larger hospital systems and larger practices have the ability to have contracting arrangements where they purchase at a lower price. … Smaller practices disproportionately pay a higher amount.”
Small practices that are slated to receive ASP plus 2.5% and that flat fee “could be losing money on all of the drugs that they buy. It will be impossible for smaller practices in rural areas to be open,” she added, noting that such a situation could cause care to be shifted to hospital outpatient departments, raising costs as well as access issues. “Cutting provider reimbursement without addressing the ASP, the actual cost of the drug in the first place, is just the wrong approach,” Rep. Bucshon said.
According to the proposed rule, whether or not a practice is in the test or control group would be based on ZIP code. Dr. Patt also noted that if she were in a test ZIP code and a neighboring ZIP code was not, she might have to refer patients to that area still receiving ASP plus 6% and “not at my center.”
Witnesses at the hearing also condemned the way CMS devised the proposed tests. Unlike the recent work on the Medicare Access and CHIP Reauthorization Act regulations and more specialized programs like the Oncology Care Model, Dr. Schweitz and Dr. Patt both testified that CMS made no outreach to the provider community with regards to getting their input before issuing the proposed rule.
They also took exception to the scope of the proposed test, which covers 49 of the 50 states (Maryland is excluded) and provides no mechanism for physicians to opt out under the proposed rule.
“I do see this as an experiment, but we conduct clinical research at our cancer and patients have informed consent,” Dr Patt said.
Dr. Schweitz agreed. “When you look at the goals of this plan, initially it appeared that it was to direct a way to save costs. But in meeting with [the Center for Medicare & Medicaid Innovation], we were advised that this is budget neutral. … So the goal of the program is to collect information which makes it a study, a test. So if the goal is to collect information, and the patients are part of that process, they should be signing informed consent.”
Several physician organizations have called on CMS to withdraw the proposal.
“We are deeply concerned that because the new methodology will frequently not properly cover the cost of physician administration of infused drugs, they will be forced to stop offering patients the ability to receive infusion treatments,” the American College of Rheumatology wrote in comments submitted on the proposed rule. Likewise, CSRO “must oppose the Part B drug payment model as it suffers from serious procedural and substantive flaws that we believe render it unworkable – and it does nothing to actually address drug prices,” according its comments.
While the proposal has garnered backlash from several directions, rheumatologists are seeing it as particularly burdensome because of the high price of medications with very limited options to substitute for lower-cost alternatives.
“Although we certainly seek to control costs for patients and Medicare whenever possible, the proposed new methodology does not adequately consider the higher average cost many of our physicians have acquiring, handling, administering, and billing for drugs and biologics,” according to the comments submitted by the ACR.
Indeed, comments from CSRO point out that when factoring in budget sequestration, the actual reimbursement physicians are receiving is ASP plus 4.4%, and doctors are actually losing money on certain drug purchases.
Of additional concern is that the proposed rule does not address ASP itself.
“A far greater concern than the add-on percentage is the underlying ASP, and the steep, fast price increases that these medications show each quarter, according to comments from the CSRO.
From 2007 to 2016, first-quarter ASP for infliximab rose from $53.73 to $79.90; ASP for abatacept rose from $18.70 to $39.44, according to CSRO comments. “These ASP increases are unsustainable for both the Medicare program and its beneficiaries, and we would like to work with CMS to explore actual solutions to stem the increases in those underlying prices.”
In its comments, ACR proposed a number of potential paths forward, starting with certain practices that should be exempted from the proposed demonstration: physician groups with 25 or fewer physicians; physician-owned practices that are located in rural and medically underserved areas; reimbursement changes for drugs and biologics that do not have an alternative with more than a 20% ASP differential; and drugs and biologics where there are three or fewer members of the drug class or biologics.
ACR also proposed altering the add-on formula that takes into account the costs of storing and administering supplies.
“For example, CMS could use a formula for reimbursement of ASP plus 6% or $500 (whichever is lower),” ACR said in its comments. “This formula would allow CMS to effectively target spending on expensive drugs, while leaving in place reimbursement rates for cheaper drugs.”
Additionally, ACR called for CMS to delay the testing of more value-based tools until it understands the impact of the ASP changes that are to be tested under this proposal.
CSRO does not have any specific policy recommendations to replace or modify the proposed rule, but rather calls for CMS to bring together all stakeholders, including patients, providers, payers, and manufacturers to devise a system that would work to the benefit of all while ensuring the best outcomes for patients, Dr. Schweitz said in an interview.
AT HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
CMS: MACRA impact on small/solo practices not as dramatic as predicted in regs
MACRA will not be as hard on small and solo practices as it first appeared when draft implementing regulations were published, according to Andy Slavitt, administrator of the Centers for Medicare & Medicaid Services.
Mr. Slavitt testified May 11 before the House Ways & Means Health Subcommittee to address legislators’ concerns about how the government intends to implement the Medicare Access and CHIP Reauthorization Act of 2015.
Rep. Sam Johnson (R-Tex.) expressed concern that the draft regulations published April 27 project “the greatest negative impact on payments to practices with nine or fewer doctors and the least harm to large systems with 100 or more docs.”
The calculations in the draft regulation were based on data from 2014, a year in which few small and solo practices reported quality data.
“In 2015 and subsequent years, the reporting went up,” Mr. Slavitt testified. “So at best, this table would be very, very conservative. ... Reporting is going to be far easier going forward.”
Mr. Slavitt said that the CMS will do all it can to help ensure that small and solo practices have every opportunity to participate in the both the Merit-Based Incentive Payment System (MIPS) and in advanced alternative payment models.
“The question of making sure that small groups and solo practitioners can be successful is of utmost importance. Our data show that physicians who are in small and solo practices ... do just as well as physicians that are in practices that are larger than that,” he said, adding that technical assistance specific to solo and small practices is being developed to help them transition to these value-based payment models.
Other federal officials have been spreading the same message to physicians. Speaking May 7 at the annual meeting of the American College of Physicians, Dr. Thomas A. Mason, chief medical officer in the Office of the National Coordinator for Health Information Technology, pointed out that the MACRA legislation put aside $20 million a year for 5 years beginning in 2016 to help solo and small practices transition to MIPS and APMs.
“It is specifically to help with the shift and transforming practices to measuring quality and improving quality performance,” he said in an interview. “The MACRA statute specifically calls out what the dollars need to be used for and the two points are for assisting MIPS-eligible professionals and improving their MIPS composite score as well as the transition to advanced alternative payment models.”
The U.S. Department of Health & Human Services already has begun soliciting contractors to support small and solo practices, he added.
“Direct technical assistance through this program will target eligible clinicians in individual or small group practices of 15 or fewer, focusing on those practicing in historically under resourced areas,” according to a request for proposals. “Technical assistance is defined as provider outreach and education, practice readiness, practice facilitation, health information technology (HIT) optimization, practice workflow redesign, change management, strategic planning, assisting clinicians in fully transitioning to Alternative Payment Models, and enabling partnerships.”
The federal health IT office plans to provide more information on the availability of transition assistance soon, Dr. Mason said.
Dr. Michael E. Nelson, FCCP, comments: If you are unfamiliar with MACRA, or alternatively don’t feel concerned about it, you will very likely notice a reduction in your income over the next few years. As a punishment for advocating the demise of the Sustainable Growth Rate formula (SGR), the Federal Government has come up with the Quality Payment Program (QPP), which includes the Merit-Based Incentive Payment System (MIPS) and Alternative Payment Models (APM). The Physician Quality Reporting System (PQRS), Value-Based Payment Modifier (VM) and Medicare Electronic Health Record (EHR Meaningful Use) are being morphed into the Merit-based Incentive Payment System (MIPS). If you don’t like this, you may choose an Alternative Payment Model (APM). You may use either of these as an Eligible Professional (EP). These programs are being phased in between 2015 and 2021. If all of these eponyms have looked like gibberish to you, I would encourage you to go to the CMS website, Google, Facebook, or whatever information source you use and self-educate.
Dr. Michael E. Nelson, FCCP, comments: If you are unfamiliar with MACRA, or alternatively don’t feel concerned about it, you will very likely notice a reduction in your income over the next few years. As a punishment for advocating the demise of the Sustainable Growth Rate formula (SGR), the Federal Government has come up with the Quality Payment Program (QPP), which includes the Merit-Based Incentive Payment System (MIPS) and Alternative Payment Models (APM). The Physician Quality Reporting System (PQRS), Value-Based Payment Modifier (VM) and Medicare Electronic Health Record (EHR Meaningful Use) are being morphed into the Merit-based Incentive Payment System (MIPS). If you don’t like this, you may choose an Alternative Payment Model (APM). You may use either of these as an Eligible Professional (EP). These programs are being phased in between 2015 and 2021. If all of these eponyms have looked like gibberish to you, I would encourage you to go to the CMS website, Google, Facebook, or whatever information source you use and self-educate.
Dr. Michael E. Nelson, FCCP, comments: If you are unfamiliar with MACRA, or alternatively don’t feel concerned about it, you will very likely notice a reduction in your income over the next few years. As a punishment for advocating the demise of the Sustainable Growth Rate formula (SGR), the Federal Government has come up with the Quality Payment Program (QPP), which includes the Merit-Based Incentive Payment System (MIPS) and Alternative Payment Models (APM). The Physician Quality Reporting System (PQRS), Value-Based Payment Modifier (VM) and Medicare Electronic Health Record (EHR Meaningful Use) are being morphed into the Merit-based Incentive Payment System (MIPS). If you don’t like this, you may choose an Alternative Payment Model (APM). You may use either of these as an Eligible Professional (EP). These programs are being phased in between 2015 and 2021. If all of these eponyms have looked like gibberish to you, I would encourage you to go to the CMS website, Google, Facebook, or whatever information source you use and self-educate.
MACRA will not be as hard on small and solo practices as it first appeared when draft implementing regulations were published, according to Andy Slavitt, administrator of the Centers for Medicare & Medicaid Services.
Mr. Slavitt testified May 11 before the House Ways & Means Health Subcommittee to address legislators’ concerns about how the government intends to implement the Medicare Access and CHIP Reauthorization Act of 2015.
Rep. Sam Johnson (R-Tex.) expressed concern that the draft regulations published April 27 project “the greatest negative impact on payments to practices with nine or fewer doctors and the least harm to large systems with 100 or more docs.”
The calculations in the draft regulation were based on data from 2014, a year in which few small and solo practices reported quality data.
“In 2015 and subsequent years, the reporting went up,” Mr. Slavitt testified. “So at best, this table would be very, very conservative. ... Reporting is going to be far easier going forward.”
Mr. Slavitt said that the CMS will do all it can to help ensure that small and solo practices have every opportunity to participate in the both the Merit-Based Incentive Payment System (MIPS) and in advanced alternative payment models.
“The question of making sure that small groups and solo practitioners can be successful is of utmost importance. Our data show that physicians who are in small and solo practices ... do just as well as physicians that are in practices that are larger than that,” he said, adding that technical assistance specific to solo and small practices is being developed to help them transition to these value-based payment models.
Other federal officials have been spreading the same message to physicians. Speaking May 7 at the annual meeting of the American College of Physicians, Dr. Thomas A. Mason, chief medical officer in the Office of the National Coordinator for Health Information Technology, pointed out that the MACRA legislation put aside $20 million a year for 5 years beginning in 2016 to help solo and small practices transition to MIPS and APMs.
“It is specifically to help with the shift and transforming practices to measuring quality and improving quality performance,” he said in an interview. “The MACRA statute specifically calls out what the dollars need to be used for and the two points are for assisting MIPS-eligible professionals and improving their MIPS composite score as well as the transition to advanced alternative payment models.”
The U.S. Department of Health & Human Services already has begun soliciting contractors to support small and solo practices, he added.
“Direct technical assistance through this program will target eligible clinicians in individual or small group practices of 15 or fewer, focusing on those practicing in historically under resourced areas,” according to a request for proposals. “Technical assistance is defined as provider outreach and education, practice readiness, practice facilitation, health information technology (HIT) optimization, practice workflow redesign, change management, strategic planning, assisting clinicians in fully transitioning to Alternative Payment Models, and enabling partnerships.”
The federal health IT office plans to provide more information on the availability of transition assistance soon, Dr. Mason said.
MACRA will not be as hard on small and solo practices as it first appeared when draft implementing regulations were published, according to Andy Slavitt, administrator of the Centers for Medicare & Medicaid Services.
Mr. Slavitt testified May 11 before the House Ways & Means Health Subcommittee to address legislators’ concerns about how the government intends to implement the Medicare Access and CHIP Reauthorization Act of 2015.
Rep. Sam Johnson (R-Tex.) expressed concern that the draft regulations published April 27 project “the greatest negative impact on payments to practices with nine or fewer doctors and the least harm to large systems with 100 or more docs.”
The calculations in the draft regulation were based on data from 2014, a year in which few small and solo practices reported quality data.
“In 2015 and subsequent years, the reporting went up,” Mr. Slavitt testified. “So at best, this table would be very, very conservative. ... Reporting is going to be far easier going forward.”
Mr. Slavitt said that the CMS will do all it can to help ensure that small and solo practices have every opportunity to participate in the both the Merit-Based Incentive Payment System (MIPS) and in advanced alternative payment models.
“The question of making sure that small groups and solo practitioners can be successful is of utmost importance. Our data show that physicians who are in small and solo practices ... do just as well as physicians that are in practices that are larger than that,” he said, adding that technical assistance specific to solo and small practices is being developed to help them transition to these value-based payment models.
Other federal officials have been spreading the same message to physicians. Speaking May 7 at the annual meeting of the American College of Physicians, Dr. Thomas A. Mason, chief medical officer in the Office of the National Coordinator for Health Information Technology, pointed out that the MACRA legislation put aside $20 million a year for 5 years beginning in 2016 to help solo and small practices transition to MIPS and APMs.
“It is specifically to help with the shift and transforming practices to measuring quality and improving quality performance,” he said in an interview. “The MACRA statute specifically calls out what the dollars need to be used for and the two points are for assisting MIPS-eligible professionals and improving their MIPS composite score as well as the transition to advanced alternative payment models.”
The U.S. Department of Health & Human Services already has begun soliciting contractors to support small and solo practices, he added.
“Direct technical assistance through this program will target eligible clinicians in individual or small group practices of 15 or fewer, focusing on those practicing in historically under resourced areas,” according to a request for proposals. “Technical assistance is defined as provider outreach and education, practice readiness, practice facilitation, health information technology (HIT) optimization, practice workflow redesign, change management, strategic planning, assisting clinicians in fully transitioning to Alternative Payment Models, and enabling partnerships.”
The federal health IT office plans to provide more information on the availability of transition assistance soon, Dr. Mason said.
Sunshine Act: ADHD drug promotion dominates payments to pediatricians
Drugs to treat ADHD are the products most commonly promoted to general pediatricians and pediatric subspecialists by industry, according to a recent analysis of Sunshine Act payments.
Researchers examined nearly 245,000 payments made in calendar 2014 to 36,000 pediatricians and pediatric subspecialists for a total of more than $32 million. Nearly all developmental pediatricians (90%) received payment, while few radiologists (13%) did.
“Our finding of ADHD drugs as the most marketed product to pediatricians fits with our finding that developmental pediatricians were most likely to receive a payment compared with other pediatricians,” Dr. Kavita Parikh, a pediatric hospitalist at Children’s National Medical Center, Washington, D.C., and colleagues wrote in an article published online (Pediatics. 2016 May 6. doi: 10.1542/peds.2015-4440).
Three ADHD drugs accounted for nearly 25% of payments, they found. Other drugs that were commonly promoted to pediatricians included vaccinations and treatments for asthma. Pediatric pulmonologists also were in the top four subspecialists to receive payments.
Dr. Parikh and colleagues recommended further study to evaluate “whether and how industry ties influence patient care because it is reasonable to believe that these payments influence physician prescribing.”
Among primary care specialists, researchers found that 72% of family physicians received payments, followed by 58% of internists and 35% of pediatricians. In comparison, 98% of cardiologists received payments, 83% of gastroenterologists, 78% of allergy/immunologists, and 73% of hematologists/oncologists.
The authors indicated that they had no conflicts of interest.
Drugs to treat ADHD are the products most commonly promoted to general pediatricians and pediatric subspecialists by industry, according to a recent analysis of Sunshine Act payments.
Researchers examined nearly 245,000 payments made in calendar 2014 to 36,000 pediatricians and pediatric subspecialists for a total of more than $32 million. Nearly all developmental pediatricians (90%) received payment, while few radiologists (13%) did.
“Our finding of ADHD drugs as the most marketed product to pediatricians fits with our finding that developmental pediatricians were most likely to receive a payment compared with other pediatricians,” Dr. Kavita Parikh, a pediatric hospitalist at Children’s National Medical Center, Washington, D.C., and colleagues wrote in an article published online (Pediatics. 2016 May 6. doi: 10.1542/peds.2015-4440).
Three ADHD drugs accounted for nearly 25% of payments, they found. Other drugs that were commonly promoted to pediatricians included vaccinations and treatments for asthma. Pediatric pulmonologists also were in the top four subspecialists to receive payments.
Dr. Parikh and colleagues recommended further study to evaluate “whether and how industry ties influence patient care because it is reasonable to believe that these payments influence physician prescribing.”
Among primary care specialists, researchers found that 72% of family physicians received payments, followed by 58% of internists and 35% of pediatricians. In comparison, 98% of cardiologists received payments, 83% of gastroenterologists, 78% of allergy/immunologists, and 73% of hematologists/oncologists.
The authors indicated that they had no conflicts of interest.
Drugs to treat ADHD are the products most commonly promoted to general pediatricians and pediatric subspecialists by industry, according to a recent analysis of Sunshine Act payments.
Researchers examined nearly 245,000 payments made in calendar 2014 to 36,000 pediatricians and pediatric subspecialists for a total of more than $32 million. Nearly all developmental pediatricians (90%) received payment, while few radiologists (13%) did.
“Our finding of ADHD drugs as the most marketed product to pediatricians fits with our finding that developmental pediatricians were most likely to receive a payment compared with other pediatricians,” Dr. Kavita Parikh, a pediatric hospitalist at Children’s National Medical Center, Washington, D.C., and colleagues wrote in an article published online (Pediatics. 2016 May 6. doi: 10.1542/peds.2015-4440).
Three ADHD drugs accounted for nearly 25% of payments, they found. Other drugs that were commonly promoted to pediatricians included vaccinations and treatments for asthma. Pediatric pulmonologists also were in the top four subspecialists to receive payments.
Dr. Parikh and colleagues recommended further study to evaluate “whether and how industry ties influence patient care because it is reasonable to believe that these payments influence physician prescribing.”
Among primary care specialists, researchers found that 72% of family physicians received payments, followed by 58% of internists and 35% of pediatricians. In comparison, 98% of cardiologists received payments, 83% of gastroenterologists, 78% of allergy/immunologists, and 73% of hematologists/oncologists.
The authors indicated that they had no conflicts of interest.
FROM PEDIATRICS