User login
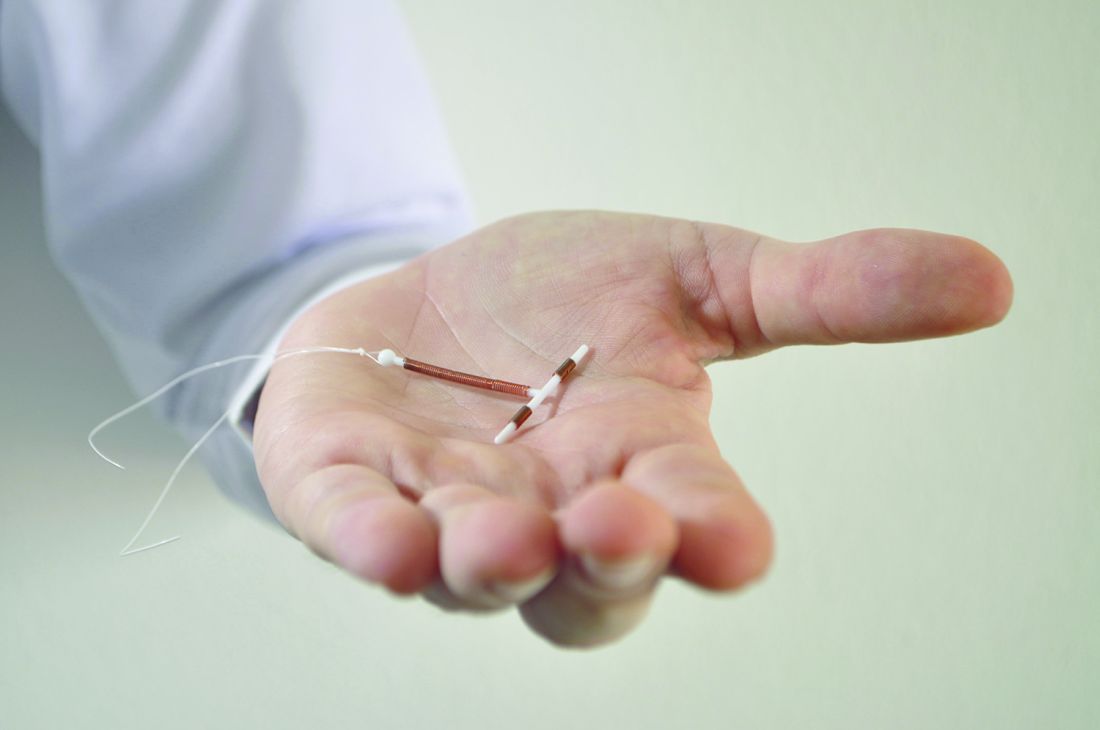
Quality group endorses new contraception measures
The National Quality Forum has endorsed – for the first time – a set of quality measures related to the prescribing of contraception.
The three new measures are intended to help reduce unintended pregnancies by ensuring that women aged 15-44 years are offered effective birth control options.
“[We] felt strongly that we needed to take a broader look at reproductive health and think about care even before someone was pregnant,” Helen Burstin, MD, the National Quality Forum’s (NQF) chief scientific officer, said in an interview. “That logically led us to thinking about what could come forward that would be useful, specifically around contraception. [We were] pleased to see this wider array of measures coming forward this round that weren’t as focused specifically on pregnancy and perinatal outcomes.”
Measure 2904 assesses the percentage of women aged 15-44 years at risk of an unintended pregnancy who receive a long-acting reversible contraceptive (LARC). The measure is intended to identify situations in which women do not have access to LARCs. The availability of LARCs varies depending on insurance coverage and availability of trained providers, according to NQF. “The measure encourages health systems to look at reporting units with very low rates of provision of LARC to identify unnecessary barriers to LARCs,” the report states. The measure does not include a target percentage for access to LARCs.
Measure 2902 is related to the postpartum period and assesses the percentage of women aged 15-44 years who have had a live birth and are provided a “most effective” or “moderately effective” method of contraception within 3 and 60 days of delivery, and the percentage of those provided with a LARC in the same time period after giving birth. “Contraceptive care for postpartum women is important to facilitate birth spacing, and this measure identifies women more clearly at risk for pregnancy,” the report states.
At the end of 2016, the NQF endorsed a total of 18 perinatal and reproductive health measures that also included measures related to chlamydia screening, labor and delivery, care of premature neonates, and postpartum care. NQF-endorsed measures are frequently used in federal public reporting and performance-based payment programs, including by Medicaid. They are also used in the private sector by hospitals and health plans.
The measures are a good foundation for understanding how women are accessing and using contraception, said Nikki Zite, MD, a professor and director of the obstetrics and gynecology residency program at the University of Tennessee, Knoxville.
“I think it was done in a very careful and thoughtful way, that they are going to track the percent of women of childbearing age using highly effective and moderately effective contraceptive options and they are going to separately track the percent of contracepting women using long-acting reversible methods,” Dr. Zite said. “We hope that if we educate women about contraceptive options, if they are trying to prevent pregnancies, they will choose the option that works best for them, and therefore it will be the most effective.”
Another selling point for the new measures is that they are patient-centered, Dr. Zite added. “The emphasis is on giving access for women who want access, removing barriers, but then also there is no goal amount, so we are not trying to say we want every woman to have LARC because that is not the right option for every woman.” Dr. Zite is an unpaid trainer for Nexplanon and serves on an international IUD advisory board for Bayer.
The American College of Obstetricians and Gynecologists voiced its support for the new measures. “All of these measures will help us advance understanding across the health care system in order to reduce unintended pregnancy,” said Sean Currigan, quality and safety officer at ACOG. “Specifically, the LARC measure will help us understand the unnecessary variation in access to LARC across providers and systems, especially outside of the Title X system; and the postpartum contraception measure will help us understand continuing barriers to contraception like nonpayment/coverage of immediate postpartum LARC.”
In 2016, ACOG withheld its endorsement from quality measures issued by the Core Quality Measures Collaborative (a group led by the Centers for Medicare & Medicaid Services and America’s Health Insurance Plans, with input from NQF, medical societies, employer groups, and consumer groups), because contraception measures were not included in the ob.gyn. measure set.
The National Quality Forum has endorsed – for the first time – a set of quality measures related to the prescribing of contraception.
The three new measures are intended to help reduce unintended pregnancies by ensuring that women aged 15-44 years are offered effective birth control options.
“[We] felt strongly that we needed to take a broader look at reproductive health and think about care even before someone was pregnant,” Helen Burstin, MD, the National Quality Forum’s (NQF) chief scientific officer, said in an interview. “That logically led us to thinking about what could come forward that would be useful, specifically around contraception. [We were] pleased to see this wider array of measures coming forward this round that weren’t as focused specifically on pregnancy and perinatal outcomes.”
Measure 2904 assesses the percentage of women aged 15-44 years at risk of an unintended pregnancy who receive a long-acting reversible contraceptive (LARC). The measure is intended to identify situations in which women do not have access to LARCs. The availability of LARCs varies depending on insurance coverage and availability of trained providers, according to NQF. “The measure encourages health systems to look at reporting units with very low rates of provision of LARC to identify unnecessary barriers to LARCs,” the report states. The measure does not include a target percentage for access to LARCs.
Measure 2902 is related to the postpartum period and assesses the percentage of women aged 15-44 years who have had a live birth and are provided a “most effective” or “moderately effective” method of contraception within 3 and 60 days of delivery, and the percentage of those provided with a LARC in the same time period after giving birth. “Contraceptive care for postpartum women is important to facilitate birth spacing, and this measure identifies women more clearly at risk for pregnancy,” the report states.
At the end of 2016, the NQF endorsed a total of 18 perinatal and reproductive health measures that also included measures related to chlamydia screening, labor and delivery, care of premature neonates, and postpartum care. NQF-endorsed measures are frequently used in federal public reporting and performance-based payment programs, including by Medicaid. They are also used in the private sector by hospitals and health plans.
The measures are a good foundation for understanding how women are accessing and using contraception, said Nikki Zite, MD, a professor and director of the obstetrics and gynecology residency program at the University of Tennessee, Knoxville.
“I think it was done in a very careful and thoughtful way, that they are going to track the percent of women of childbearing age using highly effective and moderately effective contraceptive options and they are going to separately track the percent of contracepting women using long-acting reversible methods,” Dr. Zite said. “We hope that if we educate women about contraceptive options, if they are trying to prevent pregnancies, they will choose the option that works best for them, and therefore it will be the most effective.”
Another selling point for the new measures is that they are patient-centered, Dr. Zite added. “The emphasis is on giving access for women who want access, removing barriers, but then also there is no goal amount, so we are not trying to say we want every woman to have LARC because that is not the right option for every woman.” Dr. Zite is an unpaid trainer for Nexplanon and serves on an international IUD advisory board for Bayer.
The American College of Obstetricians and Gynecologists voiced its support for the new measures. “All of these measures will help us advance understanding across the health care system in order to reduce unintended pregnancy,” said Sean Currigan, quality and safety officer at ACOG. “Specifically, the LARC measure will help us understand the unnecessary variation in access to LARC across providers and systems, especially outside of the Title X system; and the postpartum contraception measure will help us understand continuing barriers to contraception like nonpayment/coverage of immediate postpartum LARC.”
In 2016, ACOG withheld its endorsement from quality measures issued by the Core Quality Measures Collaborative (a group led by the Centers for Medicare & Medicaid Services and America’s Health Insurance Plans, with input from NQF, medical societies, employer groups, and consumer groups), because contraception measures were not included in the ob.gyn. measure set.
The National Quality Forum has endorsed – for the first time – a set of quality measures related to the prescribing of contraception.
The three new measures are intended to help reduce unintended pregnancies by ensuring that women aged 15-44 years are offered effective birth control options.
“[We] felt strongly that we needed to take a broader look at reproductive health and think about care even before someone was pregnant,” Helen Burstin, MD, the National Quality Forum’s (NQF) chief scientific officer, said in an interview. “That logically led us to thinking about what could come forward that would be useful, specifically around contraception. [We were] pleased to see this wider array of measures coming forward this round that weren’t as focused specifically on pregnancy and perinatal outcomes.”
Measure 2904 assesses the percentage of women aged 15-44 years at risk of an unintended pregnancy who receive a long-acting reversible contraceptive (LARC). The measure is intended to identify situations in which women do not have access to LARCs. The availability of LARCs varies depending on insurance coverage and availability of trained providers, according to NQF. “The measure encourages health systems to look at reporting units with very low rates of provision of LARC to identify unnecessary barriers to LARCs,” the report states. The measure does not include a target percentage for access to LARCs.
Measure 2902 is related to the postpartum period and assesses the percentage of women aged 15-44 years who have had a live birth and are provided a “most effective” or “moderately effective” method of contraception within 3 and 60 days of delivery, and the percentage of those provided with a LARC in the same time period after giving birth. “Contraceptive care for postpartum women is important to facilitate birth spacing, and this measure identifies women more clearly at risk for pregnancy,” the report states.
At the end of 2016, the NQF endorsed a total of 18 perinatal and reproductive health measures that also included measures related to chlamydia screening, labor and delivery, care of premature neonates, and postpartum care. NQF-endorsed measures are frequently used in federal public reporting and performance-based payment programs, including by Medicaid. They are also used in the private sector by hospitals and health plans.
The measures are a good foundation for understanding how women are accessing and using contraception, said Nikki Zite, MD, a professor and director of the obstetrics and gynecology residency program at the University of Tennessee, Knoxville.
“I think it was done in a very careful and thoughtful way, that they are going to track the percent of women of childbearing age using highly effective and moderately effective contraceptive options and they are going to separately track the percent of contracepting women using long-acting reversible methods,” Dr. Zite said. “We hope that if we educate women about contraceptive options, if they are trying to prevent pregnancies, they will choose the option that works best for them, and therefore it will be the most effective.”
Another selling point for the new measures is that they are patient-centered, Dr. Zite added. “The emphasis is on giving access for women who want access, removing barriers, but then also there is no goal amount, so we are not trying to say we want every woman to have LARC because that is not the right option for every woman.” Dr. Zite is an unpaid trainer for Nexplanon and serves on an international IUD advisory board for Bayer.
The American College of Obstetricians and Gynecologists voiced its support for the new measures. “All of these measures will help us advance understanding across the health care system in order to reduce unintended pregnancy,” said Sean Currigan, quality and safety officer at ACOG. “Specifically, the LARC measure will help us understand the unnecessary variation in access to LARC across providers and systems, especially outside of the Title X system; and the postpartum contraception measure will help us understand continuing barriers to contraception like nonpayment/coverage of immediate postpartum LARC.”
In 2016, ACOG withheld its endorsement from quality measures issued by the Core Quality Measures Collaborative (a group led by the Centers for Medicare & Medicaid Services and America’s Health Insurance Plans, with input from NQF, medical societies, employer groups, and consumer groups), because contraception measures were not included in the ob.gyn. measure set.
CMS proposal seeks to stabilize individual insurance market
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
Proposed regulations from the Centers for Medicare & Medicaid Services aim to provide short-term stabilization to the individual and small group insurance markets under the Affordable Care Act.
The proposal issued Feb. 15 would make changes to special enrollment periods, open enrollment, guaranteed availability, network adequacy rules, essential community providers, and actuarial value requirements. It also changes the timeline for when insurers would need to get their qualified health plan certification. It represents a first step toward fulfilling President Trump’s Inauguration Day executive order to “minimize the unwarranted economic and regulatory burdens of the [ACA], and prepare to afford the states more flexibility and control to create a more free and open health care market.”
However, the proposed rule, if finalized as is, may not have any dramatic effect on the decision by insurers to serve the individual and small group markets.
“A plan that was going to stay is probably going to stay and be a little bit happier about these regs and a plan that was going to decide to leave, like Humana, would have left anyway,” Caroline Pearson, senior vice president at Avalere Health said in an interview. “I don’t know if it is going to materially change plan participation.”
The proposed rule would shorten open enrollment for plans purchased in the ACA marketplace. Currently, plans can be purchased from Nov. 1 to Jan. 31; the proposal would move the deadline up to Dec. 15.
“We anticipate this change could improve the risk pool because it would reduce opportunities for adverse selection by those who learn they will need services in late December and January; and will encourage healthier individuals who might have previously enrolled in partial year coverage after Dec. 15th to instead enroll in coverage for the full year,” according to the proposed rule.
CMS also proposes to tighten special enrollment by requiring preverification of special enrollment period status for all people applicants. Currently, only 50% of those seeking coverage through special enrollment are verified. The agency also is proposing to limit the plan choices available to individuals who are enrolling via a special enrollment period as a way of minimizing adverse selection.
Another proposal aimed at keeping people covered is one that allows insurers to collect unpaid premiums in the prior coverage year before enrolling a patient in the next year’s plan with the same insurer.
CMS noted in the rule that a recent survey “concluded that approximately 21% of consumers stopped premium payments in 2015. Approximately 87% of those individuals repurchased plans in 2016, while 49% of these consumers purchased the same plan they had previously stopped payment on.”
On the network adequacy front, CMS is shifting the conduct of network adequacy reviews to states, or to an accrediting entity recognized by the Department of Health & Human Services in the case of states that do not have sufficient resources to conduct adequacy reviews. Further, the proposal reduces the minimum percentage of essential community providers (those who serve predominantly low-income and medically underserved populations) in a network to 20% from the 30% instituted in 2015.
CMS said in the proposal that if these rules are finalized, it will issue separate guidance on changes to the timeline for plans to submit their bids for 2018.
Avalere’s Ms. Pearson said that she sees these proposed changes merely as a stopgap measure.
“This reg is intended to stand up the exchange markets and keep them functional while the ACA replacement plan is approved and implemented,” she said. “This is meant to prevent there from being a total loss of coverage before the ACA replacement can be put into effect.”
She added that while insurers will welcome the changes, consumers and patient advocates could push back on the proposal, particularly the actuarial flexibility that could result in smaller networks and shrinking benefits.
Indeed, America’s Health Insurance Plans offered its support of the regulation. “We commend the Administration for proposing these regulatory actions as Congress considers other critical actions necessary to help stabilize and improve the individual market for 2018,” AHIP President and CEO Marilyn Tavenner said in a statement.
The proposed changes were released online Feb. 15 and are scheduled for publication in the Federal Register on Feb. 17. Comments on the proposed changes are due to CMS by March 7.
MedPAC leaning toward hybrid for paying for Part B drugs
WASHINGTON – The Medicare Payment Advisory Commission is putting the finishing touches on a recommendation for a hybrid approach to reforming how Medicare pays doctors for drugs administered in the office.
If adopted by the Centers for Medicare & Medicaid Services, MedPAC’s recommendations first would refine the current system which pays physicians for Medicare Part B drugs based on the average sales price (ASP), then eventually would allow physicians to either remain in the improved ASP system or switch to a drug value program (DVP), a revamped version of the failed Competitive Acquisition Program.
Under the potential MedPAC recommendations, new drugs – those that do not have an ASP – would be paid at wholesale acquisition cost plus 3%, which MedPAC staff said is more in line the standard ASP plus 6%.
To help stem the impact of pricing increases, the proposal calls on manufacturers to provide additional rebates when ASP growth exceeds inflation. This approach is commonly used by states collecting Medicaid drug rebates.
Finally, the proposal would consolidate billing codes to help foster competition. The plan would combine billing codes for a brand name drug and all associated generics, as well as combining biosimilars with their reference biologic products. Commission staff highlighted two cases where combining codes for a biologic/biosimilar would have an impact: Medicare’s payment rate for the biosimilar filgrastim-sndz (Zarxio) declined by 20% during the first 6 quarters on the market while the reference product (Neupogen) remained static. Further, first quarter of pricing data on the biosimilar infliximab-dyyb (Inflectra) is 22% greater than the reference product (Remicade).
MedPAC is likely to recommend that, as those changes go into effect, CMS develop the DVP, which would be voluntary beginning in 2022. In parallel with the launch of DVP, MedPAC is likely to recommend that CMS reduce the add-on percentage in the ASP program, as a stick to drive DVP participation.
Under the DVP, physicians would contract with one of a number of vendors that would negotiate prices for physicians and offer opportunities for shared savings.
Physicians would buy their drugs at the vendor-negotiated price from a network of wholesalers and distributors. Vendors would have additional negotiation tools such as formularies, step therapy, and prior authorization, and a price cap of 100% of ASP. Medicare would pay physicians at the DVP rate without any add-ons, but physicians would continue to receive fees for administrative services under the physician fee schedule or the outpatient prospective payment system.
The recommendations received general support from the MedPAC commissioners, but some of the finer details were met with concerns.
One was the possible impact of the ongoing budget sequestration, which lowers total payments from the government by 2%.
“I think the possibility that the sequester will still be in effect really casts a pall over proposals like [wholesale acquisition cost plus 3%], and we just have to think about what wording we’re going to use,” Commissioner Paul Ginsburg, PhD, of the Brookings Institution, said. “We realize that if a sequester is still in effect, we might have to make a temporary change.”
Another aspect of the DVP that raised questions was an arbitration process that would allow prices to be challenged for sole-source products.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, said she was “not in favor of the arbitration,” questioning its administrative burden, cost, and turn-around time for decisions. She added that rather than arbitration, “I would like to see us provide incentives to manufacturers to bring competition to the market versus trying to create a system that doesn’t exist today for us to go to court.”
Regarding the DVP, Commissioner Jack Hoadley, PhD, research professor at Georgetown University, Washington, said that he is “not convinced that this process is going to work,” but acknowledged the MedPAC staff’s work in developing it. “It does feel like it’s worth putting out there and trying to see if it happens or trying to set it up to happen.”
MedPAC Vice Chairman Jon Christianson, PhD, of the University of Minnesota, Minneapolis, concurred. “I think we should go ahead and continue to explore that, but maybe temper our enthusiasm a little bit,” questioning how effective multiple groups would be in negotiating separately for Medicare drug pricing.
Commissioner William Hall, MD, of the University of Rochester, N.Y., also questioned the effect on patients. “Do any of these manipulations really have a direct correlation to quality of care for the patient population that is most affected by Part B?” he asked.
MedPAC staff will take the commissioners’ comments on these options and refine them for the upcoming March 2-3 meeting and will present the final recommendations to the commissioners to vote on in April for inclusion in the June report to Congress.
WASHINGTON – The Medicare Payment Advisory Commission is putting the finishing touches on a recommendation for a hybrid approach to reforming how Medicare pays doctors for drugs administered in the office.
If adopted by the Centers for Medicare & Medicaid Services, MedPAC’s recommendations first would refine the current system which pays physicians for Medicare Part B drugs based on the average sales price (ASP), then eventually would allow physicians to either remain in the improved ASP system or switch to a drug value program (DVP), a revamped version of the failed Competitive Acquisition Program.
Under the potential MedPAC recommendations, new drugs – those that do not have an ASP – would be paid at wholesale acquisition cost plus 3%, which MedPAC staff said is more in line the standard ASP plus 6%.
To help stem the impact of pricing increases, the proposal calls on manufacturers to provide additional rebates when ASP growth exceeds inflation. This approach is commonly used by states collecting Medicaid drug rebates.
Finally, the proposal would consolidate billing codes to help foster competition. The plan would combine billing codes for a brand name drug and all associated generics, as well as combining biosimilars with their reference biologic products. Commission staff highlighted two cases where combining codes for a biologic/biosimilar would have an impact: Medicare’s payment rate for the biosimilar filgrastim-sndz (Zarxio) declined by 20% during the first 6 quarters on the market while the reference product (Neupogen) remained static. Further, first quarter of pricing data on the biosimilar infliximab-dyyb (Inflectra) is 22% greater than the reference product (Remicade).
MedPAC is likely to recommend that, as those changes go into effect, CMS develop the DVP, which would be voluntary beginning in 2022. In parallel with the launch of DVP, MedPAC is likely to recommend that CMS reduce the add-on percentage in the ASP program, as a stick to drive DVP participation.
Under the DVP, physicians would contract with one of a number of vendors that would negotiate prices for physicians and offer opportunities for shared savings.
Physicians would buy their drugs at the vendor-negotiated price from a network of wholesalers and distributors. Vendors would have additional negotiation tools such as formularies, step therapy, and prior authorization, and a price cap of 100% of ASP. Medicare would pay physicians at the DVP rate without any add-ons, but physicians would continue to receive fees for administrative services under the physician fee schedule or the outpatient prospective payment system.
The recommendations received general support from the MedPAC commissioners, but some of the finer details were met with concerns.
One was the possible impact of the ongoing budget sequestration, which lowers total payments from the government by 2%.
“I think the possibility that the sequester will still be in effect really casts a pall over proposals like [wholesale acquisition cost plus 3%], and we just have to think about what wording we’re going to use,” Commissioner Paul Ginsburg, PhD, of the Brookings Institution, said. “We realize that if a sequester is still in effect, we might have to make a temporary change.”
Another aspect of the DVP that raised questions was an arbitration process that would allow prices to be challenged for sole-source products.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, said she was “not in favor of the arbitration,” questioning its administrative burden, cost, and turn-around time for decisions. She added that rather than arbitration, “I would like to see us provide incentives to manufacturers to bring competition to the market versus trying to create a system that doesn’t exist today for us to go to court.”
Regarding the DVP, Commissioner Jack Hoadley, PhD, research professor at Georgetown University, Washington, said that he is “not convinced that this process is going to work,” but acknowledged the MedPAC staff’s work in developing it. “It does feel like it’s worth putting out there and trying to see if it happens or trying to set it up to happen.”
MedPAC Vice Chairman Jon Christianson, PhD, of the University of Minnesota, Minneapolis, concurred. “I think we should go ahead and continue to explore that, but maybe temper our enthusiasm a little bit,” questioning how effective multiple groups would be in negotiating separately for Medicare drug pricing.
Commissioner William Hall, MD, of the University of Rochester, N.Y., also questioned the effect on patients. “Do any of these manipulations really have a direct correlation to quality of care for the patient population that is most affected by Part B?” he asked.
MedPAC staff will take the commissioners’ comments on these options and refine them for the upcoming March 2-3 meeting and will present the final recommendations to the commissioners to vote on in April for inclusion in the June report to Congress.
WASHINGTON – The Medicare Payment Advisory Commission is putting the finishing touches on a recommendation for a hybrid approach to reforming how Medicare pays doctors for drugs administered in the office.
If adopted by the Centers for Medicare & Medicaid Services, MedPAC’s recommendations first would refine the current system which pays physicians for Medicare Part B drugs based on the average sales price (ASP), then eventually would allow physicians to either remain in the improved ASP system or switch to a drug value program (DVP), a revamped version of the failed Competitive Acquisition Program.
Under the potential MedPAC recommendations, new drugs – those that do not have an ASP – would be paid at wholesale acquisition cost plus 3%, which MedPAC staff said is more in line the standard ASP plus 6%.
To help stem the impact of pricing increases, the proposal calls on manufacturers to provide additional rebates when ASP growth exceeds inflation. This approach is commonly used by states collecting Medicaid drug rebates.
Finally, the proposal would consolidate billing codes to help foster competition. The plan would combine billing codes for a brand name drug and all associated generics, as well as combining biosimilars with their reference biologic products. Commission staff highlighted two cases where combining codes for a biologic/biosimilar would have an impact: Medicare’s payment rate for the biosimilar filgrastim-sndz (Zarxio) declined by 20% during the first 6 quarters on the market while the reference product (Neupogen) remained static. Further, first quarter of pricing data on the biosimilar infliximab-dyyb (Inflectra) is 22% greater than the reference product (Remicade).
MedPAC is likely to recommend that, as those changes go into effect, CMS develop the DVP, which would be voluntary beginning in 2022. In parallel with the launch of DVP, MedPAC is likely to recommend that CMS reduce the add-on percentage in the ASP program, as a stick to drive DVP participation.
Under the DVP, physicians would contract with one of a number of vendors that would negotiate prices for physicians and offer opportunities for shared savings.
Physicians would buy their drugs at the vendor-negotiated price from a network of wholesalers and distributors. Vendors would have additional negotiation tools such as formularies, step therapy, and prior authorization, and a price cap of 100% of ASP. Medicare would pay physicians at the DVP rate without any add-ons, but physicians would continue to receive fees for administrative services under the physician fee schedule or the outpatient prospective payment system.
The recommendations received general support from the MedPAC commissioners, but some of the finer details were met with concerns.
One was the possible impact of the ongoing budget sequestration, which lowers total payments from the government by 2%.
“I think the possibility that the sequester will still be in effect really casts a pall over proposals like [wholesale acquisition cost plus 3%], and we just have to think about what wording we’re going to use,” Commissioner Paul Ginsburg, PhD, of the Brookings Institution, said. “We realize that if a sequester is still in effect, we might have to make a temporary change.”
Another aspect of the DVP that raised questions was an arbitration process that would allow prices to be challenged for sole-source products.
Commissioner Amy Bricker, vice president of supply chain strategy at Express Scripts, said she was “not in favor of the arbitration,” questioning its administrative burden, cost, and turn-around time for decisions. She added that rather than arbitration, “I would like to see us provide incentives to manufacturers to bring competition to the market versus trying to create a system that doesn’t exist today for us to go to court.”
Regarding the DVP, Commissioner Jack Hoadley, PhD, research professor at Georgetown University, Washington, said that he is “not convinced that this process is going to work,” but acknowledged the MedPAC staff’s work in developing it. “It does feel like it’s worth putting out there and trying to see if it happens or trying to set it up to happen.”
MedPAC Vice Chairman Jon Christianson, PhD, of the University of Minnesota, Minneapolis, concurred. “I think we should go ahead and continue to explore that, but maybe temper our enthusiasm a little bit,” questioning how effective multiple groups would be in negotiating separately for Medicare drug pricing.
Commissioner William Hall, MD, of the University of Rochester, N.Y., also questioned the effect on patients. “Do any of these manipulations really have a direct correlation to quality of care for the patient population that is most affected by Part B?” he asked.
MedPAC staff will take the commissioners’ comments on these options and refine them for the upcoming March 2-3 meeting and will present the final recommendations to the commissioners to vote on in April for inclusion in the June report to Congress.
AT A MEDPAC MEETING
Senate confirms Price as HHS secretary
The U.S. Senate voted to confirm Rep. Tom Price, MD, as secretary of the Department of Health & Human Services.
The final tally, recorded in the early hours of Feb. 10, was a strict party-line vote, with all 52 Republicans voting in favor of Rep. Price (R-Ga.) and 47 Democrats voting against. One Democrat, Sen. Claire McCaskill of Missouri, did not vote. Only a simple majority is needed to confirm cabinet members to their posts.
The confirmation comes amid ongoing concerns presented by Senate Democrats on Dr. Price’s stock purchases, particularly of Australia-based Innate Immunotherapeutics. Dr. Price was serving as a representative from Georgia at the time of his nomination, and there have been questions of possible ethics violations related to this and other securities purchases.
Democrats also rallied against the policies that Dr. Price advocated for when he was a U.S. House member, including dismantling the Affordable Care Act and pushing for block grants to fund Medicaid. He has also supported policies that would promote more extensive use of health savings accounts linked to high-deductible health plans, and high-risk pools to help ensure that those with pre-existing conditions are able to get insurance coverage without a need for guaranteed issue.
However, Democratic objections were not enough to cause any waver in support from Senate Republicans.
The American Medical Association “looks forward to working with Secretary Price to improve the health of our nation through policies that promote access to high-quality, affordable care, delivery innovation, and reduced regulatory burdens that helps patients and their physicians,” AMA President Andrew Gurman, MD, said in a statement.
The U.S. Senate voted to confirm Rep. Tom Price, MD, as secretary of the Department of Health & Human Services.
The final tally, recorded in the early hours of Feb. 10, was a strict party-line vote, with all 52 Republicans voting in favor of Rep. Price (R-Ga.) and 47 Democrats voting against. One Democrat, Sen. Claire McCaskill of Missouri, did not vote. Only a simple majority is needed to confirm cabinet members to their posts.
The confirmation comes amid ongoing concerns presented by Senate Democrats on Dr. Price’s stock purchases, particularly of Australia-based Innate Immunotherapeutics. Dr. Price was serving as a representative from Georgia at the time of his nomination, and there have been questions of possible ethics violations related to this and other securities purchases.
Democrats also rallied against the policies that Dr. Price advocated for when he was a U.S. House member, including dismantling the Affordable Care Act and pushing for block grants to fund Medicaid. He has also supported policies that would promote more extensive use of health savings accounts linked to high-deductible health plans, and high-risk pools to help ensure that those with pre-existing conditions are able to get insurance coverage without a need for guaranteed issue.
However, Democratic objections were not enough to cause any waver in support from Senate Republicans.
The American Medical Association “looks forward to working with Secretary Price to improve the health of our nation through policies that promote access to high-quality, affordable care, delivery innovation, and reduced regulatory burdens that helps patients and their physicians,” AMA President Andrew Gurman, MD, said in a statement.
The U.S. Senate voted to confirm Rep. Tom Price, MD, as secretary of the Department of Health & Human Services.
The final tally, recorded in the early hours of Feb. 10, was a strict party-line vote, with all 52 Republicans voting in favor of Rep. Price (R-Ga.) and 47 Democrats voting against. One Democrat, Sen. Claire McCaskill of Missouri, did not vote. Only a simple majority is needed to confirm cabinet members to their posts.
The confirmation comes amid ongoing concerns presented by Senate Democrats on Dr. Price’s stock purchases, particularly of Australia-based Innate Immunotherapeutics. Dr. Price was serving as a representative from Georgia at the time of his nomination, and there have been questions of possible ethics violations related to this and other securities purchases.
Democrats also rallied against the policies that Dr. Price advocated for when he was a U.S. House member, including dismantling the Affordable Care Act and pushing for block grants to fund Medicaid. He has also supported policies that would promote more extensive use of health savings accounts linked to high-deductible health plans, and high-risk pools to help ensure that those with pre-existing conditions are able to get insurance coverage without a need for guaranteed issue.
However, Democratic objections were not enough to cause any waver in support from Senate Republicans.
The American Medical Association “looks forward to working with Secretary Price to improve the health of our nation through policies that promote access to high-quality, affordable care, delivery innovation, and reduced regulatory burdens that helps patients and their physicians,” AMA President Andrew Gurman, MD, said in a statement.
Dems force delay in vote for HHS secretary
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
HHS Secretary-nominee avoids specifics on Medicaid funding during second hearing
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
Senate takes first step toward repealing ACA
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
CMS nixes Part B drug payment demonstration
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
President Trump hits ground running on ACA repeal
WASHINGTON – President Trump wasted no time in getting the executive branch’s wheels in motion toward repeal of the Affordable Care Act.
Within hours of being sworn in as the 45th president of the United States on Jan. 20, he signed an executive order that announced the incoming administration’s policy “to seek the prompt repeal of the Patient Protection and Affordable Care Act.”
The order opens the door for federal agencies to tackle ACA provisions such as the individual mandate and its tax penalties for not carrying insurance, as well as other financial aspects of the ACA that impact patients, providers, insurers, and manufacturers.
The order directs the secretaries of HHS, the Treasury department, and the Labor department to “exercise all authority and discretion available to them to provide greater flexibility to States and cooperate with them in implementing healthcare programs.”
With this order, President Trump also set the stage for creating a framework to sell insurance products across state lines by directing secretaries with oversight of insurance markets to “encourage the development of a free and open market in interstate commerce for the offering of healthcare services and health insurance, with the goal of achieving and preserving maximum options for patients and consumers.”
Little action is expected on the executive order until secretaries are approved for HHS, Treasury, and Labor. Rep. Tom Price (R-Ga.) is scheduled to appear before the Senate Finance Committee on Jan. 24.
WASHINGTON – President Trump wasted no time in getting the executive branch’s wheels in motion toward repeal of the Affordable Care Act.
Within hours of being sworn in as the 45th president of the United States on Jan. 20, he signed an executive order that announced the incoming administration’s policy “to seek the prompt repeal of the Patient Protection and Affordable Care Act.”
The order opens the door for federal agencies to tackle ACA provisions such as the individual mandate and its tax penalties for not carrying insurance, as well as other financial aspects of the ACA that impact patients, providers, insurers, and manufacturers.
The order directs the secretaries of HHS, the Treasury department, and the Labor department to “exercise all authority and discretion available to them to provide greater flexibility to States and cooperate with them in implementing healthcare programs.”
With this order, President Trump also set the stage for creating a framework to sell insurance products across state lines by directing secretaries with oversight of insurance markets to “encourage the development of a free and open market in interstate commerce for the offering of healthcare services and health insurance, with the goal of achieving and preserving maximum options for patients and consumers.”
Little action is expected on the executive order until secretaries are approved for HHS, Treasury, and Labor. Rep. Tom Price (R-Ga.) is scheduled to appear before the Senate Finance Committee on Jan. 24.
WASHINGTON – President Trump wasted no time in getting the executive branch’s wheels in motion toward repeal of the Affordable Care Act.
Within hours of being sworn in as the 45th president of the United States on Jan. 20, he signed an executive order that announced the incoming administration’s policy “to seek the prompt repeal of the Patient Protection and Affordable Care Act.”
The order opens the door for federal agencies to tackle ACA provisions such as the individual mandate and its tax penalties for not carrying insurance, as well as other financial aspects of the ACA that impact patients, providers, insurers, and manufacturers.
The order directs the secretaries of HHS, the Treasury department, and the Labor department to “exercise all authority and discretion available to them to provide greater flexibility to States and cooperate with them in implementing healthcare programs.”
With this order, President Trump also set the stage for creating a framework to sell insurance products across state lines by directing secretaries with oversight of insurance markets to “encourage the development of a free and open market in interstate commerce for the offering of healthcare services and health insurance, with the goal of achieving and preserving maximum options for patients and consumers.”
Little action is expected on the executive order until secretaries are approved for HHS, Treasury, and Labor. Rep. Tom Price (R-Ga.) is scheduled to appear before the Senate Finance Committee on Jan. 24.
Repeal and replace: House bills offer potential road maps
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.