User login
Consider Melatonin for Migraine Prevention
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
Consider melatonin for migraine prevention
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend nightly melatonin 3 mg to your patients with chronic migraines, as it appears to be as effective as amitriptyline in reducing headaches and causes fewer adverse effects.
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality randomized controlled trial.
Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.1
Steroids During Late Preterm Labor? Better Later Than Never
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
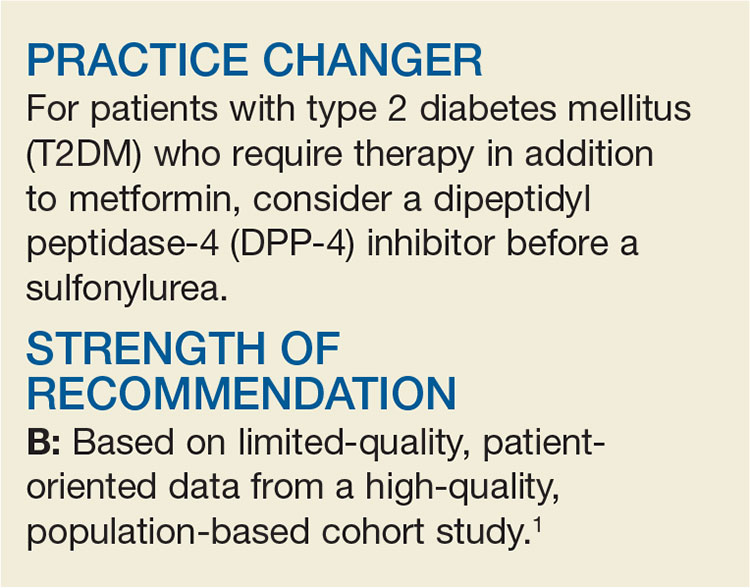
Need an Add-on to Metformin? Consider This
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
Steroids during late preterm labor: Better later than never
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Use steroids in women at risk of preterm delivery, even if they are 36 weeks, 6 days’ pregnant, because steroids may reduce respiratory complications in the newborn with minimal risk for neonatal or maternal complications.
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.1
STRENGTH OF RECOMMENDATION
A: Based on a good quality randomized controlled trial and consistent with a meta-analysis.
Need an add-on to metformin? Consider this
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Consider a dipeptidyl peptidase-4 inhibitor before a sulfonylurea for patients with type 2 diabetes mellitus who require therapy in addition to metformin.
Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.1
STRENGTH OF RECOMMENDATION
B: Based on limited-quality, patient-oriented data from a high-quality, population-based cohort study.
Monitoring Home BP Readings Just Got Easier
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Mildly pruritic palmar rash
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)
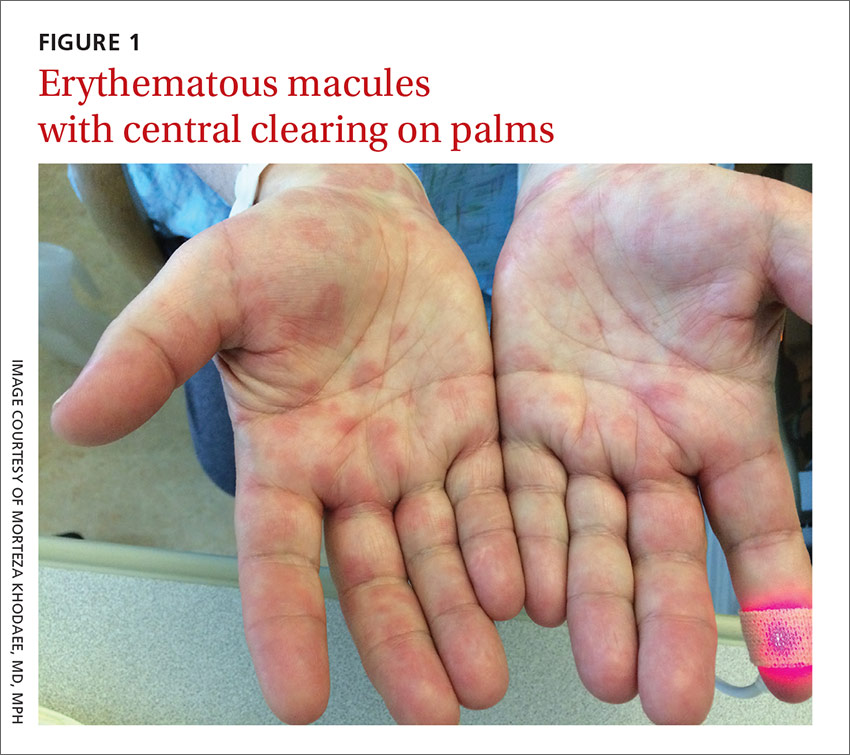
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7
Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7

Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7

Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
Monitoring home BP readings just got easier
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Do corticosteroid injections improve carpal tunnel syndrome symptoms?
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Evidence-based answers from the Family Physicians Inquiries Network