User login
Which detoxification regimens are effective for alcohol withdrawal syndrome?
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE-BASED ANSWER:
Benzodiazepines remain the first-line regimen for alcohol withdrawal syndrome (AWS) and are the only class more effective than placebo for reducing seizure (strength of recommendation [SOR]: B, based on 3 medium-quality randomized controlled trials [RCTs]). Anticonvulsants are no more effective than placebo at reducing seizures (SOR: B, based on 10 moderate-quality RCTs). Gabapentin reduces withdrawal symptoms and is less sedating than benzodiazepines (SOR: B, based on 1 medium-quality RCT). Carbamazepine also reduces withdrawal symptoms (SOR: B, based on 3 RCTs). Evidence of benzodiazepine superiority to other drugs with respect to safety is lacking (SOR: A, based on a meta-analysis).
Does evidence support the use of supplements to aid in BP control?
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
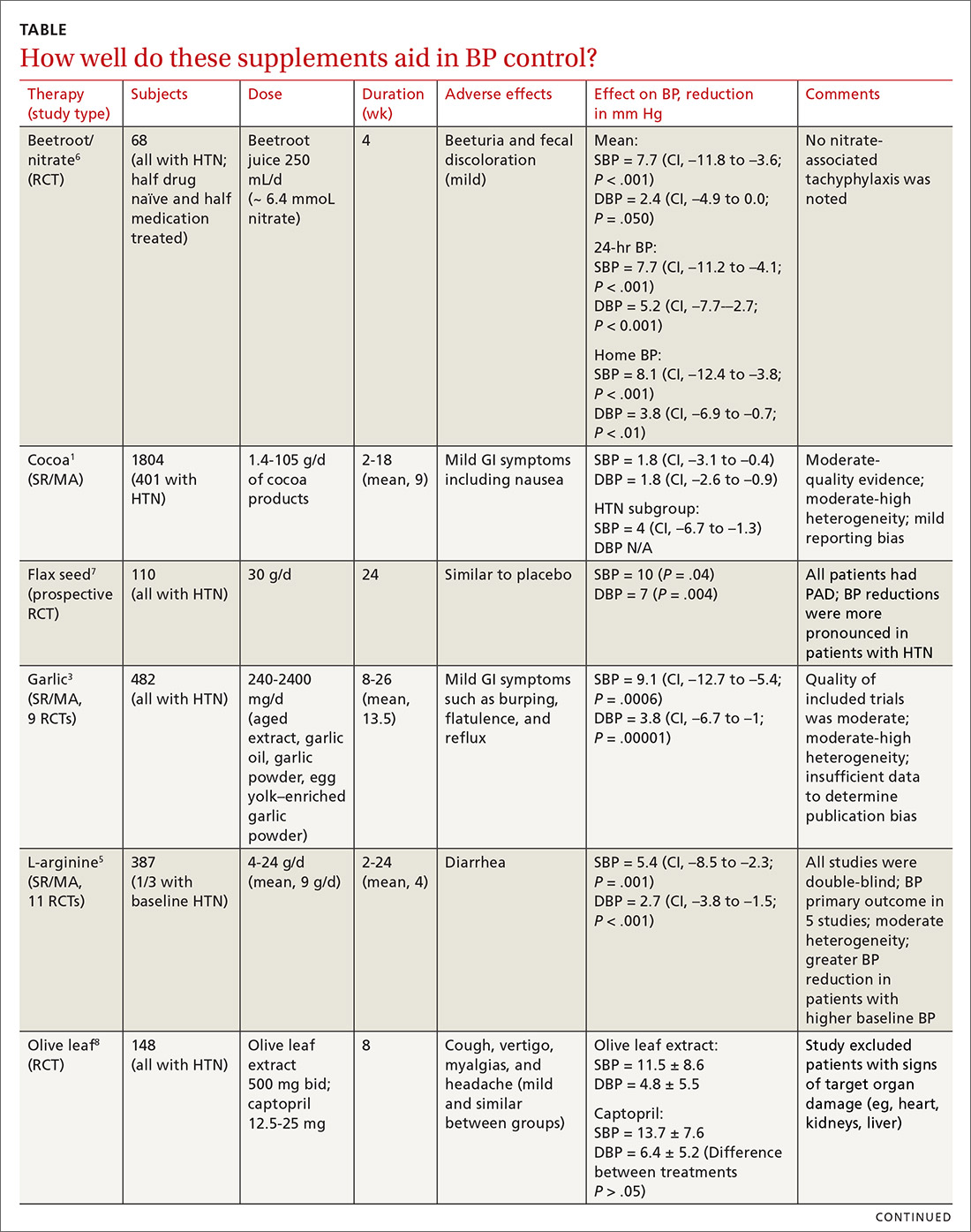
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
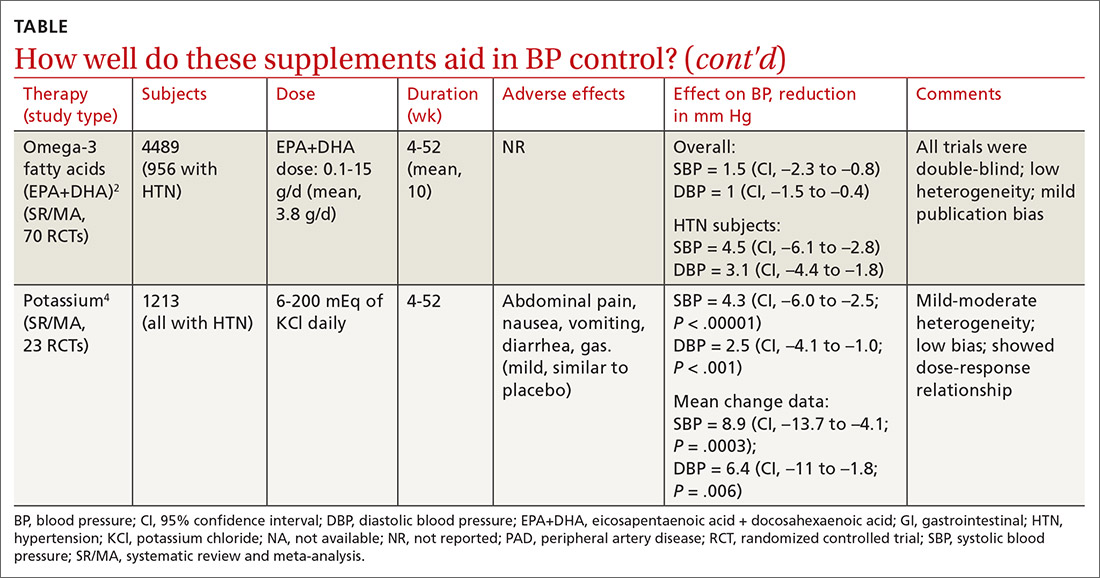
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE-BASED ANSWER:
Yes. A number of well-tolerated natural therapies have been shown to reduce systolic and diastolic blood pressure (BP). (See Table1-8 for summary.) However, the studies don’t provide direct evidence of whether the decrease in BP is linked to patient-oriented outcomes. Nor do they allow definitive conclusions concerning the lasting nature of the reductions, because most studies were fewer than 6 months in duration (strength of recommendation: C, disease-oriented evidence).
Which medications work best for menorrhagia?
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE-BASED ANSWER:
Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized controlled trials (RCTs): the levonorgestrel-releasing intrauterine system (LNG-IUS), tranexamic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and danazol, a synthetic steroid (strength of recommendation: A, meta-analyses of RCTs).
A single trial showed that the LNG-IUS reduced MBL by about 100 mL, compared with placebo. In a meta-analysis of 4 placebo-controlled RCTs, tranexamic acid reduced MBL by about 53 mL, roughly a 40% to 50% decrease. The 8 NSAID trials (5 mefenamic acid, 2 naproxen, 1 ibuprofen) demonstrated effectiveness, but the effect size is difficult to quantify. The single danazol RCT used a subjective scoring system without reporting MBL.
No studies compared all effective medical therapies against one another. In head-to-head comparisons, women were more likely to experience improvement with the LNG-IUS than with tranexamic acid (number needed to treat [NNT] = 2 to 6). Both treatments are superior to NSAIDs. Danazol is also more efficacious than NSAIDs, but its use is limited by its adverse effects, including teratogenicity.
No placebo-controlled trials have studied oral contraceptive pills (OCPs) or oral progesterone to treat menorrhagia. However, multiple comparative RCTs have demonstrated that these commonly prescribed medications significantly decrease MBL. Trials have shown the reduction to be inferior to LNG-IUS and danazol and equivalent to NSAIDs.
Do cinnamon supplements improve glycemic control in adults with T2DM?
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
EVIDENCE-BASED ANSWER:
The answer isn’t clear. Cinnamon supplements for adults with type 2 diabetes haven’t been shown to decrease hemoglobin A1C (strength of recommendation [SOR]: C, multiple systematic reviews of disease-oriented outcomes).
Cinnamon supplements have shown inconsistent effects on fasting glucose levels (SOR: C, multiple systematic reviews and a single meta-analysis of disease-oriented outcomes). Supplements decreased fasting glucose levels in some studies, but the evidence isn’t consistent and hasn’t been correlated with clinically significant improvements in glycemic control.
Can unintended pregnancies be reduced by dispensing a year’s worth of hormonal contraception?
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE-BASED ANSWER:
Probably, although studies that looked directly at this outcome are limited. A systematic review showed that women who received a larger number of pills at one time were more likely to continue using combined hormonal contraception 7 to 15 months later (strength of recommendation [SOR]: A, consistent evidence from 2 cohort studies and 1 randomized, controlled trial), which might be extrapolated to indicate lower unintended pregnancy rates.
One of the large retrospective cohort studies included in the review demonstrated a significantly lower rate of pregnancy among women who received 12 or 13 packs of oral contraceptives at an office visit compared with 1 or 3 packs (SOR: B, large retrospective cohort study).
Do A-fib patients continue to benefit from vitamin K antagonists with advancing age?
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE-BASED ANSWER:
Yes, patients with atrial fibrilla- tion who are between the ages of 50 and 90 years continue to benefit from vitamin K antagonist therapy (warfarin) (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and large cohorts). Regardless of age, warfarin produces a reduction in risk of thrombotic events that is 2- to 4-fold greater than the risk of hemorrhagic events.
Does left atrial appendage closure reduce stroke rates as well as oral anticoagulants and antiplatelet meds in A-fib patients?
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
EVIDENCE SUMMARY
A 2017 network meta-analysis included 19 RCTs and 87,831 patients receiving anticoagulation, antiplatelet therapy, or LAAC for NVAF.1 LAAC was superior to antiplatelet therapy (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.23-0.86; P<.05) and similar to NOACs (HR=1.01; 95% CI, 0.53-1.92; P=.969) for reducing risk of stroke.
LAAC and NOACs found “most effective”
A network meta-analysis of 21 RCTs, which included data from 96,017 patients, examined the effectiveness of 7 interventions to prevent stroke in patients with NVAF: 4 NOACs, VKA, aspirin, and LAAC; the analysis compared VKA with the other interventions.2 The 2 trials that investigated LAAC accounted for only 1114 patients.
When the 7 interventions were ranked simultaneously on 2 efficacy outcomes (stroke/systemic embolism and all-cause mortality), all 4 NOACs and LAAC clustered together as “the most effective and lifesaving.”
Fewer hemorrhagic strokes with LAAC than VKA
A 2016 meta-analysis of 6 RCTs compared risk of stroke for adults with NVAF who received LAAC, VKA, or NOACs.3 No significant differences were found between NOACs and VKA or LAAC and VKA. The LAAC group had a significantly smaller number of patients.
A 2015 meta-analysis of 2406 patients with NVAF found that patients who received LAAC had significantly fewer hemorrhagic strokes (HR=0.22; P<.05) than patients who received VKA.4 No differences in all-cause stroke were found between the 2 groups during an average follow-up of 2.69 years.
LAAC found superior to warfarin for stroke prevention in one trial
A 2014 multicenter, randomized study (PROTECT-AF) of 707 patients with NVAF plus 1 additional stroke risk factor compared LAAC with VKA (warfarin).5 LAAC met criteria at 3.8 years for both noninferiority and superiority in preventing stroke, based on 2.3 events per 100 patient-years compared with 3.8 events per 100 patient-years for VKA. The number needed to treat with LAAC was 67 to result in 1 less event per patient-year.
A 2014 RCT (PREVAIL) evaluated patients with NVAF plus 1 additional stroke risk factor. LAAC was noninferior to warfarin for ischemic stroke prevention.6
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Cardiology (ACC) recommends LAAC for patients with NVAF who are not candidates for long-term anticoagulation.7 Similarly, the 2016 European Society of Cardiology guidelines issued a Class IIb recommendation for LAAC for stroke prevention in those with contraindications for long-term anticoagulation.8 Lastly, in a 2014 guideline, the American Heart Association, ACC, and the Heart Rhythm Society issued a Class IIb recommendation for surgical excision of the left atrial appendage in patients with atrial fibrillation undergoing cardiac surgery, but did not provide recommendations regarding LAAC.9
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
1. Sahay S, Nombela-Franco L, Rodes-Cabau J, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart. 2017;103:139-147.
2. Tereshchenko LG, Henrikson CA, Cigarroa, J, et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016; 5:e003206.
3. Bajaj NS, Kalra R, Patel N, et al. Comparison of approaches for stroke prophylaxis in patients with non-valvular atrial fibrillation: network meta-analyses of randomized clinical trials. PLoS One. 2016;11:e0163608.
4. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
5. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
6. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
7. Panaich S, Holmes DR. Left atrial appendage occlusion: Expert analysis. http://www.acc.org/latest-in-cardiology/articles/2017/ 01/31/13/08/left-atrial-appendage-occlusion. Accessed April 5, 2018.
8. Kirchof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678.
9. January CT, Wann LS, Alpert LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. JACC. 2014;64:2246-2280.
EVIDENCE-BASED ANSWER:
Yes. Left atrial appendage closure (LAAC) with the Watchman device is noninferior to vitamin K antagonists (VKAs) and non-VKA oral anticoagulants (NOACs) for adults with nonvalvular atrial fibrillation (NVAF) and 1 additional stroke risk factor (strength of recommendation [SOR]: A, multiple meta-analyses).
LAAC has consistently been shown to be superior to antiplatelet therapy (SOR: A, single meta-analysis). One randomized controlled trial (RCT) demonstrated superiority of LAAC to VKA (SOR: B, single RCT).
Does prophylactic azithromycin reduce the number of COPD exacerbations or hospitalizations?
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
EVIDENCE-BASED ANSWER:
Yes for exacerbations, no for hospitalizations. Prophylactic azithromycin reduces the number of exacerbations by about 25%. It also extends the time between exacerbations by approximately 90 days for patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Azithromycin benefits patients who are >65 years, patients with Global Initiative for Obstructive Lung Disease (GOLD) stage II or III COPD, former smokers, and patients using long-term oxygen; it doesn’t benefit patients ≤65 years, patients with GOLD stage IV COPD, current smokers, or patients not using oxygen (strength of recommendation [SOR]: B, randomized controlled trials [RCTs]).
Prophylactic azithromycin doesn’t reduce hospitalizations overall (SOR: B, single small RCT).
Does exercise relieve vasomotor menopausal symptoms?
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Exercise doesn’t decrease the frequency or severity of vasomotor menopausal symptoms (VMS) in perimenopausal and postmenopausal women (strength of recommendation: A, systematic review of randomized controlled trials [RCTs] and consistent RCT).
Is megestrol acetate safe and effective for malnourished nursing home residents?
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Megestrol acetate (MA) is neither safe nor effective for stimulating appetite in malnourished nursing home residents. It increases the risk of deep vein thrombosis (DVT) (strength of recommendation [SOR]: C, 2 retrospective chart reviews), but isn’t associated with other new or worsening events or disorders (SOR: B, single randomized controlled trial [RCT]).
Over a 25-week period, MA wasn’t associated with increased mortality (SOR: B, single RCT). After 44 months, however, MA-treated patients showed decreased median survival (SOR: B, single case-control study).
Consistent, meaningful weight gain was not observed with MA treatment (SOR: B, single case-control study, single RCT, 2 retrospective chart reviews, single prospective case-series).