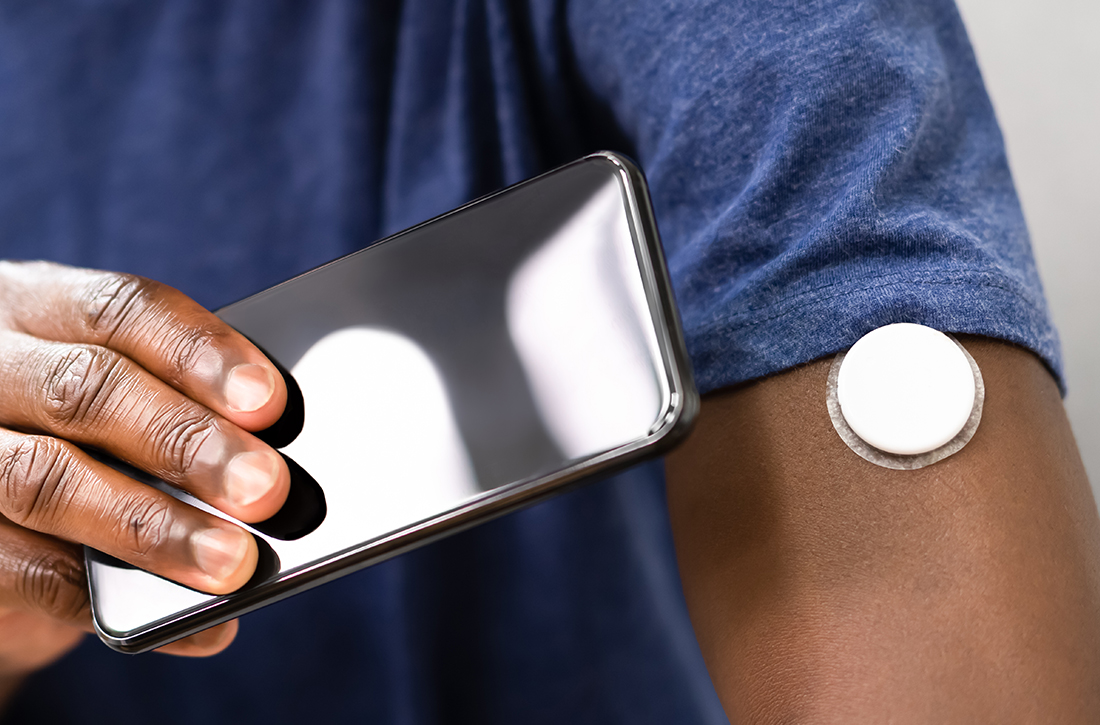
User login
Does use of continuous or flash glucose monitors decrease hypoglycemia episodes in T2D?
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
EVIDENCE-BASED REVIEW:
NO. In adults with insulin-treated type 2 diabetes (T2D), continuous glucose monitoring (CGM) and flash glucose monitoring (FGM) do not decrease symptomatic hypoglycemia episodes (strength of recommendation [SOR], B) but do lower time in hypoglycemia (SOR, C; disease-oriented evidence).
CGM, in which glucose levels are sent automatically in numeric and graphic format to a patient’s smart device for their potential action, did not change the hypoglycemic event rate (SOR, B; 2 prospective studies). CGM significantly reduced hypoglycemia duration in an 8-month randomized controlled trial (RCT; SOR, C) but not in a 1-year prospective study (SOR, C).
FGM, in which glucose levels are sent on demand to a device, did not significantly reduce hypoglycemic episodes (SOR, B; 1 small RCT and 1 prospective study). Hypoglycemia duration was reduced significantly with FGM in a 6-month RCT (SOR, B) but not in a 1-year prospective study (SOR, B).
Is megestrol acetate safe and effective for malnourished nursing home residents?
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
EVIDENCE SUMMARY
A 25-week double-blind, placebo-controlled RCT of 51 nursing home patients (mean age 76 years, range 50 to 95 years; 96% men) in 2000 found no difference in all-cause mortality between the MA treatment group and the placebo group (absolute risk reduction [ARR]=13.4%; 95% confidence interval [CI], -12.9% to 37.3%; number needed to harm [NNH]=7; 95% CI, -8 to 3).1
A 2007 case-control study of 17,328 nursing home residents (mean age 84 years [standard deviation, 9]; 71% women) found increased mortality for residents treated with at least 6 days of MA (median survival=23.9 months; 95% CI, 20.2-27.5) compared with untreated residents (median survival=31.2 months; 95% CI, 27.8-35.9).2 The decrease in median survival remained after adjusting for demographic variables, medical diagnoses, and cognitive and physical functioning (hazard ratio=1.37; 95% CI, 1.17-1.59). Follow-up ranged from 30 days to 44 months.
Risks related to megestrol acetate include deep vein thrombosis
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference in adverse events between the MA group and the placebo group (absolute risk increase=6.3%; 95% CI, -14.7% to 27.3%).1 No DVTs were reported as adverse events.
A 2003 retrospective chart review of 246 nursing home residents (mean age 87 years, 77% women) who were given MA 400 mg/d found an overall incidence of DVT of 4.1% (10 residents); 3.2% (8) residents were on MA at the time of DVT occurrence.3
A 2000 retrospective chart review of 19 nursing home residents who were prescribed MA (mean age 83 years, range 66 to 92 years; 84% women) found 32% (6) who developed Doppler-confirmed DVT after 50 days of therapy.4 DVT was not associated with known risk factors, age, body mass index, numbers of medications, or other medical diagnoses. The authors didn’t report MA dosage.
Patients on megestrol acetate don’t gain weight...
The 2000 double-blind, placebo-controlled RCT of 51 nursing home patients found no difference between the MA (800 mg/d for 12 weeks) and placebo groups in percentage of patients who gained ≥1.82 kg (ARR=-6.6%; 95% CI, -30.2% to 18.2%).1 At the 25-week follow-up (after the MA patients had been off the therapy for 13 weeks), a statistically, but not clinically, significant difference was observed in the number of MA patients who gained ≥1.82 kg (absolute benefit increase=40.2%; 95% CI, 13.4%-66.9%; number needed to treat [NNT]=2; 95% CI, 1-8). Of note, the authors based their statistics on a weight gain of ≥1.82 kg whereas 5 kg or 5% weight gain is the more commonly used definition for clinical significance.5
The 2007 case-control cohort study of 17,328 nursing home residents, who had lost 5% of total body weight in 3 months or 10% of total body weight in 6 months, also found no significant difference in weight gain between MA-treated patients (median dose=486 mg, range 20 to 2400 mg; median duration=90 days, range 7 to 934 days; median change=1 lb, interquartile range [IQR]=-8 to 10) and controls (median change=2 lb, IQR=-4 to 9) after 6 months of treatment.2
...And some lose weight
In a 2005 prospective case series of 17 nursing home residents (mean age 92 years [standard deviation, 6], 88% women), MA (400 mg/d for 63 days) was associated with weight loss (mean=-2.13±9.32 lb).6 Nine patients (53%) lost weight (mean=9.3±5.4 lb), and 8 patients (47%) gained weight (mean=5.9±4.9 lb).
A retrospective chart review in 2000 of 14 nursing home residents (mean age 84 years, range 74 to 97 years; 85% women) who received MA 40 to 800 mg/d for one to 15 weeks showed that 43% gained weight (mean=3.1 kg), 43% lost weight (mean=2.0 kg), and 14% had no weight change.7
A 2002 retrospective chart review of 50 nursing home residents (mean age 79 years, range 31 to 93 years; 74% women) who were treated with MA 200 to 2400 mg/d for at least 6 months found a mean weight loss of 1.1 to 2.2 kg.8 In the 6 months after MA discontinuation, weight gain for available subjects (5 to 16 patients) varied (mean monthly change=-0.17 kg to 3.07 kg). The study had a high attrition rate (26 patients were lost 6 months after MA initiation; 39 were lost 6 months after MA discontinuation).
RECOMMENDATIONS
The 2015 American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults strongly advises against the use of MA because of limited increases in weight and increased risk of thrombotic events.9
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind placebo controlled study. J Am Geriatr Soc. 2000;48:485-492.
2. Bodenner D, Spencer T, Riggs AT, et al., A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5:137-146.
3. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255-256.
4. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248-252.
5. Colman E. Food and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156-2164.
6. Simmons SF, Walker KA, Osterwell D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3 Suppl):S5-S11.
7. Cicero LA, Rosenberg JM, Miyashiro A, et al. Megestrol acetate suspension for the treatment of involuntary weight loss in elderly nursing home residents: a retrospective chart review. Consult Pharm. 2000;15:811-814.
8. Dickerson LM, Jones KW. Retrospective review and intervention in the use of megestrol acetate in residents of skilled nursing facilities in South Carolina. Consult Pharm. 2002;17:1040-1042.
9. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Megestrol acetate (MA) is neither safe nor effective for stimulating appetite in malnourished nursing home residents. It increases the risk of deep vein thrombosis (DVT) (strength of recommendation [SOR]: C, 2 retrospective chart reviews), but isn’t associated with other new or worsening events or disorders (SOR: B, single randomized controlled trial [RCT]).
Over a 25-week period, MA wasn’t associated with increased mortality (SOR: B, single RCT). After 44 months, however, MA-treated patients showed decreased median survival (SOR: B, single case-control study).
Consistent, meaningful weight gain was not observed with MA treatment (SOR: B, single case-control study, single RCT, 2 retrospective chart reviews, single prospective case-series).