User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
FDA approves Arikayce for MAC lung diseases
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
Adalimumab safety update finds no new signals
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
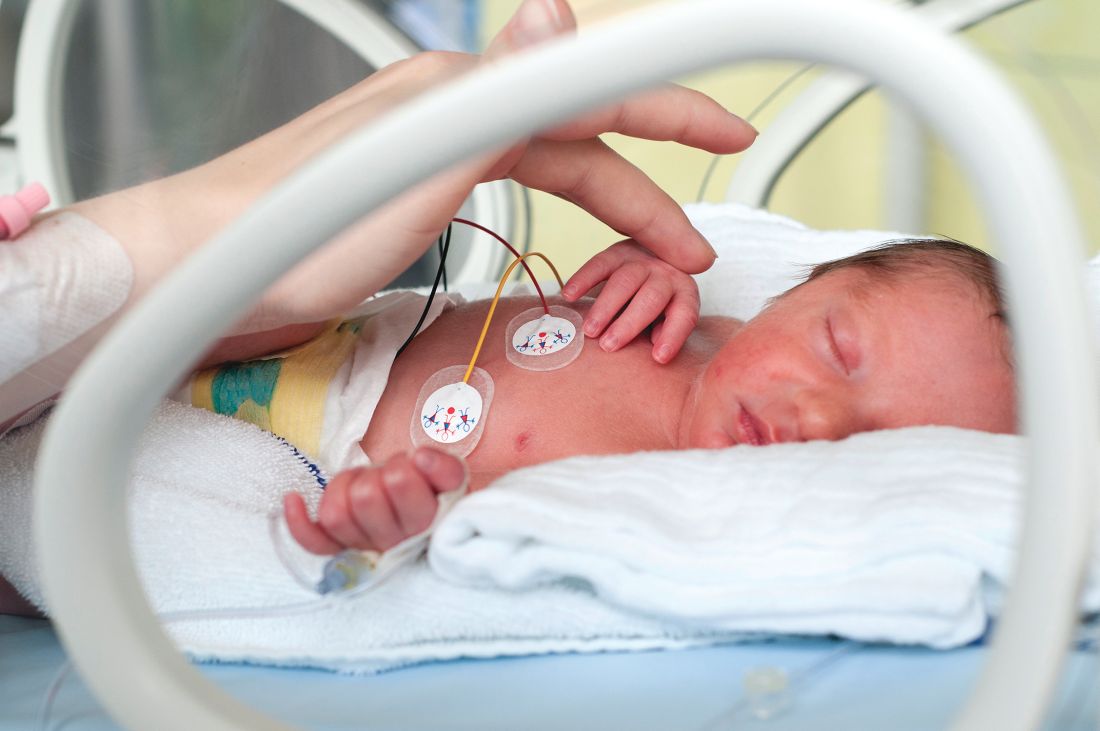
Methadone may shorten NICU stays for neonatal abstinence syndrome
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
FROM THE JOURNAL OF PEDIATRICS
New AAP policy statement addresses teen driver risks
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
FROM PEDIATRICS
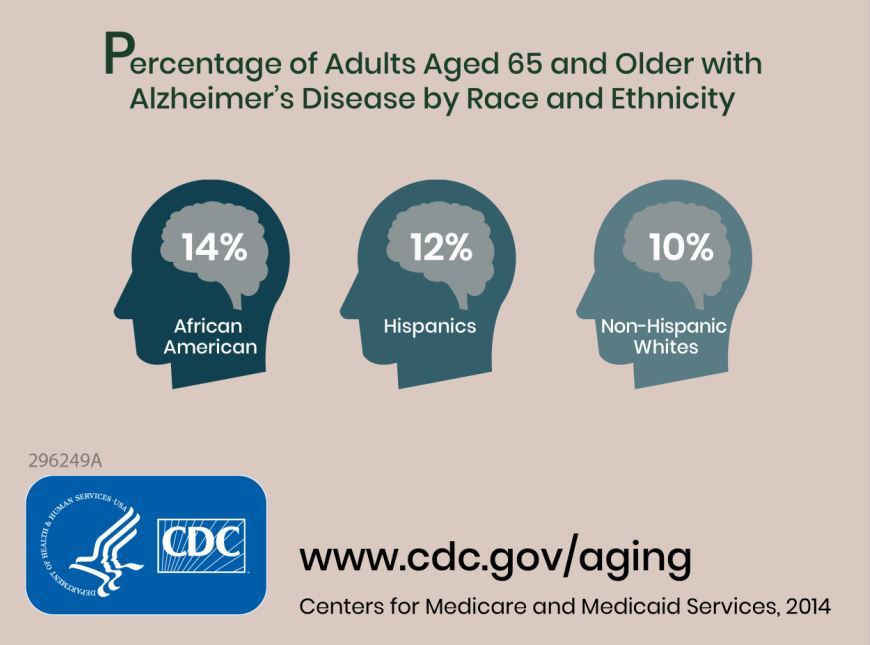
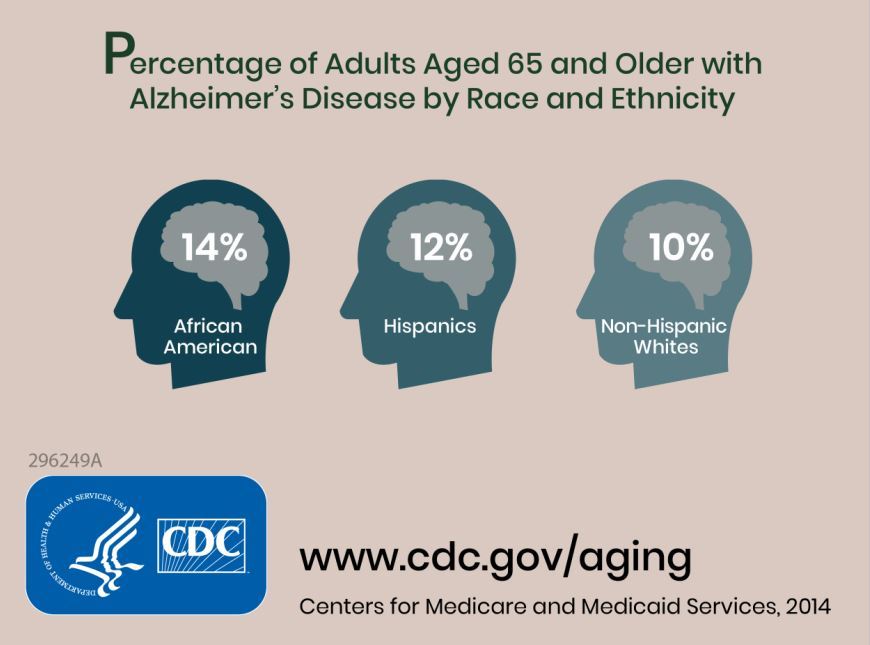
Burden of dementia will shift more to minorities by 2060
, according to a study in Alzheimer’s and Dementia.
Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
FROM ALZHEIMER’S AND DEMENTIA
FDA clears gold microparticles for acne vulgaris
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
ICYMI: Canakinumab reduced risk of gout attacks
Canakinumab, an interleukin-1–beta blocker, reduced the risk of a gout attack by 52% when administered every 3 months, according to a secondary exploratory analysis of data from the multicenter, randomized, double-blind, placebo-controlled CANTOS trial (NCT01327846). The results of this analysis were published Sept. 17 in the Annals of Internal Medicine (doi: 10.7326/M18-1167).
We covered this story before it was published in the journal. Find our conference coverage at the links below.
Canakinumab, an interleukin-1–beta blocker, reduced the risk of a gout attack by 52% when administered every 3 months, according to a secondary exploratory analysis of data from the multicenter, randomized, double-blind, placebo-controlled CANTOS trial (NCT01327846). The results of this analysis were published Sept. 17 in the Annals of Internal Medicine (doi: 10.7326/M18-1167).
We covered this story before it was published in the journal. Find our conference coverage at the links below.
Canakinumab, an interleukin-1–beta blocker, reduced the risk of a gout attack by 52% when administered every 3 months, according to a secondary exploratory analysis of data from the multicenter, randomized, double-blind, placebo-controlled CANTOS trial (NCT01327846). The results of this analysis were published Sept. 17 in the Annals of Internal Medicine (doi: 10.7326/M18-1167).
We covered this story before it was published in the journal. Find our conference coverage at the links below.
FROM THE ANNALS OF INTERNAL MEDICINE
FDA approves device for coronary artery perforations
according to an announcement from the agency.
These small tears in the arterial wall are rare but life-threatening complications of certain procedures, such as percutaneous coronary interventions. The device uses a balloon catheter like that used with PCI and, when successful, it can spare patients more invasive procedures, such as open heart surgery.
The approval is based on survey data from 80 patients treated with this device. In 76 of the patients (95%), the device was successfully delivered to the site of the acute coronary arterial perforation, and it successfully healed the tear in 73 patients (91%). Two patients died during the procedure, and five whose perforations were sealed successfully died in the hospital after the procedure, as did one whose perforation was not sealed.
The agency noted that this is the first device approved for the indication in 17 years. More information can be found in the full FDA announcement.
according to an announcement from the agency.
These small tears in the arterial wall are rare but life-threatening complications of certain procedures, such as percutaneous coronary interventions. The device uses a balloon catheter like that used with PCI and, when successful, it can spare patients more invasive procedures, such as open heart surgery.
The approval is based on survey data from 80 patients treated with this device. In 76 of the patients (95%), the device was successfully delivered to the site of the acute coronary arterial perforation, and it successfully healed the tear in 73 patients (91%). Two patients died during the procedure, and five whose perforations were sealed successfully died in the hospital after the procedure, as did one whose perforation was not sealed.
The agency noted that this is the first device approved for the indication in 17 years. More information can be found in the full FDA announcement.
according to an announcement from the agency.
These small tears in the arterial wall are rare but life-threatening complications of certain procedures, such as percutaneous coronary interventions. The device uses a balloon catheter like that used with PCI and, when successful, it can spare patients more invasive procedures, such as open heart surgery.
The approval is based on survey data from 80 patients treated with this device. In 76 of the patients (95%), the device was successfully delivered to the site of the acute coronary arterial perforation, and it successfully healed the tear in 73 patients (91%). Two patients died during the procedure, and five whose perforations were sealed successfully died in the hospital after the procedure, as did one whose perforation was not sealed.
The agency noted that this is the first device approved for the indication in 17 years. More information can be found in the full FDA announcement.
FDA grants praliciguat Fast Track Designation for HFpEF
fraction (HFpEF), according to its developer Ironwood.
A phase 2, randomized, double-blind, placebo-controlled trial is currently enrolling patients to evaluate praliciguat as a treatment for HFpEF. The trial aims to enroll about 175 patients and intends to evaluate safety and efficacy, and topline data is expected later in 2019.
Praliciguat is an oral, once-daily, soluble guanylate cyclase (sGC) stimulator. It is being studied in patients with diabetic nephropathy and in patients with HFpEF. The condition affects an estimated 3 million Americans, but there are no approved therapies at this time to treat it; however, praliciguat may have the potential to treat the underlying causes by improving nitric oxide signaling, according to the press release from Ironwood.
fraction (HFpEF), according to its developer Ironwood.
A phase 2, randomized, double-blind, placebo-controlled trial is currently enrolling patients to evaluate praliciguat as a treatment for HFpEF. The trial aims to enroll about 175 patients and intends to evaluate safety and efficacy, and topline data is expected later in 2019.
Praliciguat is an oral, once-daily, soluble guanylate cyclase (sGC) stimulator. It is being studied in patients with diabetic nephropathy and in patients with HFpEF. The condition affects an estimated 3 million Americans, but there are no approved therapies at this time to treat it; however, praliciguat may have the potential to treat the underlying causes by improving nitric oxide signaling, according to the press release from Ironwood.
fraction (HFpEF), according to its developer Ironwood.
A phase 2, randomized, double-blind, placebo-controlled trial is currently enrolling patients to evaluate praliciguat as a treatment for HFpEF. The trial aims to enroll about 175 patients and intends to evaluate safety and efficacy, and topline data is expected later in 2019.
Praliciguat is an oral, once-daily, soluble guanylate cyclase (sGC) stimulator. It is being studied in patients with diabetic nephropathy and in patients with HFpEF. The condition affects an estimated 3 million Americans, but there are no approved therapies at this time to treat it; however, praliciguat may have the potential to treat the underlying causes by improving nitric oxide signaling, according to the press release from Ironwood.