User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
FDA names 40 ARBs that are free of nitrosamines
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
New renal, CV disease indication sought for canagliflozin
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
Some cardiac devices vulnerable to cybersecurity threats
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
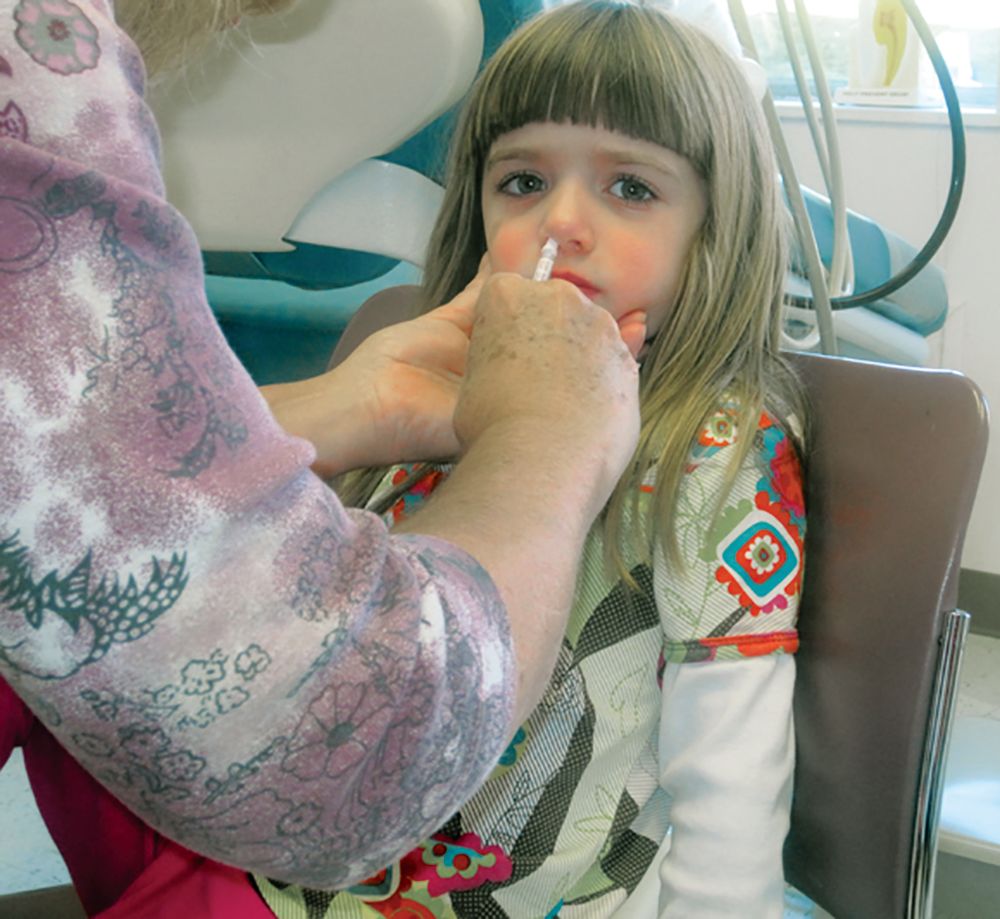
AAP updates 2019-2020 flu vaccine recommendations to include nasal spray
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
FDA: Programmable heart failure device approved
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
Rapid preeclampsia urine test is simple, noninvasive
according to a research letter in EClinicalMedicine.
The research team, led by Kara M. Rood, MD, of the department of obstetrics and gynecology at the Ohio State University, Columbus, said that their pragmatic study in 346 consecutive pregnant patients demonstrated that the test is not only inexpensive, but also easy to use and well received by the nursing staff. A positive Congo Red Dot Rapid Paper Test had 80% sensitivity, 89% specificity, 92% negative predictive value and 87% accuracy to correctly diagnose preeclampsia.
The patients were recruited from the labor and delivery triage unit at the Ohio State University Wexner Medical Center. Certain misfolded proteins typically are found in the urine of women with preeclampsia, so in prior research, the researchers had hypothesized that a urine test that could detect these proteins would carry “diagnostic and prognostic potential for” preeclampsia. The researchers were able to show that this was possible with a laboratory test that used Congo Red dye because those misfolded proteins bind with it. This current study explored the accuracy of a 3-minute, point-of-care urine test that uses a dot of Congo Red dye on a piece of paper.
Other serum and urine tests, which often have been more complicated or time intensive, have failed to gain traction in real-world practice, as well as in low-resource countries where mortality and morbidity from preeclampsia are highest, the authors noted. By contrast, the researchers hope the rapid paper test they studied in the current research will fulfill that unmet need.
The study was funded by the Saving Lives at Birth grant and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Rood KM et al. EClinicalMedicine. 2019. doi: 10.1016/j.eclinm.2019.02.004.
according to a research letter in EClinicalMedicine.
The research team, led by Kara M. Rood, MD, of the department of obstetrics and gynecology at the Ohio State University, Columbus, said that their pragmatic study in 346 consecutive pregnant patients demonstrated that the test is not only inexpensive, but also easy to use and well received by the nursing staff. A positive Congo Red Dot Rapid Paper Test had 80% sensitivity, 89% specificity, 92% negative predictive value and 87% accuracy to correctly diagnose preeclampsia.
The patients were recruited from the labor and delivery triage unit at the Ohio State University Wexner Medical Center. Certain misfolded proteins typically are found in the urine of women with preeclampsia, so in prior research, the researchers had hypothesized that a urine test that could detect these proteins would carry “diagnostic and prognostic potential for” preeclampsia. The researchers were able to show that this was possible with a laboratory test that used Congo Red dye because those misfolded proteins bind with it. This current study explored the accuracy of a 3-minute, point-of-care urine test that uses a dot of Congo Red dye on a piece of paper.
Other serum and urine tests, which often have been more complicated or time intensive, have failed to gain traction in real-world practice, as well as in low-resource countries where mortality and morbidity from preeclampsia are highest, the authors noted. By contrast, the researchers hope the rapid paper test they studied in the current research will fulfill that unmet need.
The study was funded by the Saving Lives at Birth grant and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Rood KM et al. EClinicalMedicine. 2019. doi: 10.1016/j.eclinm.2019.02.004.
according to a research letter in EClinicalMedicine.
The research team, led by Kara M. Rood, MD, of the department of obstetrics and gynecology at the Ohio State University, Columbus, said that their pragmatic study in 346 consecutive pregnant patients demonstrated that the test is not only inexpensive, but also easy to use and well received by the nursing staff. A positive Congo Red Dot Rapid Paper Test had 80% sensitivity, 89% specificity, 92% negative predictive value and 87% accuracy to correctly diagnose preeclampsia.
The patients were recruited from the labor and delivery triage unit at the Ohio State University Wexner Medical Center. Certain misfolded proteins typically are found in the urine of women with preeclampsia, so in prior research, the researchers had hypothesized that a urine test that could detect these proteins would carry “diagnostic and prognostic potential for” preeclampsia. The researchers were able to show that this was possible with a laboratory test that used Congo Red dye because those misfolded proteins bind with it. This current study explored the accuracy of a 3-minute, point-of-care urine test that uses a dot of Congo Red dye on a piece of paper.
Other serum and urine tests, which often have been more complicated or time intensive, have failed to gain traction in real-world practice, as well as in low-resource countries where mortality and morbidity from preeclampsia are highest, the authors noted. By contrast, the researchers hope the rapid paper test they studied in the current research will fulfill that unmet need.
The study was funded by the Saving Lives at Birth grant and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Rood KM et al. EClinicalMedicine. 2019. doi: 10.1016/j.eclinm.2019.02.004.
FROM ECLINICALMEDICINE
Sjögren’s syndrome risk increases with infections
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
FROM THE JOURNAL OF INTERNAL MEDICINE
Match Day 2019: Pediatrics up from last year
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.
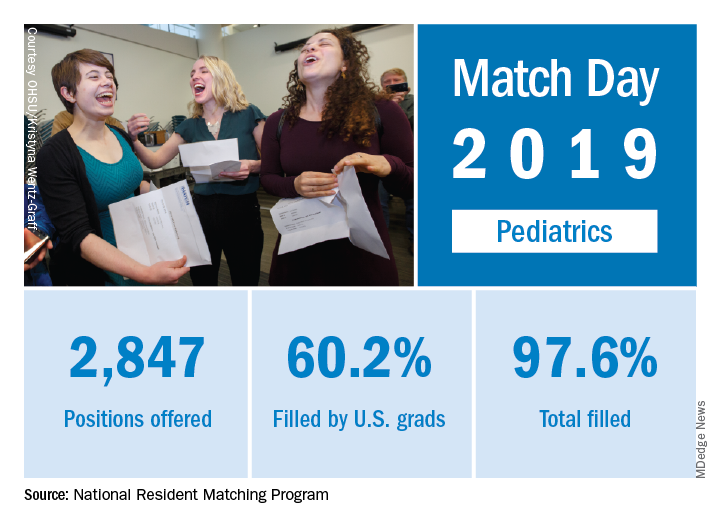
Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.

Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.

Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019: Ob.gyn. up from last year
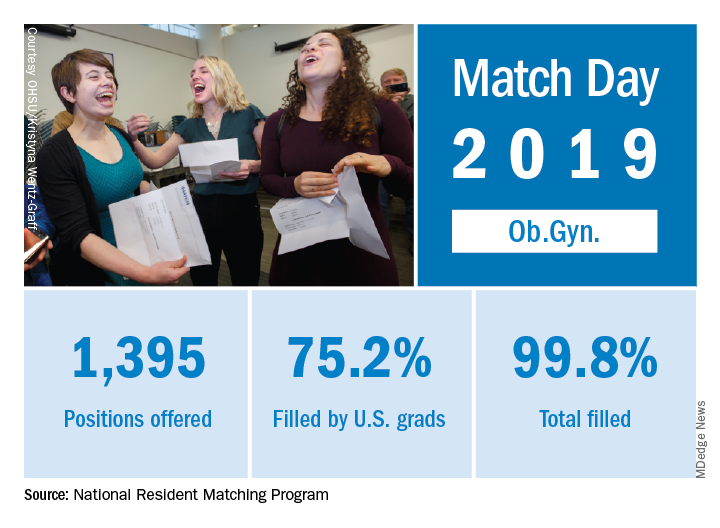
Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.

Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.

Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
ICYMI: Noninferior tuberculosis prevention in HIV has shorter duration
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
FROM NEW ENGLAND JOURNAL OF MEDICINE