User login
TMS tied to ‘marked’ antidepressant, anxiolytic effects in anxious depression
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Psychiatric illnesses share common brain network
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.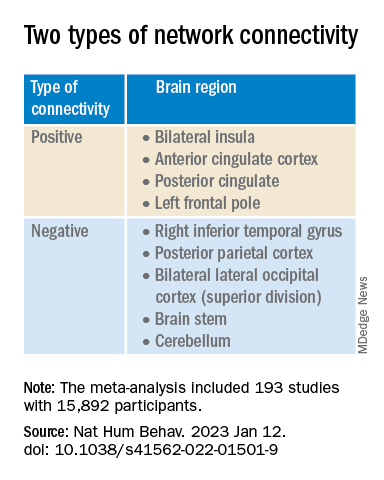
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE HUMAN BEHAVIOR
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.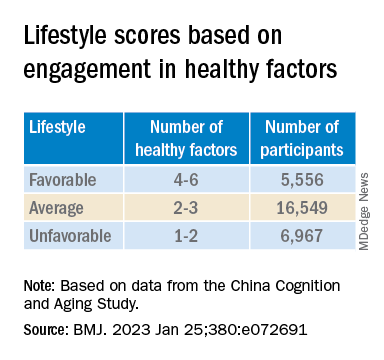
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.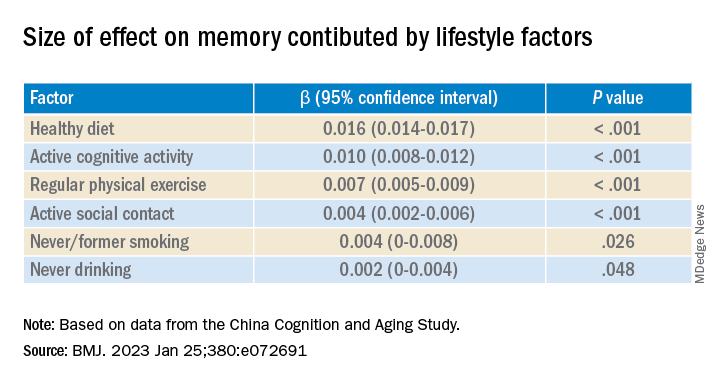
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Social isolation hikes dementia risk in older adults
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HRT may prevent Alzheimer’s in high-risk women
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Postconcussion symptoms tied to high risk of depression
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Sleep complaints in major depression flag risk for other psychiatric disorders
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Is the FDA serotonin syndrome warning unnecessary?
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
Results from a study that included more than 1,100 patients who were prescribed linezolid, about 20% of whom were also taking antidepressants, showed that serotonin syndrome occurred in fewer than 0.5% of participants – and that the percentage was actually lower among those who took antidepressants, compared with those who did not.
A comparison of participants who took antidepressants to propensity-matched patients who did not take antidepressants showed similar rates of altered mental status, hospitalization, and death between the two groups.
“In this cohort study of older patients who were prescribed linezolid, serotonin syndrome occurred rarely [and] concurrent antidepressants did not significantly increase the risk of serotonin syndrome,” Anthony Bai, MD, division of infectious diseases, department of medicine, Queen’s University, Kingston, Ont., and colleagues write.
“These findings suggested that linezolid is likely safe for patients receiving antidepressants. Nevertheless, prescribers should remain vigilant for this potential drug interaction,” they warn.
The findings were published online in JAMA Network Open.
Scarce data
Linezolid, a synthetic oxazolidinone antibiotic active against resistant gram-positive bacteria, has bioavailability of 100%, “making it ideal as first-line or step-down oral antibiotic therapy for bacteremia and pneumonia as well as skin and soft tissue infections,” the researchers write.
However, they note its use has been “limited because of concerns of drug interactions,” since it can reversibly inhibit monoamine oxidase (MAO).
Thus, “coadministration with antidepressants, such as nonselective MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion, may precipitate serotonin syndrome,” they write.
The investigators note that many patients who were taking antidepressants and who also needed linezolid for an infection “could not receive it because of this relative contraindication.” They add that data on the risk of serotonin syndrome associated with linezolid are “scarce” and are based largely on case reports or case series from passive surveillance.
Although a previous review of linezolid trials found “no conclusive evidence” that it increased risk for serotonin syndrome in patients taking serotonergic medication, data on patients outside of trials “are lacking.” In addition, an observational study suggested that an increased risk had a small sample size that “likely led to imprecise estimates with a wide CI and inconclusive results,” the researchers write.
Therefore, they sought to fill the knowledge gap by retrospectively analyzing data drawn from the ICES database, an independent nonprofit research institute funded by the Ontario Ministry of Health. This was done in order to “estimate the incidence of serotonin syndrome and how this risk changes because of concomitant antidepressant use in patients receiving linezolid treatment,” they write.
The study included a convenience sample of Ontario-based adults (n = 1,134, 52.5% men) who were dispensed oral linezolid 600 mg twice daily between Oct. 1, 2014, and Jan. 1, 2021. All patients were followed for 30 days.
Of these participants, 19% were also taking antidepressants. Close to half (47.9%) were taking an SSRI, 16.7% were taking an SNRI, 7% were taking a tricyclic antidepressant, and 3.3% were taking a norepinephrine and dopamine reuptake inhibitor.
Patients were divided into groups on the basis of age: 66-69 years (19.8%), 70-79 years (41.7%), and 80 years or older (38.4%).
Reassuring findings
Serotonin syndrome occurred in fewer than six patients (< .5%), although the exact numbers were not reported, owing to patient privacy concerns. However, on the basis of fewer than six events, the investigators calculated the risk difference for serotonin syndrome as ranging from −0.5% to 2.3%.
Fewer patients who were taking antidepressants experienced serotonin syndrome, compared with those who were not taking antidepressants.
The investigators estimated a propensity score for antidepressant use that incorporated several patient baseline characteristics, including age, sex, rural home address, Charlson Comorbidity Index, estimated glomerular filtration rate, history of substance use disorder, and days of use of linezolid and other serotonergic medications. They then matched patients who were not taking antidepressants with those who were taking antidepressants (n = 166 each).
The adjusted risk difference for serotonin syndrome was lower in the antidepressant group than in the no-antidepressant group (−1.2%; 95% confidence interval, −2.9% to 0.5%).
“Within this 95% CI, the worst-case scenario would be a 0.5% increase in the risk of serotonin syndrome due to antidepressants, which is equivalent to a number needed to harm of 200,” the researchers write.
For secondary outcomes, they found “similar rates” of altered mental status or confusion, hospitalization, and death within 30 days between the two propensity score–matched groups.
The investigators note that their findings have “limitations, due to the nature of retrospective observational studies.” Moreover, these types of studies are “not efficient because they often focus on a particular adverse event.”
Future research should move beyond observational studies to phase 4 studies, which would “prospectively monitor for all types of adverse events,” they write.
Still, “while waiting for higher-quality evidence, our study adds to the existing evidence for the safety of linezolid even in the context of concomitant antidepressants,” the researchers note.
“Based on the existing evidence, clinicians should be reassured that it appears safe to prescribe oral linezolid to patients taking antidepressants, especially if there are limited antibiotic options or alternative antibiotic options would be inferior,” they add.
‘Consequential relevance’
Commenting on the study, Ipsit Vahia, MD, associate chief of geriatric psychiatry and director of digital psychiatry translation at McLean Hospital, Boston, noted that although studies of drug interactions across age groups “may not accurately reflect the rates of risk for older adults,” the current study focused on linezolid use among older patients.
“One may expect higher rates of serotonin syndrome in older adults, who generally tend to be more sensitive to adverse reactions,” said Dr. Vahia, who is also director of the Technology and Aging Lab at McLean and was not involved with the current research.
“However, the study finds the risk to be low with a number needed to harm of 200,” Dr. Vahia said.
“This retrospective epidemiologic study does not shed light on why this number may be lower than expected, but it has consequential relevance in clinical practice for the management of severe infections among older adults using antidepressants,” he added.
The study was funded by a Queen’s University Research Initiation Grant. Dr. Bai and three of the four other investigators report no relevant financial relationships. Coinvestigator Mark Loeb, MD, reports having received personal fees from the Paladin Labs Advisory Committee, the International Centre for Professional Development in Health and Medicine Advisory Committee, and the Sunovion Advisory Committee outside the submitted work. Dr. Vahia serves as a consultant for Otsuka, has a research collaboration with Emerald Innovations, and receives honorarium as editor for The American Journal of Geriatric Psychiatry.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Antiepileptic drugs tied to increased Parkinson’s disease risk
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Greater handgrip strength tied to lower risk for depression
, new research suggests.
In a study of more than 115,000 adults, there was a significant association between stronger handgrip, up to 40 kg in men and 27 kg in women, and lower depression risk.
Investigators add that there was a “dose-response” association between physical strength and risk for depression.
“Being physically strong may serve as a preventive factor for depression in older adults, but this is limited to a maximum specific threshold for men and women,” Ruben Lopez-Bueno, PhD, of the department of physical medicine and nursing, University of Zaragoza, Spain, and colleagues write.
The findings were published online in the British Journal of Psychiatry.
Easy, fast, reliable
Depression is a major public health problem, and studies “aimed at examining preventive factors to tackle the increase in depression are required,” the investigators write.
They add that a “growing body of research” is examining the link between depression and muscle strength, with handgrip as an estimator, in healthy middle-aged and older adults.
Handgrip strength is an “easy-to-use, fast and reliable indicator of both sarcopenia (age-related loss of muscle mass) and dynapenia (age-related loss of muscle strength), both of which have been associated with depression,” the researchers note.
It is plausible that there is a “regulatory role of skeletal muscle on brain function affecting this condition,” they add.
They note that exercise seems to play a “key role” because it can improve muscle strength as well as muscle mass, downregulate systemic inflammation, and improve neuroplasticity, neuroendocrine, and oxidative stress responses.
Previous studies have relied either on cross-sectional or prospective cohort models and have focused mostly on a specific country, “not accounting for time-varying changes of both handgrip strength and relevant covariables.”
Moreover, previous evidence has been mixed regarding the “extent to which handgrip strength levels may associate with lower risk of depression, with study results ranging from weak to strong associations,” the investigators write.
So “higher-quality research with representative samples from different countries is required to better clarify the strength of such an association and to confirm directionality,” they add.
SHARE data
To fill this gap, the researchers turned to data from waves 1, 2, 4, 5, 6, and 7 of the Survey of Health, Ageing and Retirement in Europe (SHARE). This encompassed 115,601 individuals aged 50 years and older (mean age, 64.3 years; 54.3% women) residing in European countries and Israel (24 countries total).
Data from wave 3 were not used because handgrip measures were not used in that wave. In the other waves, a handheld dynamometer was used to measure handgrip strength.
The participants were divided into tertiles of handgrip strength, with the “first third” being the lowest tertile of strength and the “final third” representing the highest strength.
All participants were followed for a median of 7.3 years (792,459 person-years), during which 26.1% experienced a risk for depression, as reflected by scores on the EURO-D 12-item scale.
The investigators set the time scale as the months from study entry until either a first depression onset or the end of follow-up.
Covariates that the researchers accounted for included gender, age, education, country, body mass index, physical inactivity, smoking, alcohol consumption, whether living with a partner, wave of inclusion, chronic diseases, consumption of prescribed drugs, and fruit and vegetable consumption.
The researchers used two models: the first adjusted for gender and age at time of the interview, and the second adjusted for all confounders.
In the model that was adjusted only for gender and age, greater handgrip strength was associated with a significantly reduced risk for depression among participants in the second, third, and the final third in comparison with the first third (hazard ratio, 0.65; 95% confidence interval, 0.63-0.68; and HR, 0.50; 95% CI, 0.48-0.53, respectively).
The associations remained consistent in the fully adjusted model, although risk for depression was slightly attenuated in the second and final thirds compared with the first third (HR, 0.76; 95% CI, 0.71-0.81; and HR, 0.64; 95% CI, 0.59-0.69, respectively).
When the researchers conducted analyses using restricted cubic spline modeling, they found a significant association for each kilogram increase of handgrip strength and depression, up to 40 kg in men and 27 kg in women (HR, 1.39; 95% CI, 1.08-1.71; and HR, 1.28; 95% CI, 1.05-1.55, respectively).
There was no greater reduction in depression risk in those with handgrip strength above those values.
Potential depression screen
The investigators suggest several explanations for their findings. For example, handgrip strength has “been used as an overall indicator of health status, including sarcopenia,” they write.
Adults with sarcopenia have been found to be at greater risk for depression because of reduced muscle strength, since neurotrophins are produced by skeletal muscle, among other tissues, and are associated with improvement in mood.
From a psychological point of view, “being physically strong may lead to a sensation of psychological wellbeing,” the researchers write.
Moreover, being physically active “across the lifespan also promotes structural and functional changes in the brain, benefiting cognitive functioning and reducing the risk of neurodegeneration,” they write.
This can be important because aging adults with cognitive impairments can also experience neuromuscular impairments that “presumably will contribute to becoming weaker,” they note.
Overall, the findings “warrant strength training programmes aimed at older adults to reduce depression risk,” the investigators write. Clinicians “may consider using the observed handgrip strength thresholds to screen for potential depression risk in older adults,” they add.
Protective factor?
Commenting for this news organization, Julian Mutz, PhD, postdoctoral research associate at the Social, Genetic and Developmental Psychiatry Centre, King’s College, London, said the study “provides further evidence that physical strength may be a protective factor against depression in older adults.”
This confirms a “plethora of cross-sectional and longitudinal studies,” including one recently conducted by Dr. Mutz’s group.
The design of the current study “allowed the authors to address a number of key limitations of previous studies, for example, by including repeated measurements of grip strength and adjustment for potential confounding factors over time,” said Dr. Mutz, who was not involved with the research.
Additionally, “an important contribution of this study is that the authors show that higher grip strength is only associated with a lower risk of depression up to a specific threshold,” he noted.
“The clinical implication of this finding is that only individuals with grip strength below this threshold are at a higher risk of depression. These individuals especially may benefit from interventions aimed at increasing physical strength,” Dr. Mutz said.
The SHARE data collection has been funded by the European Commission and by DG Employment, Social Affairs and Inclusion. Additional funding was obtained from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, and the U.S. National Institute on Aging. Dr. Lopez-Bueno is supported by the European Union – Next Generation EU. The other investigators and Dr. Mutz have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a study of more than 115,000 adults, there was a significant association between stronger handgrip, up to 40 kg in men and 27 kg in women, and lower depression risk.
Investigators add that there was a “dose-response” association between physical strength and risk for depression.
“Being physically strong may serve as a preventive factor for depression in older adults, but this is limited to a maximum specific threshold for men and women,” Ruben Lopez-Bueno, PhD, of the department of physical medicine and nursing, University of Zaragoza, Spain, and colleagues write.
The findings were published online in the British Journal of Psychiatry.
Easy, fast, reliable
Depression is a major public health problem, and studies “aimed at examining preventive factors to tackle the increase in depression are required,” the investigators write.
They add that a “growing body of research” is examining the link between depression and muscle strength, with handgrip as an estimator, in healthy middle-aged and older adults.
Handgrip strength is an “easy-to-use, fast and reliable indicator of both sarcopenia (age-related loss of muscle mass) and dynapenia (age-related loss of muscle strength), both of which have been associated with depression,” the researchers note.
It is plausible that there is a “regulatory role of skeletal muscle on brain function affecting this condition,” they add.
They note that exercise seems to play a “key role” because it can improve muscle strength as well as muscle mass, downregulate systemic inflammation, and improve neuroplasticity, neuroendocrine, and oxidative stress responses.
Previous studies have relied either on cross-sectional or prospective cohort models and have focused mostly on a specific country, “not accounting for time-varying changes of both handgrip strength and relevant covariables.”
Moreover, previous evidence has been mixed regarding the “extent to which handgrip strength levels may associate with lower risk of depression, with study results ranging from weak to strong associations,” the investigators write.
So “higher-quality research with representative samples from different countries is required to better clarify the strength of such an association and to confirm directionality,” they add.
SHARE data
To fill this gap, the researchers turned to data from waves 1, 2, 4, 5, 6, and 7 of the Survey of Health, Ageing and Retirement in Europe (SHARE). This encompassed 115,601 individuals aged 50 years and older (mean age, 64.3 years; 54.3% women) residing in European countries and Israel (24 countries total).
Data from wave 3 were not used because handgrip measures were not used in that wave. In the other waves, a handheld dynamometer was used to measure handgrip strength.
The participants were divided into tertiles of handgrip strength, with the “first third” being the lowest tertile of strength and the “final third” representing the highest strength.
All participants were followed for a median of 7.3 years (792,459 person-years), during which 26.1% experienced a risk for depression, as reflected by scores on the EURO-D 12-item scale.
The investigators set the time scale as the months from study entry until either a first depression onset or the end of follow-up.
Covariates that the researchers accounted for included gender, age, education, country, body mass index, physical inactivity, smoking, alcohol consumption, whether living with a partner, wave of inclusion, chronic diseases, consumption of prescribed drugs, and fruit and vegetable consumption.
The researchers used two models: the first adjusted for gender and age at time of the interview, and the second adjusted for all confounders.
In the model that was adjusted only for gender and age, greater handgrip strength was associated with a significantly reduced risk for depression among participants in the second, third, and the final third in comparison with the first third (hazard ratio, 0.65; 95% confidence interval, 0.63-0.68; and HR, 0.50; 95% CI, 0.48-0.53, respectively).
The associations remained consistent in the fully adjusted model, although risk for depression was slightly attenuated in the second and final thirds compared with the first third (HR, 0.76; 95% CI, 0.71-0.81; and HR, 0.64; 95% CI, 0.59-0.69, respectively).
When the researchers conducted analyses using restricted cubic spline modeling, they found a significant association for each kilogram increase of handgrip strength and depression, up to 40 kg in men and 27 kg in women (HR, 1.39; 95% CI, 1.08-1.71; and HR, 1.28; 95% CI, 1.05-1.55, respectively).
There was no greater reduction in depression risk in those with handgrip strength above those values.
Potential depression screen
The investigators suggest several explanations for their findings. For example, handgrip strength has “been used as an overall indicator of health status, including sarcopenia,” they write.
Adults with sarcopenia have been found to be at greater risk for depression because of reduced muscle strength, since neurotrophins are produced by skeletal muscle, among other tissues, and are associated with improvement in mood.
From a psychological point of view, “being physically strong may lead to a sensation of psychological wellbeing,” the researchers write.
Moreover, being physically active “across the lifespan also promotes structural and functional changes in the brain, benefiting cognitive functioning and reducing the risk of neurodegeneration,” they write.
This can be important because aging adults with cognitive impairments can also experience neuromuscular impairments that “presumably will contribute to becoming weaker,” they note.
Overall, the findings “warrant strength training programmes aimed at older adults to reduce depression risk,” the investigators write. Clinicians “may consider using the observed handgrip strength thresholds to screen for potential depression risk in older adults,” they add.
Protective factor?
Commenting for this news organization, Julian Mutz, PhD, postdoctoral research associate at the Social, Genetic and Developmental Psychiatry Centre, King’s College, London, said the study “provides further evidence that physical strength may be a protective factor against depression in older adults.”
This confirms a “plethora of cross-sectional and longitudinal studies,” including one recently conducted by Dr. Mutz’s group.
The design of the current study “allowed the authors to address a number of key limitations of previous studies, for example, by including repeated measurements of grip strength and adjustment for potential confounding factors over time,” said Dr. Mutz, who was not involved with the research.
Additionally, “an important contribution of this study is that the authors show that higher grip strength is only associated with a lower risk of depression up to a specific threshold,” he noted.
“The clinical implication of this finding is that only individuals with grip strength below this threshold are at a higher risk of depression. These individuals especially may benefit from interventions aimed at increasing physical strength,” Dr. Mutz said.
The SHARE data collection has been funded by the European Commission and by DG Employment, Social Affairs and Inclusion. Additional funding was obtained from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, and the U.S. National Institute on Aging. Dr. Lopez-Bueno is supported by the European Union – Next Generation EU. The other investigators and Dr. Mutz have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a study of more than 115,000 adults, there was a significant association between stronger handgrip, up to 40 kg in men and 27 kg in women, and lower depression risk.
Investigators add that there was a “dose-response” association between physical strength and risk for depression.
“Being physically strong may serve as a preventive factor for depression in older adults, but this is limited to a maximum specific threshold for men and women,” Ruben Lopez-Bueno, PhD, of the department of physical medicine and nursing, University of Zaragoza, Spain, and colleagues write.
The findings were published online in the British Journal of Psychiatry.
Easy, fast, reliable
Depression is a major public health problem, and studies “aimed at examining preventive factors to tackle the increase in depression are required,” the investigators write.
They add that a “growing body of research” is examining the link between depression and muscle strength, with handgrip as an estimator, in healthy middle-aged and older adults.
Handgrip strength is an “easy-to-use, fast and reliable indicator of both sarcopenia (age-related loss of muscle mass) and dynapenia (age-related loss of muscle strength), both of which have been associated with depression,” the researchers note.
It is plausible that there is a “regulatory role of skeletal muscle on brain function affecting this condition,” they add.
They note that exercise seems to play a “key role” because it can improve muscle strength as well as muscle mass, downregulate systemic inflammation, and improve neuroplasticity, neuroendocrine, and oxidative stress responses.
Previous studies have relied either on cross-sectional or prospective cohort models and have focused mostly on a specific country, “not accounting for time-varying changes of both handgrip strength and relevant covariables.”
Moreover, previous evidence has been mixed regarding the “extent to which handgrip strength levels may associate with lower risk of depression, with study results ranging from weak to strong associations,” the investigators write.
So “higher-quality research with representative samples from different countries is required to better clarify the strength of such an association and to confirm directionality,” they add.
SHARE data
To fill this gap, the researchers turned to data from waves 1, 2, 4, 5, 6, and 7 of the Survey of Health, Ageing and Retirement in Europe (SHARE). This encompassed 115,601 individuals aged 50 years and older (mean age, 64.3 years; 54.3% women) residing in European countries and Israel (24 countries total).
Data from wave 3 were not used because handgrip measures were not used in that wave. In the other waves, a handheld dynamometer was used to measure handgrip strength.
The participants were divided into tertiles of handgrip strength, with the “first third” being the lowest tertile of strength and the “final third” representing the highest strength.
All participants were followed for a median of 7.3 years (792,459 person-years), during which 26.1% experienced a risk for depression, as reflected by scores on the EURO-D 12-item scale.
The investigators set the time scale as the months from study entry until either a first depression onset or the end of follow-up.
Covariates that the researchers accounted for included gender, age, education, country, body mass index, physical inactivity, smoking, alcohol consumption, whether living with a partner, wave of inclusion, chronic diseases, consumption of prescribed drugs, and fruit and vegetable consumption.
The researchers used two models: the first adjusted for gender and age at time of the interview, and the second adjusted for all confounders.
In the model that was adjusted only for gender and age, greater handgrip strength was associated with a significantly reduced risk for depression among participants in the second, third, and the final third in comparison with the first third (hazard ratio, 0.65; 95% confidence interval, 0.63-0.68; and HR, 0.50; 95% CI, 0.48-0.53, respectively).
The associations remained consistent in the fully adjusted model, although risk for depression was slightly attenuated in the second and final thirds compared with the first third (HR, 0.76; 95% CI, 0.71-0.81; and HR, 0.64; 95% CI, 0.59-0.69, respectively).
When the researchers conducted analyses using restricted cubic spline modeling, they found a significant association for each kilogram increase of handgrip strength and depression, up to 40 kg in men and 27 kg in women (HR, 1.39; 95% CI, 1.08-1.71; and HR, 1.28; 95% CI, 1.05-1.55, respectively).
There was no greater reduction in depression risk in those with handgrip strength above those values.
Potential depression screen
The investigators suggest several explanations for their findings. For example, handgrip strength has “been used as an overall indicator of health status, including sarcopenia,” they write.
Adults with sarcopenia have been found to be at greater risk for depression because of reduced muscle strength, since neurotrophins are produced by skeletal muscle, among other tissues, and are associated with improvement in mood.
From a psychological point of view, “being physically strong may lead to a sensation of psychological wellbeing,” the researchers write.
Moreover, being physically active “across the lifespan also promotes structural and functional changes in the brain, benefiting cognitive functioning and reducing the risk of neurodegeneration,” they write.
This can be important because aging adults with cognitive impairments can also experience neuromuscular impairments that “presumably will contribute to becoming weaker,” they note.
Overall, the findings “warrant strength training programmes aimed at older adults to reduce depression risk,” the investigators write. Clinicians “may consider using the observed handgrip strength thresholds to screen for potential depression risk in older adults,” they add.
Protective factor?
Commenting for this news organization, Julian Mutz, PhD, postdoctoral research associate at the Social, Genetic and Developmental Psychiatry Centre, King’s College, London, said the study “provides further evidence that physical strength may be a protective factor against depression in older adults.”
This confirms a “plethora of cross-sectional and longitudinal studies,” including one recently conducted by Dr. Mutz’s group.
The design of the current study “allowed the authors to address a number of key limitations of previous studies, for example, by including repeated measurements of grip strength and adjustment for potential confounding factors over time,” said Dr. Mutz, who was not involved with the research.
Additionally, “an important contribution of this study is that the authors show that higher grip strength is only associated with a lower risk of depression up to a specific threshold,” he noted.
“The clinical implication of this finding is that only individuals with grip strength below this threshold are at a higher risk of depression. These individuals especially may benefit from interventions aimed at increasing physical strength,” Dr. Mutz said.
The SHARE data collection has been funded by the European Commission and by DG Employment, Social Affairs and Inclusion. Additional funding was obtained from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, and the U.S. National Institute on Aging. Dr. Lopez-Bueno is supported by the European Union – Next Generation EU. The other investigators and Dr. Mutz have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF PSYCHIATRY