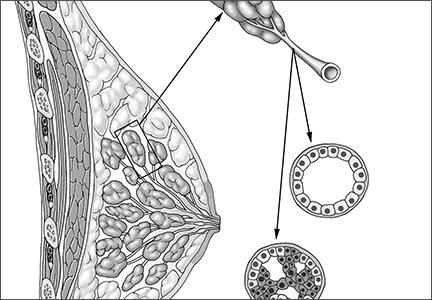
User login
When is it time to stop hormone therapy?
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
2016 Update on menopause
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
In this article
• JoAnn E. Manson discusses new data on HT benefits vs risks
• Use of compounded hormones growing
How gynecologic procedures and pharmacologic treatments can affect the uterus
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
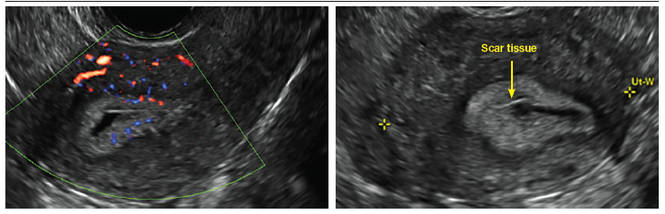
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
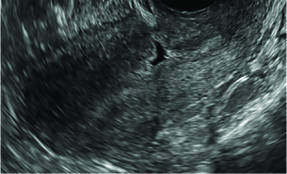
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
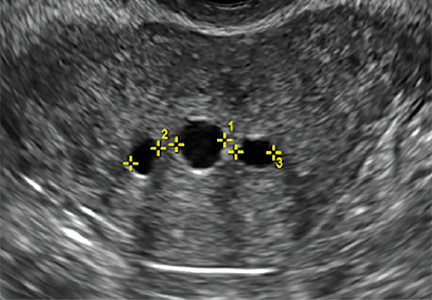
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
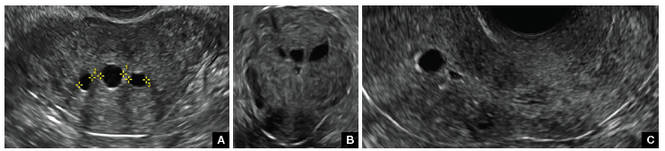
Postablation endometrial destruction
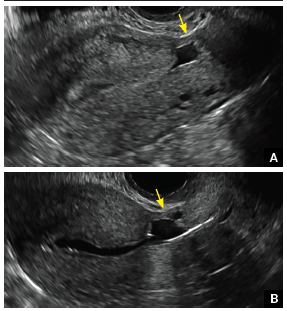
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
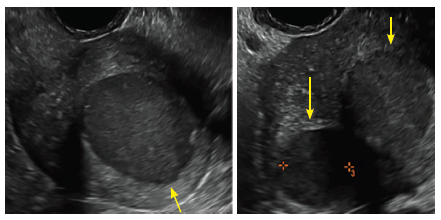
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
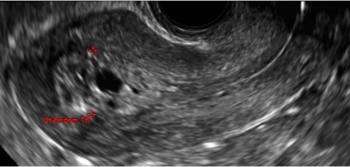 | 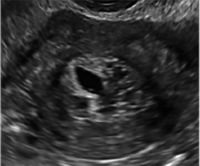 | 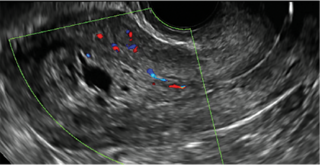 | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
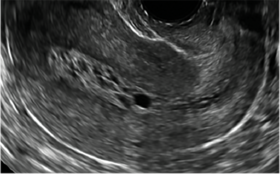 | 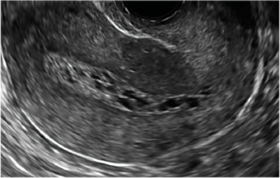 | 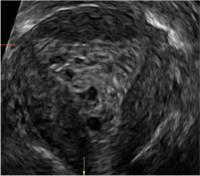 | ||
ADDITIONAL CASES - Postablation
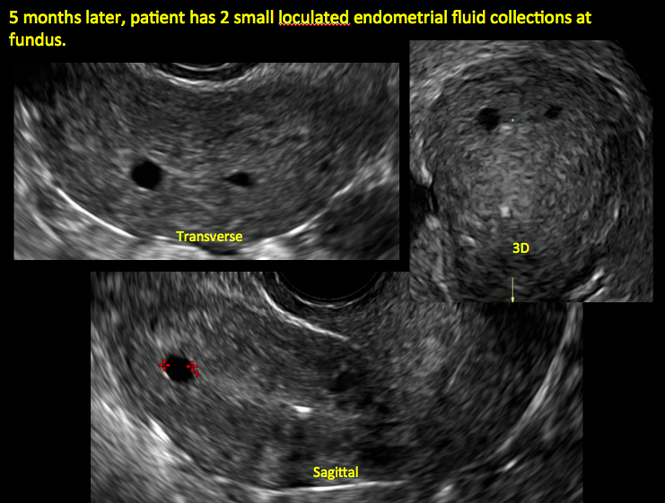
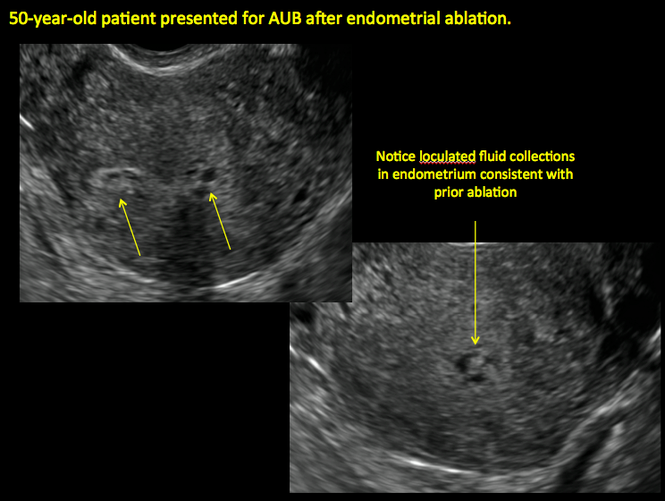
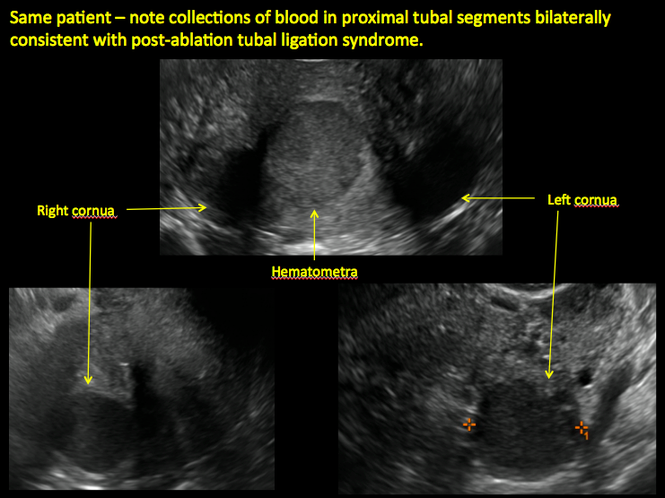
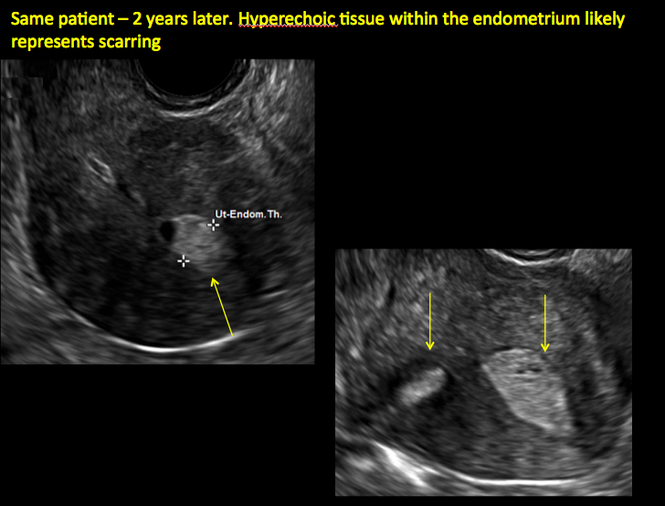
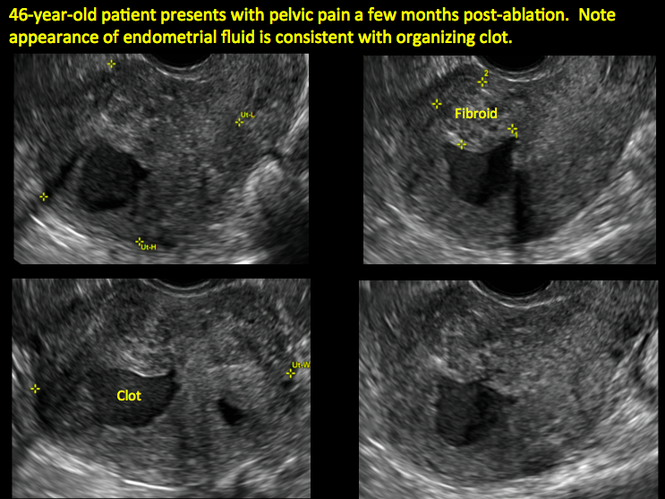
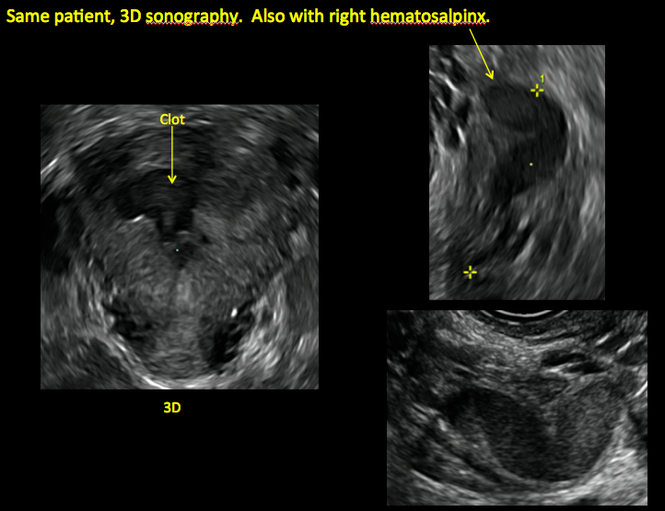
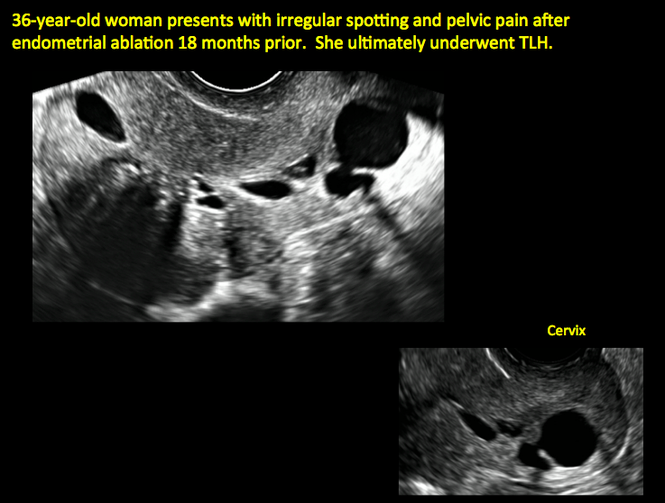

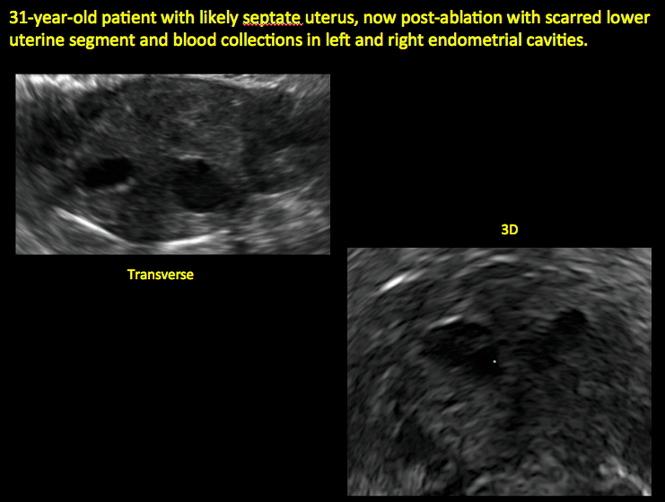
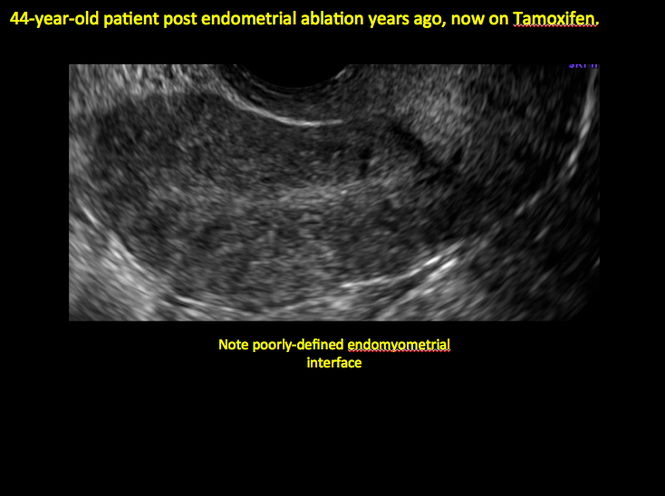
ADDITIONAL CASES - Cesarean scar defect
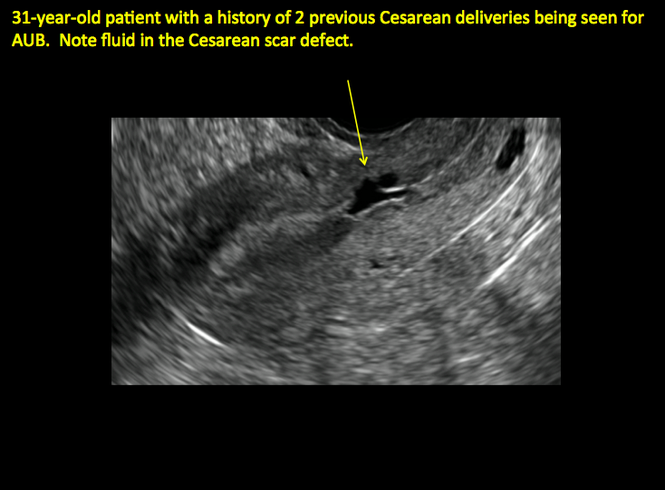
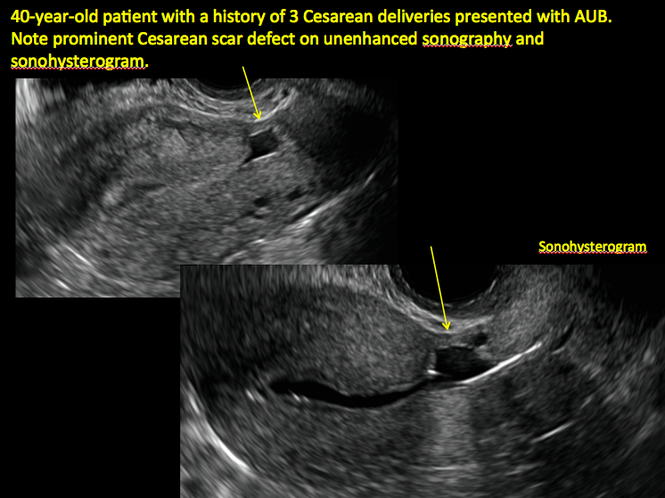
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use
Is BRCA testing causing women to undergo unnecessary prophylactic mastectomy?
Because the prevalence of BRCA1 and BRCA2 mutations is elevated among young women diagnosed with breast cancer, guidelines recommend carrier testing for women diagnosed with this disease at age 50 years or younger.1 Are women being tested, however, and what are their treatment decisions surrounding those test results? The Young Women’s Breast Cancer Study (YWBCS) seeks to answer such questions.
Details of the study
Study investigators recruited women diagnosed with breast cancer at age 40 or younger from 11 academic and community hospitals in the United States and Canada beginning in 2006. There were 897 evaluable participants who were recruited between 2006 and 2014. Their mean age at diagnosis was 35.5 years and 86.1% of them were white non-Hispanic. A respective 84.5% and 99.8% of women had at least a college education and were insured.
Overall, BRCA testing was performed within 1 year of breast cancer diagnosis in 87% of participants, with rates rising from 77% in 2006 to 95% in 2013. Among participants tested, 7.6% had a BRCA1 mutation, 4.5% had a BRCA2 mutation, 4.6% had an indeterminate result of unknown clinical significance, and 81.3% had a negative test result.
A total of 86.4% of women found to be mutation carriers proceeded with risk-reducing bilateral mastectomy; 51.2% found not to be mutation carriers had this same prophylactic surgery.
What this evidence means for practice
Although it is encouraging to see that the proportion of young women with breast cancer who are receiving counseling and genetic testing is rising, the findings from this study of highly educated, largely white and affluent women is not generalizable to all US women diagnosed with breast cancer at a young age.
That more than half of BRCA-negative women in this study chose bilateral prophylactic mastectomy, a procedure not recommended in this population, is concerning, and reflects nationwide trends.2 The increasing use of next-generation sequencing (which yields information on moderate- and low-penetrance genes in addition to BRCA status) means that women and their providers increasingly are being confronted with genetic testing results that call for formal genetics expertise. Unfortunately, genetics counselors remain in short supply and many clinicians without specific genetics training are offering these tests. As editorialists appropriately point out, these trends may further increase the number of relatively low-risk women proceeding with unwarranted bilateral mastectomy.3 In my practice, I continue to refer women whose family or personal histories indicate high-risk status to a cancer genetics counselor for formal counseling and possible testing.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Clinical Summary of USPSTF Recommendation. AHRQ Publication No. 12-05164-EF-3. http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/brcatestsumm.htm. Published December 2013. Accessed February 25, 2016.
- Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367.
- Blazer KR, Slavin T, Weitzel JN. Increased reach of genetic cancer risk assessment as a tool for precision management of hereditary breast cancer [published online ahead of print February 11, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2015.5975.
Because the prevalence of BRCA1 and BRCA2 mutations is elevated among young women diagnosed with breast cancer, guidelines recommend carrier testing for women diagnosed with this disease at age 50 years or younger.1 Are women being tested, however, and what are their treatment decisions surrounding those test results? The Young Women’s Breast Cancer Study (YWBCS) seeks to answer such questions.
Details of the study
Study investigators recruited women diagnosed with breast cancer at age 40 or younger from 11 academic and community hospitals in the United States and Canada beginning in 2006. There were 897 evaluable participants who were recruited between 2006 and 2014. Their mean age at diagnosis was 35.5 years and 86.1% of them were white non-Hispanic. A respective 84.5% and 99.8% of women had at least a college education and were insured.
Overall, BRCA testing was performed within 1 year of breast cancer diagnosis in 87% of participants, with rates rising from 77% in 2006 to 95% in 2013. Among participants tested, 7.6% had a BRCA1 mutation, 4.5% had a BRCA2 mutation, 4.6% had an indeterminate result of unknown clinical significance, and 81.3% had a negative test result.
A total of 86.4% of women found to be mutation carriers proceeded with risk-reducing bilateral mastectomy; 51.2% found not to be mutation carriers had this same prophylactic surgery.
What this evidence means for practice
Although it is encouraging to see that the proportion of young women with breast cancer who are receiving counseling and genetic testing is rising, the findings from this study of highly educated, largely white and affluent women is not generalizable to all US women diagnosed with breast cancer at a young age.
That more than half of BRCA-negative women in this study chose bilateral prophylactic mastectomy, a procedure not recommended in this population, is concerning, and reflects nationwide trends.2 The increasing use of next-generation sequencing (which yields information on moderate- and low-penetrance genes in addition to BRCA status) means that women and their providers increasingly are being confronted with genetic testing results that call for formal genetics expertise. Unfortunately, genetics counselors remain in short supply and many clinicians without specific genetics training are offering these tests. As editorialists appropriately point out, these trends may further increase the number of relatively low-risk women proceeding with unwarranted bilateral mastectomy.3 In my practice, I continue to refer women whose family or personal histories indicate high-risk status to a cancer genetics counselor for formal counseling and possible testing.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Because the prevalence of BRCA1 and BRCA2 mutations is elevated among young women diagnosed with breast cancer, guidelines recommend carrier testing for women diagnosed with this disease at age 50 years or younger.1 Are women being tested, however, and what are their treatment decisions surrounding those test results? The Young Women’s Breast Cancer Study (YWBCS) seeks to answer such questions.
Details of the study
Study investigators recruited women diagnosed with breast cancer at age 40 or younger from 11 academic and community hospitals in the United States and Canada beginning in 2006. There were 897 evaluable participants who were recruited between 2006 and 2014. Their mean age at diagnosis was 35.5 years and 86.1% of them were white non-Hispanic. A respective 84.5% and 99.8% of women had at least a college education and were insured.
Overall, BRCA testing was performed within 1 year of breast cancer diagnosis in 87% of participants, with rates rising from 77% in 2006 to 95% in 2013. Among participants tested, 7.6% had a BRCA1 mutation, 4.5% had a BRCA2 mutation, 4.6% had an indeterminate result of unknown clinical significance, and 81.3% had a negative test result.
A total of 86.4% of women found to be mutation carriers proceeded with risk-reducing bilateral mastectomy; 51.2% found not to be mutation carriers had this same prophylactic surgery.
What this evidence means for practice
Although it is encouraging to see that the proportion of young women with breast cancer who are receiving counseling and genetic testing is rising, the findings from this study of highly educated, largely white and affluent women is not generalizable to all US women diagnosed with breast cancer at a young age.
That more than half of BRCA-negative women in this study chose bilateral prophylactic mastectomy, a procedure not recommended in this population, is concerning, and reflects nationwide trends.2 The increasing use of next-generation sequencing (which yields information on moderate- and low-penetrance genes in addition to BRCA status) means that women and their providers increasingly are being confronted with genetic testing results that call for formal genetics expertise. Unfortunately, genetics counselors remain in short supply and many clinicians without specific genetics training are offering these tests. As editorialists appropriately point out, these trends may further increase the number of relatively low-risk women proceeding with unwarranted bilateral mastectomy.3 In my practice, I continue to refer women whose family or personal histories indicate high-risk status to a cancer genetics counselor for formal counseling and possible testing.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Clinical Summary of USPSTF Recommendation. AHRQ Publication No. 12-05164-EF-3. http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/brcatestsumm.htm. Published December 2013. Accessed February 25, 2016.
- Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367.
- Blazer KR, Slavin T, Weitzel JN. Increased reach of genetic cancer risk assessment as a tool for precision management of hereditary breast cancer [published online ahead of print February 11, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2015.5975.
- U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Clinical Summary of USPSTF Recommendation. AHRQ Publication No. 12-05164-EF-3. http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/brcatestsumm.htm. Published December 2013. Accessed February 25, 2016.
- Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367.
- Blazer KR, Slavin T, Weitzel JN. Increased reach of genetic cancer risk assessment as a tool for precision management of hereditary breast cancer [published online ahead of print February 11, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2015.5975.
Can CA 125 screening reduce mortality from ovarian cancer?
To date, screening has not been found effective in reducing mortality from ovarian cancer. Collaborative trial investigators in the United Kingdom studied postmenopausal women in the general population to assess whether early detection by screening could decrease ovarian cancer mortality.
Details of the study
During 2001 to 2005, more than 200,000 UK postmenopausal women aged 50 to 74 years (mean age at baseline, 60.6 years) were randomly assigned to no screening, annual transvaginal ultrasound screening (TVUS), or annual multimodal screening (MMS) with serum CA 125 using the Risk of Ovarian Cancer Algorithm (ROCA), which takes into account changes in CA 125 levels over time. When ROCA scores indicated normal risk for ovarian cancer, women were advised to undergo repeat CA 125 assessment in 1 year. Women with intermediate risk were advised to repeat CA 125 assessment in 3 months, while high-risk women were advised to undergo TVUS.
With a median of 11.1 years of follow-up, ovarian cancer (including fallopian tube malignancies) was diagnosed in 1,282 participants (0.6%), with fatal outcomes among the 3 groups as follows: 0.34% in the no-screening group, 0.30% in the TVUS group, and 0.29% in the MMS group. Based on the results of a planned secondary analysis that excluded prevalent cases of ovarian cancer, annual MMS was associated with an overall average mortality reduction of 20% compared with no screening (P = .021). When the mortality reduction was broken down by years of annual screening, 0 to 7 years was associated with an 8% mortality reduction over no screening, and this jumped to 28% for 7 to 14 annual MMS screening years.
The overall average mortality reduction with TVUS compared with no screening was smaller than with MMS. With MMS, the number needed to screen to prevent 1 death from ovarian cancer was 641.
Assessing unnecessary treatment
False-positive screens that resulted in surgical intervention with findings of benign adnexal pathology or normal adnexa occurred in 14 and 50 per 10,000 screens in the MMS and TVUS groups, respectively. For each ovarian cancer detected in the MMS and TVUS groups, an additional 2 and 10 women, respectively, underwent surgery based on false-positive results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This massive trial’s findings provide optimism that screening for ovarian cancer can indeed reduce mortality from this uncommon but too-often lethal disease. There are unanswered questions, however, which include the cost-effectiveness of MMS screening and how well this strategy can be implemented outside of a highly centralized and controlled clinical trial. While encouraging, these trial results should be viewed as preliminary until additional efficacy and cost-effectiveness data—and guidance from professional organizations—are available.
—ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
To date, screening has not been found effective in reducing mortality from ovarian cancer. Collaborative trial investigators in the United Kingdom studied postmenopausal women in the general population to assess whether early detection by screening could decrease ovarian cancer mortality.
Details of the study
During 2001 to 2005, more than 200,000 UK postmenopausal women aged 50 to 74 years (mean age at baseline, 60.6 years) were randomly assigned to no screening, annual transvaginal ultrasound screening (TVUS), or annual multimodal screening (MMS) with serum CA 125 using the Risk of Ovarian Cancer Algorithm (ROCA), which takes into account changes in CA 125 levels over time. When ROCA scores indicated normal risk for ovarian cancer, women were advised to undergo repeat CA 125 assessment in 1 year. Women with intermediate risk were advised to repeat CA 125 assessment in 3 months, while high-risk women were advised to undergo TVUS.
With a median of 11.1 years of follow-up, ovarian cancer (including fallopian tube malignancies) was diagnosed in 1,282 participants (0.6%), with fatal outcomes among the 3 groups as follows: 0.34% in the no-screening group, 0.30% in the TVUS group, and 0.29% in the MMS group. Based on the results of a planned secondary analysis that excluded prevalent cases of ovarian cancer, annual MMS was associated with an overall average mortality reduction of 20% compared with no screening (P = .021). When the mortality reduction was broken down by years of annual screening, 0 to 7 years was associated with an 8% mortality reduction over no screening, and this jumped to 28% for 7 to 14 annual MMS screening years.
The overall average mortality reduction with TVUS compared with no screening was smaller than with MMS. With MMS, the number needed to screen to prevent 1 death from ovarian cancer was 641.
Assessing unnecessary treatment
False-positive screens that resulted in surgical intervention with findings of benign adnexal pathology or normal adnexa occurred in 14 and 50 per 10,000 screens in the MMS and TVUS groups, respectively. For each ovarian cancer detected in the MMS and TVUS groups, an additional 2 and 10 women, respectively, underwent surgery based on false-positive results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This massive trial’s findings provide optimism that screening for ovarian cancer can indeed reduce mortality from this uncommon but too-often lethal disease. There are unanswered questions, however, which include the cost-effectiveness of MMS screening and how well this strategy can be implemented outside of a highly centralized and controlled clinical trial. While encouraging, these trial results should be viewed as preliminary until additional efficacy and cost-effectiveness data—and guidance from professional organizations—are available.
—ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
To date, screening has not been found effective in reducing mortality from ovarian cancer. Collaborative trial investigators in the United Kingdom studied postmenopausal women in the general population to assess whether early detection by screening could decrease ovarian cancer mortality.
Details of the study
During 2001 to 2005, more than 200,000 UK postmenopausal women aged 50 to 74 years (mean age at baseline, 60.6 years) were randomly assigned to no screening, annual transvaginal ultrasound screening (TVUS), or annual multimodal screening (MMS) with serum CA 125 using the Risk of Ovarian Cancer Algorithm (ROCA), which takes into account changes in CA 125 levels over time. When ROCA scores indicated normal risk for ovarian cancer, women were advised to undergo repeat CA 125 assessment in 1 year. Women with intermediate risk were advised to repeat CA 125 assessment in 3 months, while high-risk women were advised to undergo TVUS.
With a median of 11.1 years of follow-up, ovarian cancer (including fallopian tube malignancies) was diagnosed in 1,282 participants (0.6%), with fatal outcomes among the 3 groups as follows: 0.34% in the no-screening group, 0.30% in the TVUS group, and 0.29% in the MMS group. Based on the results of a planned secondary analysis that excluded prevalent cases of ovarian cancer, annual MMS was associated with an overall average mortality reduction of 20% compared with no screening (P = .021). When the mortality reduction was broken down by years of annual screening, 0 to 7 years was associated with an 8% mortality reduction over no screening, and this jumped to 28% for 7 to 14 annual MMS screening years.
The overall average mortality reduction with TVUS compared with no screening was smaller than with MMS. With MMS, the number needed to screen to prevent 1 death from ovarian cancer was 641.
Assessing unnecessary treatment
False-positive screens that resulted in surgical intervention with findings of benign adnexal pathology or normal adnexa occurred in 14 and 50 per 10,000 screens in the MMS and TVUS groups, respectively. For each ovarian cancer detected in the MMS and TVUS groups, an additional 2 and 10 women, respectively, underwent surgery based on false-positive results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This massive trial’s findings provide optimism that screening for ovarian cancer can indeed reduce mortality from this uncommon but too-often lethal disease. There are unanswered questions, however, which include the cost-effectiveness of MMS screening and how well this strategy can be implemented outside of a highly centralized and controlled clinical trial. While encouraging, these trial results should be viewed as preliminary until additional efficacy and cost-effectiveness data—and guidance from professional organizations—are available.
—ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
No surprises from the USPSTF with new guidance on screening mammography
In 2009, the US Preventive Services Task Force (USPSTF) recommended that biennial mammography screening in average-risk women begin at age 50.1 New guidelines, that take into account reviews and modeling studies, clarify the earlier USPSTF recommendations, paying particular attention to individualized screening for women aged 40 to 49, use of tomosynthesis, and supplemental evaluation for women with radiologically dense breasts.
The new guidance only applies to women at average risk for breast cancer (not to those at substantially higher-than-average risk), including those with prior breast cancer or biopsy-confirmed high-risk lesions (eg, atypical hyperplasia), certain genetic conditions (such as BRCA1 or BRCA2 mutation), or histories of chest irradiation (eg, Hodgkin lymphoma).
Major statements:
- Biennial screening is recommended for women aged 50 to 74 (B recommendation; definitions of USPSTF grades are available online at ).
- Initiation of screening before age 50 should be individualized depending on patient preferences (C recommendation).
- For women aged ≥75, current evidence is insufficient to assess benefits and harms of screening (I statement).
- Current evidence is insufficient to assess the benefits and harms of adding tomosynthesis to conventional screening mammography (I statement).
- For women with radiologically dense breasts, current evidence is insufficient to assess the benefits and harms of adjunctive ultrasound, magnetic resonance imaging (MRI), or tomosynthesis (I statement).2
The Task Force generated controversy with its 2009 recommendation that screening begin at age 50 in average-risk women. The current guidance clarifies that repetitive screening of women through 10 years reduces breast cancer deaths by 4 (aged 40–49), 8 (aged 50–59), and 21 (aged 60–69) per 10,000 women, respectively.2
The term “overdiagnosis” refers to detection and treatment of invasive and noninvasive (usually ductal carcinoma in situ) lesions that would have gone undetected without screening and would not have caused health problems. The USPSTF acknowledges that, while overdiagnosis represents the principal harm from screening, estimating overdiagnosis rates is challenging (best estimates range from 1 in 5 to 1 in 8 breast cancers diagnosed in screened women).2–4 False-positive results, which lead to unnecessary additional imaging and biopsies,3,4 can represent an additional harm of screening mammography.
The rationale for recommending that average-risk women begin screening at age 50 is based on the relatively smaller benefits and greater harms incurred when younger women are screened;3,4 however, in noting that most of the screening benefits for women in their 40s are realized starting at age 45, the USPSTF guidance opens the door to average-risk women to begin screening at that age (congruent with the November 2015 American Cancer Society recommendations5). Also, women with a first-degree relative with breast cancer may want to initiate screening at age 40.
Regarding screening frequency, annual screening generates minimal if any benefit while increasing the potential for harm3,4; thus, for most women at average risk for breast cancer, biennial screening provides the best benefit–harm balance.
What about use of tomosynthesis and women with dense breasts?
Tomosynthesis, which can be performed along with conventional digital screening mammography, seems to diminish the need for follow-up imaging while also increasing cancer detection rates.6 However, whether these additional cancers represent overdiagnosis remains unknown. Furthermore, tomosynthesis can expose women to about twice the radiation as conventional digital screening.7
Twenty-four states currently mandate that patients with dense breasts identified at screening be notified. Although increased breast density is a common independent risk factor for breast cancer, the degree of radiographic density can vary substantially from one screen to the next in the same woman. Evidence for or against adjunctive imaging is very limited in women found to have dense breasts in an otherwise negative mammogram, and suggests that ultrasonography and MRI (as well as tomosynthesis) can detect additional breast cancers while also generating more false-positive results.8 Thus, the USPSTF does not recommend specific screening strategies for women with dense breasts.
How I counsel my patients
I plan to continue recommending screening based on USPSTF guidance. However, I also will continue to support the preferences of many of my patients to:
- initiate screening before age 50
- undergo screening annually
- continue screening after age 74.
You and your patients alike may find the USPSTF’s Summary for Patients9 (http://annals.org/article.aspx?articleid=2480981&resultClick=3) to be helpful when navigating this territory.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716−726.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement [published online ahead of print January 12, 2016]. Ann Intern Med. doi:10.7326/M15-2886.
- Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0970.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1536.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599−1614.
- Nelson HD, OMeara ES, Kerlikowski K, Balch S, Miglioretti D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0971.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1241.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-1789.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force Recommendation Statement (Summary for Patients). Ann Intern Med. 2016:164:279–296. http://annals.org/article.aspx?articleid=2480981&resultClick=3. Published January 12, 2016. Accessed January 25, 2016.
In 2009, the US Preventive Services Task Force (USPSTF) recommended that biennial mammography screening in average-risk women begin at age 50.1 New guidelines, that take into account reviews and modeling studies, clarify the earlier USPSTF recommendations, paying particular attention to individualized screening for women aged 40 to 49, use of tomosynthesis, and supplemental evaluation for women with radiologically dense breasts.
The new guidance only applies to women at average risk for breast cancer (not to those at substantially higher-than-average risk), including those with prior breast cancer or biopsy-confirmed high-risk lesions (eg, atypical hyperplasia), certain genetic conditions (such as BRCA1 or BRCA2 mutation), or histories of chest irradiation (eg, Hodgkin lymphoma).
Major statements:
- Biennial screening is recommended for women aged 50 to 74 (B recommendation; definitions of USPSTF grades are available online at ).
- Initiation of screening before age 50 should be individualized depending on patient preferences (C recommendation).
- For women aged ≥75, current evidence is insufficient to assess benefits and harms of screening (I statement).
- Current evidence is insufficient to assess the benefits and harms of adding tomosynthesis to conventional screening mammography (I statement).
- For women with radiologically dense breasts, current evidence is insufficient to assess the benefits and harms of adjunctive ultrasound, magnetic resonance imaging (MRI), or tomosynthesis (I statement).2
The Task Force generated controversy with its 2009 recommendation that screening begin at age 50 in average-risk women. The current guidance clarifies that repetitive screening of women through 10 years reduces breast cancer deaths by 4 (aged 40–49), 8 (aged 50–59), and 21 (aged 60–69) per 10,000 women, respectively.2
The term “overdiagnosis” refers to detection and treatment of invasive and noninvasive (usually ductal carcinoma in situ) lesions that would have gone undetected without screening and would not have caused health problems. The USPSTF acknowledges that, while overdiagnosis represents the principal harm from screening, estimating overdiagnosis rates is challenging (best estimates range from 1 in 5 to 1 in 8 breast cancers diagnosed in screened women).2–4 False-positive results, which lead to unnecessary additional imaging and biopsies,3,4 can represent an additional harm of screening mammography.
The rationale for recommending that average-risk women begin screening at age 50 is based on the relatively smaller benefits and greater harms incurred when younger women are screened;3,4 however, in noting that most of the screening benefits for women in their 40s are realized starting at age 45, the USPSTF guidance opens the door to average-risk women to begin screening at that age (congruent with the November 2015 American Cancer Society recommendations5). Also, women with a first-degree relative with breast cancer may want to initiate screening at age 40.
Regarding screening frequency, annual screening generates minimal if any benefit while increasing the potential for harm3,4; thus, for most women at average risk for breast cancer, biennial screening provides the best benefit–harm balance.
What about use of tomosynthesis and women with dense breasts?
Tomosynthesis, which can be performed along with conventional digital screening mammography, seems to diminish the need for follow-up imaging while also increasing cancer detection rates.6 However, whether these additional cancers represent overdiagnosis remains unknown. Furthermore, tomosynthesis can expose women to about twice the radiation as conventional digital screening.7
Twenty-four states currently mandate that patients with dense breasts identified at screening be notified. Although increased breast density is a common independent risk factor for breast cancer, the degree of radiographic density can vary substantially from one screen to the next in the same woman. Evidence for or against adjunctive imaging is very limited in women found to have dense breasts in an otherwise negative mammogram, and suggests that ultrasonography and MRI (as well as tomosynthesis) can detect additional breast cancers while also generating more false-positive results.8 Thus, the USPSTF does not recommend specific screening strategies for women with dense breasts.
How I counsel my patients
I plan to continue recommending screening based on USPSTF guidance. However, I also will continue to support the preferences of many of my patients to:
- initiate screening before age 50
- undergo screening annually
- continue screening after age 74.
You and your patients alike may find the USPSTF’s Summary for Patients9 (http://annals.org/article.aspx?articleid=2480981&resultClick=3) to be helpful when navigating this territory.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In 2009, the US Preventive Services Task Force (USPSTF) recommended that biennial mammography screening in average-risk women begin at age 50.1 New guidelines, that take into account reviews and modeling studies, clarify the earlier USPSTF recommendations, paying particular attention to individualized screening for women aged 40 to 49, use of tomosynthesis, and supplemental evaluation for women with radiologically dense breasts.
The new guidance only applies to women at average risk for breast cancer (not to those at substantially higher-than-average risk), including those with prior breast cancer or biopsy-confirmed high-risk lesions (eg, atypical hyperplasia), certain genetic conditions (such as BRCA1 or BRCA2 mutation), or histories of chest irradiation (eg, Hodgkin lymphoma).
Major statements:
- Biennial screening is recommended for women aged 50 to 74 (B recommendation; definitions of USPSTF grades are available online at ).
- Initiation of screening before age 50 should be individualized depending on patient preferences (C recommendation).
- For women aged ≥75, current evidence is insufficient to assess benefits and harms of screening (I statement).
- Current evidence is insufficient to assess the benefits and harms of adding tomosynthesis to conventional screening mammography (I statement).
- For women with radiologically dense breasts, current evidence is insufficient to assess the benefits and harms of adjunctive ultrasound, magnetic resonance imaging (MRI), or tomosynthesis (I statement).2
The Task Force generated controversy with its 2009 recommendation that screening begin at age 50 in average-risk women. The current guidance clarifies that repetitive screening of women through 10 years reduces breast cancer deaths by 4 (aged 40–49), 8 (aged 50–59), and 21 (aged 60–69) per 10,000 women, respectively.2
The term “overdiagnosis” refers to detection and treatment of invasive and noninvasive (usually ductal carcinoma in situ) lesions that would have gone undetected without screening and would not have caused health problems. The USPSTF acknowledges that, while overdiagnosis represents the principal harm from screening, estimating overdiagnosis rates is challenging (best estimates range from 1 in 5 to 1 in 8 breast cancers diagnosed in screened women).2–4 False-positive results, which lead to unnecessary additional imaging and biopsies,3,4 can represent an additional harm of screening mammography.
The rationale for recommending that average-risk women begin screening at age 50 is based on the relatively smaller benefits and greater harms incurred when younger women are screened;3,4 however, in noting that most of the screening benefits for women in their 40s are realized starting at age 45, the USPSTF guidance opens the door to average-risk women to begin screening at that age (congruent with the November 2015 American Cancer Society recommendations5). Also, women with a first-degree relative with breast cancer may want to initiate screening at age 40.
Regarding screening frequency, annual screening generates minimal if any benefit while increasing the potential for harm3,4; thus, for most women at average risk for breast cancer, biennial screening provides the best benefit–harm balance.
What about use of tomosynthesis and women with dense breasts?
Tomosynthesis, which can be performed along with conventional digital screening mammography, seems to diminish the need for follow-up imaging while also increasing cancer detection rates.6 However, whether these additional cancers represent overdiagnosis remains unknown. Furthermore, tomosynthesis can expose women to about twice the radiation as conventional digital screening.7
Twenty-four states currently mandate that patients with dense breasts identified at screening be notified. Although increased breast density is a common independent risk factor for breast cancer, the degree of radiographic density can vary substantially from one screen to the next in the same woman. Evidence for or against adjunctive imaging is very limited in women found to have dense breasts in an otherwise negative mammogram, and suggests that ultrasonography and MRI (as well as tomosynthesis) can detect additional breast cancers while also generating more false-positive results.8 Thus, the USPSTF does not recommend specific screening strategies for women with dense breasts.
How I counsel my patients
I plan to continue recommending screening based on USPSTF guidance. However, I also will continue to support the preferences of many of my patients to:
- initiate screening before age 50
- undergo screening annually
- continue screening after age 74.
You and your patients alike may find the USPSTF’s Summary for Patients9 (http://annals.org/article.aspx?articleid=2480981&resultClick=3) to be helpful when navigating this territory.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716−726.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement [published online ahead of print January 12, 2016]. Ann Intern Med. doi:10.7326/M15-2886.
- Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0970.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1536.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599−1614.
- Nelson HD, OMeara ES, Kerlikowski K, Balch S, Miglioretti D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0971.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1241.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-1789.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force Recommendation Statement (Summary for Patients). Ann Intern Med. 2016:164:279–296. http://annals.org/article.aspx?articleid=2480981&resultClick=3. Published January 12, 2016. Accessed January 25, 2016.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716−726.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement [published online ahead of print January 12, 2016]. Ann Intern Med. doi:10.7326/M15-2886.
- Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0970.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1536.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599−1614.
- Nelson HD, OMeara ES, Kerlikowski K, Balch S, Miglioretti D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-0971.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326 /M15-1241.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force [published online ahead of print January 12, 2016]. Ann Intern Med. doi: 10.7326/M15-1789.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force Recommendation Statement (Summary for Patients). Ann Intern Med. 2016:164:279–296. http://annals.org/article.aspx?articleid=2480981&resultClick=3. Published January 12, 2016. Accessed January 25, 2016.
Does the Mediterranean diet reduce the risk of breast cancer?
The Mediterranean diet, characterized by an emphasis on plant foods, fish, and olive oil, is known to have cardiovascular benefits. This study by Toledo and colleagues is a secondary analysis of a large randomized trial that assessed the impact of the Mediterranean diet versus a recommended low-fat diet in patients at elevated risk for cardiovascular disease (CVD). The larger trial was stopped after 4.8 years of follow-up, when findings of early cardiovascular benefit became evident.
The 4,282 women in the trial, all of whom were white, were randomly allocated to one of the following groups:
- Mediterranean diet supplemented by EVOO. Participants were given 1 L of EVOO a week for the study duration. At baseline and quarterly thereafter, dieticians ran individual and group sessions. In individual sessions, participants completed a 14-item dietary screening questionnaire to assess adherence to the diet.
- Mediterranean diet supplemented by mixed nuts. Participants were given 30 g of mixed nuts per day (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds). They also took part in individual and group sessions at baseline and quarterly thereafter, and completed the same screening questionnaire as the first group.
- A control group. Participants were advised to reduce dietary fat. They also underwent dietary training at the baseline visit and completed the 14-item screener. Thereafter, during the first 3 years of the trial, they received annual mailing of a leaflet explaining the low-fat diet. In 2006, however, the protocol was amended to include personalized advice and quarterly group sessions, with use of a separate 9-item dietary screener. The control group also received gifts of nonfood items as incentives.
Physical activity was not promoted in any group.
Findings of the trialAfter a median follow-up of 4.8 years, 35 confirmed cases of invasive breast cancer occurred among participants in the trial, who ranged in age from 60 to 80 years. The observed rate (per 1,000 person-years) of breast cancer was 1.1 for women following the Mediterranean diet supplemented with EVOO, 1.8 for women on the diet supplemented by mixed nuts, and 2.9 for the control group.
Women allocated to the Mediterranean diet with EVOO had a 62% reduced risk of invasive breast cancer (95% confidence interval [CI], 0.16–0.87). Women allocated to the same diet with mixed nuts had a 38% reduced risk of breast cancer, but this finding was not statistically significant.
Note that women in the control group failed to reduce their total fat intake substantially, even though they were advised to do so, although saturated fat intake remained below 10%.
Strengths and limitations of the trialAlthough the incidence of breast cancer is lower in Mediterranean countries, this is the first randomized trial to assess the impact of the Mediterranean diet on risk of this disease.
Because breast cancer was not the primary outcome, investigators were unable to verify if or when participants underwent mammography screening. However, randomization resulted in large groups of participants between whom mammographic status likely was comparable.
The lack of ethnic diversity represents another limitation.
Toledo and colleagues describe differences between olive oils in general and EVOO, including biologic mechanisms that might result in breast cancer prophylaxis.
WHAT THIS EVIDENCE MEANS FOR PRACTICEGiven the known cardiovascular benefits, it is reasonable to suggest to our menopausal patients that a Mediterranean diet with EVOO may reduce their risk of breast cancer.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The Mediterranean diet, characterized by an emphasis on plant foods, fish, and olive oil, is known to have cardiovascular benefits. This study by Toledo and colleagues is a secondary analysis of a large randomized trial that assessed the impact of the Mediterranean diet versus a recommended low-fat diet in patients at elevated risk for cardiovascular disease (CVD). The larger trial was stopped after 4.8 years of follow-up, when findings of early cardiovascular benefit became evident.
The 4,282 women in the trial, all of whom were white, were randomly allocated to one of the following groups:
- Mediterranean diet supplemented by EVOO. Participants were given 1 L of EVOO a week for the study duration. At baseline and quarterly thereafter, dieticians ran individual and group sessions. In individual sessions, participants completed a 14-item dietary screening questionnaire to assess adherence to the diet.
- Mediterranean diet supplemented by mixed nuts. Participants were given 30 g of mixed nuts per day (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds). They also took part in individual and group sessions at baseline and quarterly thereafter, and completed the same screening questionnaire as the first group.
- A control group. Participants were advised to reduce dietary fat. They also underwent dietary training at the baseline visit and completed the 14-item screener. Thereafter, during the first 3 years of the trial, they received annual mailing of a leaflet explaining the low-fat diet. In 2006, however, the protocol was amended to include personalized advice and quarterly group sessions, with use of a separate 9-item dietary screener. The control group also received gifts of nonfood items as incentives.
Physical activity was not promoted in any group.
Findings of the trialAfter a median follow-up of 4.8 years, 35 confirmed cases of invasive breast cancer occurred among participants in the trial, who ranged in age from 60 to 80 years. The observed rate (per 1,000 person-years) of breast cancer was 1.1 for women following the Mediterranean diet supplemented with EVOO, 1.8 for women on the diet supplemented by mixed nuts, and 2.9 for the control group.
Women allocated to the Mediterranean diet with EVOO had a 62% reduced risk of invasive breast cancer (95% confidence interval [CI], 0.16–0.87). Women allocated to the same diet with mixed nuts had a 38% reduced risk of breast cancer, but this finding was not statistically significant.
Note that women in the control group failed to reduce their total fat intake substantially, even though they were advised to do so, although saturated fat intake remained below 10%.
Strengths and limitations of the trialAlthough the incidence of breast cancer is lower in Mediterranean countries, this is the first randomized trial to assess the impact of the Mediterranean diet on risk of this disease.
Because breast cancer was not the primary outcome, investigators were unable to verify if or when participants underwent mammography screening. However, randomization resulted in large groups of participants between whom mammographic status likely was comparable.
The lack of ethnic diversity represents another limitation.
Toledo and colleagues describe differences between olive oils in general and EVOO, including biologic mechanisms that might result in breast cancer prophylaxis.
WHAT THIS EVIDENCE MEANS FOR PRACTICEGiven the known cardiovascular benefits, it is reasonable to suggest to our menopausal patients that a Mediterranean diet with EVOO may reduce their risk of breast cancer.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The Mediterranean diet, characterized by an emphasis on plant foods, fish, and olive oil, is known to have cardiovascular benefits. This study by Toledo and colleagues is a secondary analysis of a large randomized trial that assessed the impact of the Mediterranean diet versus a recommended low-fat diet in patients at elevated risk for cardiovascular disease (CVD). The larger trial was stopped after 4.8 years of follow-up, when findings of early cardiovascular benefit became evident.
The 4,282 women in the trial, all of whom were white, were randomly allocated to one of the following groups:
- Mediterranean diet supplemented by EVOO. Participants were given 1 L of EVOO a week for the study duration. At baseline and quarterly thereafter, dieticians ran individual and group sessions. In individual sessions, participants completed a 14-item dietary screening questionnaire to assess adherence to the diet.
- Mediterranean diet supplemented by mixed nuts. Participants were given 30 g of mixed nuts per day (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds). They also took part in individual and group sessions at baseline and quarterly thereafter, and completed the same screening questionnaire as the first group.
- A control group. Participants were advised to reduce dietary fat. They also underwent dietary training at the baseline visit and completed the 14-item screener. Thereafter, during the first 3 years of the trial, they received annual mailing of a leaflet explaining the low-fat diet. In 2006, however, the protocol was amended to include personalized advice and quarterly group sessions, with use of a separate 9-item dietary screener. The control group also received gifts of nonfood items as incentives.
Physical activity was not promoted in any group.
Findings of the trialAfter a median follow-up of 4.8 years, 35 confirmed cases of invasive breast cancer occurred among participants in the trial, who ranged in age from 60 to 80 years. The observed rate (per 1,000 person-years) of breast cancer was 1.1 for women following the Mediterranean diet supplemented with EVOO, 1.8 for women on the diet supplemented by mixed nuts, and 2.9 for the control group.
Women allocated to the Mediterranean diet with EVOO had a 62% reduced risk of invasive breast cancer (95% confidence interval [CI], 0.16–0.87). Women allocated to the same diet with mixed nuts had a 38% reduced risk of breast cancer, but this finding was not statistically significant.
Note that women in the control group failed to reduce their total fat intake substantially, even though they were advised to do so, although saturated fat intake remained below 10%.
Strengths and limitations of the trialAlthough the incidence of breast cancer is lower in Mediterranean countries, this is the first randomized trial to assess the impact of the Mediterranean diet on risk of this disease.
Because breast cancer was not the primary outcome, investigators were unable to verify if or when participants underwent mammography screening. However, randomization resulted in large groups of participants between whom mammographic status likely was comparable.
The lack of ethnic diversity represents another limitation.
Toledo and colleagues describe differences between olive oils in general and EVOO, including biologic mechanisms that might result in breast cancer prophylaxis.
WHAT THIS EVIDENCE MEANS FOR PRACTICEGiven the known cardiovascular benefits, it is reasonable to suggest to our menopausal patients that a Mediterranean diet with EVOO may reduce their risk of breast cancer.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
This recently published study from Finland generated headlines when its authors concluded that stopping HT elevates the risk of mortality from cardiovascular disease (CVD), including cardiac and cerebrovascular events. Using nationwide data, investigators compared the CVD mortality rate among women who discontinued HT during the years 1994 through 2009 (n = 332,202) with expected (not actual) CVD mortality rates in the background population.
Within the first year after HT discontinuation, elevations in death rates from cardiac events and stroke were noted (standardized mortality ratio, 1.26 and 1.63, respectively), while in the subsequent year, reductions in such mortality were observed (P<.05 for all comparisons).
The absolute increased risk of death from cardiac events reported within the first year after discontinuation of HT was 4 deaths per 10,000 woman-years of exposure. The absolute risk of death from stroke was 5 additional events per 10,000 woman-years. This level of risk is considered to be rare.
How these data compare to those of other studiesIn contrast with these Finnish data, findings from the Women’s Health Initiative—the largest randomized trial of menopausal HT—do not indicate an increase in mortality or an increase in coronary heart or stroke events among women stopping HT.1,2
It seems likely that limitations associated with the Finnish observational data account for this discordance. For example, Mikkola and colleagues did not know why women discontinued HT, raising the possibility that women with symptoms suggestive of CVD or development of new risk factors preferentially stopped HT, potentially introducing important bias into the Finnish analysis.
What this evidence means for practiceWomen and their clinicians should make decisions regarding whether to continue, reduce the dose, or discontinue HT through shared decision making, focusing on individual patient quality of life parameters as well as changing risk concerns related to such entities as cancer, CVD, and osteoporosis.3 Dramatic as they are, findings from this Finnish report should not impact how we counsel women regarding use or discontinuation of HT.
—Andrew M. Kaunitz, MD; JoAnn E. Manson, MD, DrPH; and Cynthia A. Stuenkel, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305(13):1305–1314.
- Kaunitz AM. Extended duration use of menopausal hormone therapy. Menopause. 2014;21(6):679–68.
This recently published study from Finland generated headlines when its authors concluded that stopping HT elevates the risk of mortality from cardiovascular disease (CVD), including cardiac and cerebrovascular events. Using nationwide data, investigators compared the CVD mortality rate among women who discontinued HT during the years 1994 through 2009 (n = 332,202) with expected (not actual) CVD mortality rates in the background population.
Within the first year after HT discontinuation, elevations in death rates from cardiac events and stroke were noted (standardized mortality ratio, 1.26 and 1.63, respectively), while in the subsequent year, reductions in such mortality were observed (P<.05 for all comparisons).
The absolute increased risk of death from cardiac events reported within the first year after discontinuation of HT was 4 deaths per 10,000 woman-years of exposure. The absolute risk of death from stroke was 5 additional events per 10,000 woman-years. This level of risk is considered to be rare.
How these data compare to those of other studiesIn contrast with these Finnish data, findings from the Women’s Health Initiative—the largest randomized trial of menopausal HT—do not indicate an increase in mortality or an increase in coronary heart or stroke events among women stopping HT.1,2
It seems likely that limitations associated with the Finnish observational data account for this discordance. For example, Mikkola and colleagues did not know why women discontinued HT, raising the possibility that women with symptoms suggestive of CVD or development of new risk factors preferentially stopped HT, potentially introducing important bias into the Finnish analysis.
What this evidence means for practiceWomen and their clinicians should make decisions regarding whether to continue, reduce the dose, or discontinue HT through shared decision making, focusing on individual patient quality of life parameters as well as changing risk concerns related to such entities as cancer, CVD, and osteoporosis.3 Dramatic as they are, findings from this Finnish report should not impact how we counsel women regarding use or discontinuation of HT.
—Andrew M. Kaunitz, MD; JoAnn E. Manson, MD, DrPH; and Cynthia A. Stuenkel, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
This recently published study from Finland generated headlines when its authors concluded that stopping HT elevates the risk of mortality from cardiovascular disease (CVD), including cardiac and cerebrovascular events. Using nationwide data, investigators compared the CVD mortality rate among women who discontinued HT during the years 1994 through 2009 (n = 332,202) with expected (not actual) CVD mortality rates in the background population.
Within the first year after HT discontinuation, elevations in death rates from cardiac events and stroke were noted (standardized mortality ratio, 1.26 and 1.63, respectively), while in the subsequent year, reductions in such mortality were observed (P<.05 for all comparisons).
The absolute increased risk of death from cardiac events reported within the first year after discontinuation of HT was 4 deaths per 10,000 woman-years of exposure. The absolute risk of death from stroke was 5 additional events per 10,000 woman-years. This level of risk is considered to be rare.
How these data compare to those of other studiesIn contrast with these Finnish data, findings from the Women’s Health Initiative—the largest randomized trial of menopausal HT—do not indicate an increase in mortality or an increase in coronary heart or stroke events among women stopping HT.1,2
It seems likely that limitations associated with the Finnish observational data account for this discordance. For example, Mikkola and colleagues did not know why women discontinued HT, raising the possibility that women with symptoms suggestive of CVD or development of new risk factors preferentially stopped HT, potentially introducing important bias into the Finnish analysis.
What this evidence means for practiceWomen and their clinicians should make decisions regarding whether to continue, reduce the dose, or discontinue HT through shared decision making, focusing on individual patient quality of life parameters as well as changing risk concerns related to such entities as cancer, CVD, and osteoporosis.3 Dramatic as they are, findings from this Finnish report should not impact how we counsel women regarding use or discontinuation of HT.
—Andrew M. Kaunitz, MD; JoAnn E. Manson, MD, DrPH; and Cynthia A. Stuenkel, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305(13):1305–1314.
- Kaunitz AM. Extended duration use of menopausal hormone therapy. Menopause. 2014;21(6):679–68.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305(13):1305–1314.
- Kaunitz AM. Extended duration use of menopausal hormone therapy. Menopause. 2014;21(6):679–68.
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
The minimally invasive approach to hysterectomy (laparoscopic, robot-assisted, or vaginal) is associated with less blood loss, a shorter length of stay, and quicker recovery than the abdominal approach (laparotomy). In this study, Esselen and colleagues drew from the 2012 National Inpatient Sample, the largest national all-payer database of hospital discharges, which samples some 20% of hospital discharges. Of an estimated 28,160 hysterectomies performed in 2012 for endometrial cancer, 50% were abdominal and 50% involved MIS (38% robot-assisted, 11% laparoscopic, and 1% vaginal).
MIS was used less often for black women (adjusted odds ratio [OR], 0.50) and Native American women (0.56), compared with white women. Similarly, Medicaid patients were less likely to undergo MIS (adjusted OR, 0.58) than those who were covered by commercial medical insurance.
MIS was used 3.68 times more often in women cared for in urban teaching hospitals, compared with women undergoing hysterectomy for endometrial cancer in rural hospitals (P<.04 for all comparisons).
Length of stay was substantially longer and total costs were higher for women undergoing abdominal hysterectomy.
Study did not control for stage of cancer at presentation
These striking findings parallel higher cancer-specific mortality and other disparities faced by minority patients. Esselen and colleagues point out that higher stage at presentation sometimes mandates use of an abdominal approach for hysterectomy and that minority and low-income women often present with higher-stage disease; accordingly, their inability to control for stage represents an important limitation.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This report indicates that, for US women of different ethnic and socioeconomic status, important differences are present with respect to access to MIS for gynecologic malignancy.
— Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The minimally invasive approach to hysterectomy (laparoscopic, robot-assisted, or vaginal) is associated with less blood loss, a shorter length of stay, and quicker recovery than the abdominal approach (laparotomy). In this study, Esselen and colleagues drew from the 2012 National Inpatient Sample, the largest national all-payer database of hospital discharges, which samples some 20% of hospital discharges. Of an estimated 28,160 hysterectomies performed in 2012 for endometrial cancer, 50% were abdominal and 50% involved MIS (38% robot-assisted, 11% laparoscopic, and 1% vaginal).
MIS was used less often for black women (adjusted odds ratio [OR], 0.50) and Native American women (0.56), compared with white women. Similarly, Medicaid patients were less likely to undergo MIS (adjusted OR, 0.58) than those who were covered by commercial medical insurance.
MIS was used 3.68 times more often in women cared for in urban teaching hospitals, compared with women undergoing hysterectomy for endometrial cancer in rural hospitals (P<.04 for all comparisons).
Length of stay was substantially longer and total costs were higher for women undergoing abdominal hysterectomy.
Study did not control for stage of cancer at presentation
These striking findings parallel higher cancer-specific mortality and other disparities faced by minority patients. Esselen and colleagues point out that higher stage at presentation sometimes mandates use of an abdominal approach for hysterectomy and that minority and low-income women often present with higher-stage disease; accordingly, their inability to control for stage represents an important limitation.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This report indicates that, for US women of different ethnic and socioeconomic status, important differences are present with respect to access to MIS for gynecologic malignancy.
— Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The minimally invasive approach to hysterectomy (laparoscopic, robot-assisted, or vaginal) is associated with less blood loss, a shorter length of stay, and quicker recovery than the abdominal approach (laparotomy). In this study, Esselen and colleagues drew from the 2012 National Inpatient Sample, the largest national all-payer database of hospital discharges, which samples some 20% of hospital discharges. Of an estimated 28,160 hysterectomies performed in 2012 for endometrial cancer, 50% were abdominal and 50% involved MIS (38% robot-assisted, 11% laparoscopic, and 1% vaginal).
MIS was used less often for black women (adjusted odds ratio [OR], 0.50) and Native American women (0.56), compared with white women. Similarly, Medicaid patients were less likely to undergo MIS (adjusted OR, 0.58) than those who were covered by commercial medical insurance.
MIS was used 3.68 times more often in women cared for in urban teaching hospitals, compared with women undergoing hysterectomy for endometrial cancer in rural hospitals (P<.04 for all comparisons).
Length of stay was substantially longer and total costs were higher for women undergoing abdominal hysterectomy.
Study did not control for stage of cancer at presentation
These striking findings parallel higher cancer-specific mortality and other disparities faced by minority patients. Esselen and colleagues point out that higher stage at presentation sometimes mandates use of an abdominal approach for hysterectomy and that minority and low-income women often present with higher-stage disease; accordingly, their inability to control for stage represents an important limitation.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This report indicates that, for US women of different ethnic and socioeconomic status, important differences are present with respect to access to MIS for gynecologic malignancy.
— Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
How to individualize cancer risk reduction after a diagnosis of DCIS
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
In this Article
- What is DCIS?
- Why did some deaths occur after diagnosis of DCIS?
- Why did some deaths occur after diagnosis of DCIS?