User login
Adenomyosis in the spotlight, but which sign is featured?
A) Globular enlarged uterus INCORRECT
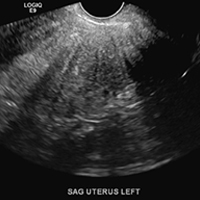
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1
B) Cystic myometrial spaces INCORRECT
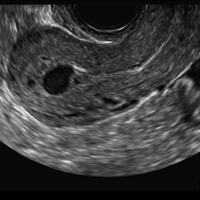
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2
C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1
D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

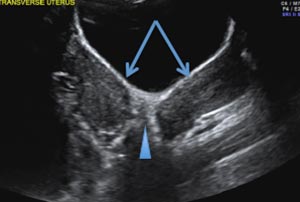
E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2
- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
A) Globular enlarged uterus INCORRECT
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1

B) Cystic myometrial spaces INCORRECT
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2

C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1

D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2

A) Globular enlarged uterus INCORRECT
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1

B) Cystic myometrial spaces INCORRECT
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2

C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1

D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2

- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
A 37-year-old woman with heavy menstrual bleeding and dysmenorrhea presents to her gynecologist. Physical exam suggests an enlarged uterus; the gynecologist orders pelvic ultrasonography.
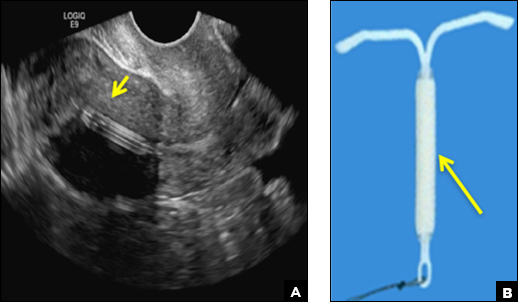
Eye spy: Name that IUD
A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
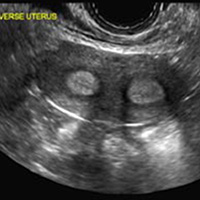
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4
B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7
- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4

B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7

A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4

B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7

- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
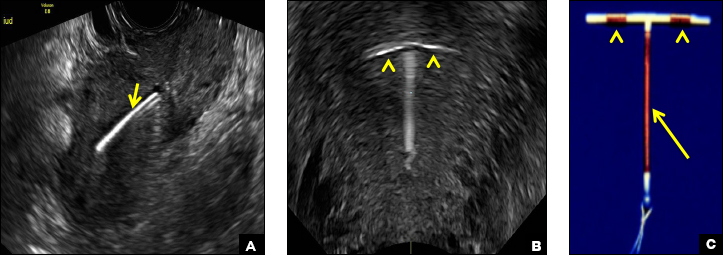
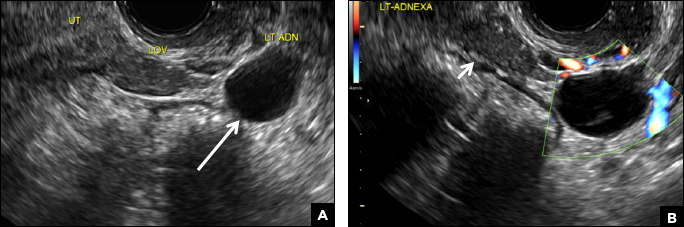
A 25-year-old woman using an IUD presented to her ObGyn’s office for follow-up care of a 4- to 5-cm left simple ovarian cyst. A pelvic ultrasound was performed. The ovarian cyst had resolved and a fundally-positioned IUD was imaged.
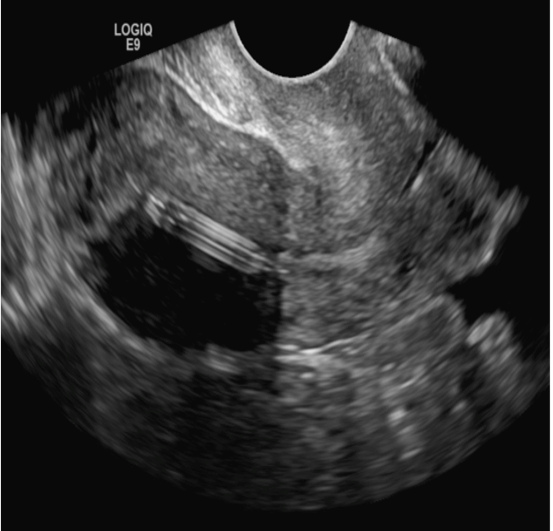
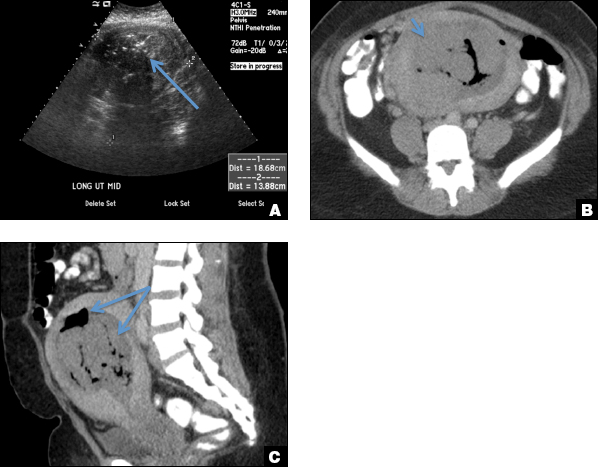
A woman with heavy noncyclical bleeding 6 weeks after abortion
A) Retained products of conception (RPOC) INCORRECT
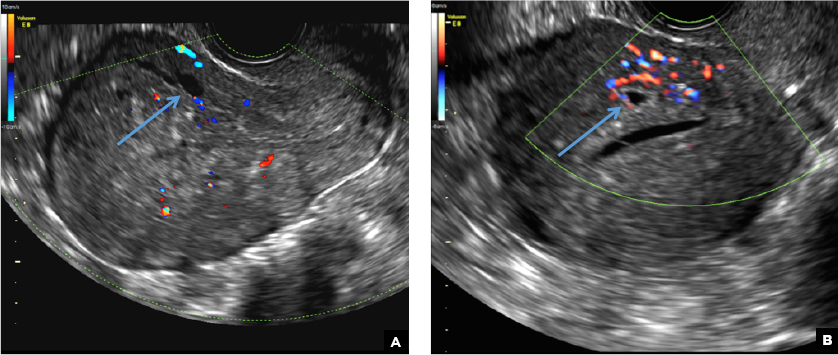
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.
B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3
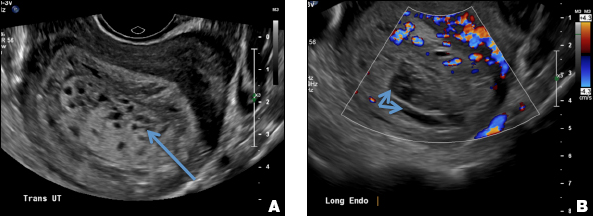
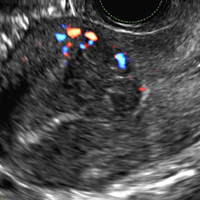
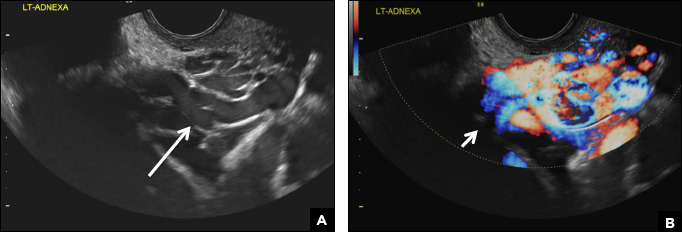
C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.
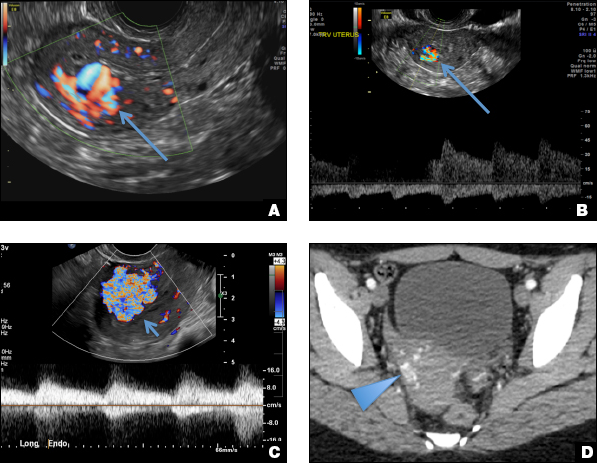
D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.
- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
A) Retained products of conception (RPOC) INCORRECT
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.

B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3

C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.

D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.

A) Retained products of conception (RPOC) INCORRECT
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.

B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3

C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.

D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.

- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
A 25-year-old woman presents to her ObGyn’s office with heavy noncyclical bleeding 6 weeks after a first-trimester suction curettage abortion. Transvaginal pelvic ultrasonography of the uterus with grayscale (A) and color Doppler (B) are performed.
Multiple miscarriages in a 29-year-old woman
A) Didelphys uterus INCORRECT
A didelphys uterus occurs as a result of complete nonfusion of the Müllerian duct, resulting in duplicated uterine horns, duplicated cervices, and occasionally the proximal vagina, which is often associated with a transverse or longitudinal vaginal septum.1,2 Fusion anomalies (didelphys and bicornuate) can be differentiated from resorption anomalies (septate and arcuate) on ultrasonography (US) by a deep fundal cleft (>1 cm).1 3D US is highly accurate in identifying Müllerian duct anomalies (MDAs), it correlates well with magnetic resonance imaging (MRI), and it is more cost effective and accessible.3 For the didelphys uterus, 3D US obtained coronal to the uterine fundus often demonstrates widely divergent uterine horns separated by a deep serosal fundal cleft (>1 cm) and noncommunicating endometrial cavities with 2 cervices.1 Identifying a duplicated vaginal canal can help differentiate a didelphys uterus from a bicornuate bicollis uterus.1
B) Unicornuate uterus INCORRECT
A unicornuate uterus results from arrested development of one Müllerian duct and normal development of the other. There are 4 different subtypes: no rudimentary horn, rudimentary horn without endometrial cavity, rudimentary horn with communicating endometrial cavity, and rudimentary horn with noncommunicating uterine cavity.1 Renal anomalies, especially renal agenesis when present, are usually ipsilateral to the rudimentary horn.1 On US, unicornuate uterus appears as a lobular oblong (banana-shaped) structure away from the midline. The rudimentary horn is often difficult to identify and, when seen, the presence or absence of endometrium is an important finding.1
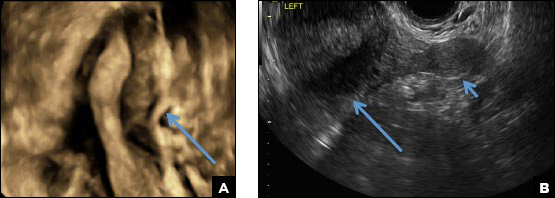
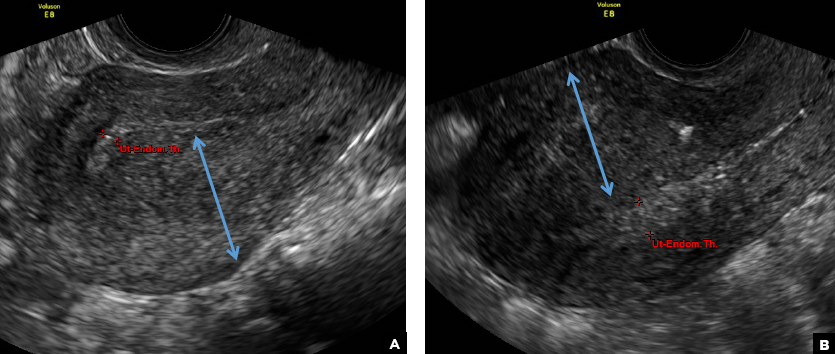
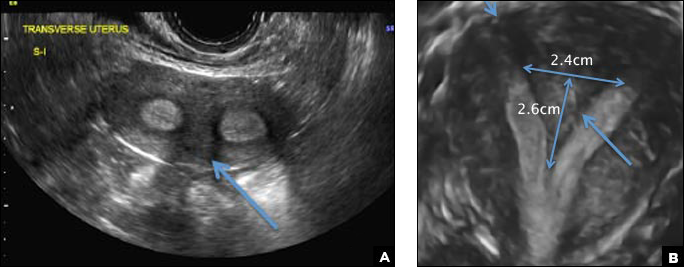
C) Partial septate uterus CORRECT
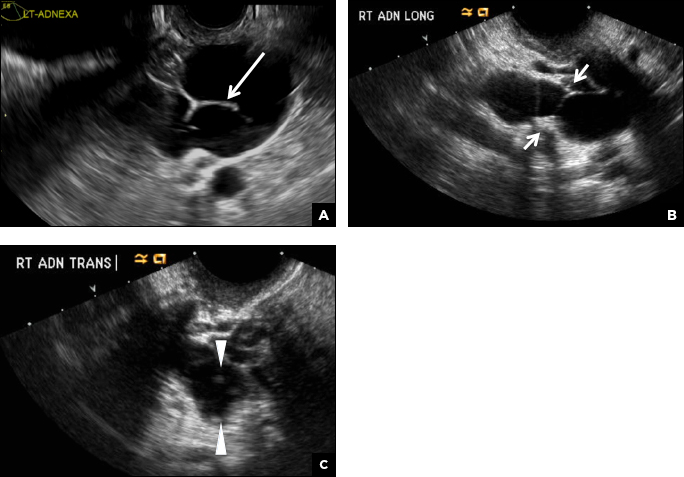
A septate uterus is a result of a resorption anomaly. The septum may be partial (subseptate) or complete secondary to failure of resorption of the uterovaginal septum. A complete septum extends to the external cervical os and occasionally to the vagina.1 US demonstrates interruption of the myometrium by a hypoechoic fibrous septum and/or a muscular septum which is isoechoic to the myometrium and arises midline from the fundus.1 3D US often clearly delineates the septum and improves visualization of the external fundal contour.2 The external fundal contour in septate uterus is convex (little or no serosal fundal cleft) and the apex is greater than 5 mm above the interostial line compared with a bicornuate or didelphys uterus, where the apex is less than 5 mm from the interostial line.1,2 Additionally, the intercornual distance is less than 4 cm.4 A septate uterus with duplicated cervix can appear similar to a bicornuate bicollis uterus and can be correctly diagnosed by evaluating the external fundal contour.1
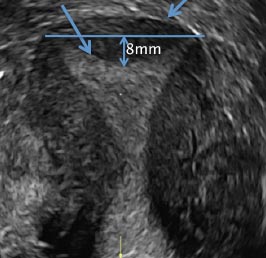
D) Arcuate uterus INCORRECT
The arcuate uterus also represents a resorption anomaly as a sequela of near-complete resorption of the uterovaginal septum.1 On 3D US, the fundal indentation in an arcuate uterus is well delineated and appears broad based with an obtuse angle at the most central point, which can help differentiate it from a septate uterus that has an acute angle.2,3 Both an arcuate and a septate uterus have a normal external (serosal) fundal contour.2,3 With an arcuate uterus, the length of the vertical measurement from the tubal ostia line, which is drawn horizontally between the tubal ostia to the fundal midline component of the endometrial cavity, is 1 cm or less. With a septate uterus, this vertical distance is greater than 1 cm.
- CambriaBehr SC, Courtier JL, Qayyum A. Imaging of MMS PGothicüCambriallerian duct anomalies. RadioGraphics. 2012;32(6):e23.
- Bermejo C, Martinez Ten P, Diaz D, et al. Three-dimensional ultrasound in the diagnosis of MMS PGothicüCambriallerian duct anomaly and concordance with MRI. Ultrasound Obstet Gynecol.Cambria 2010;35(5):593–601.
- Deutch TD, Abuhamed AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of MMS PGothicüCambriallerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- CambriaChandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. MMS PGothicüCambriallerian duct anomalies: from diagnosis to intervention.Cambria Br J Radiol. 2009;82(984):1034–1042.
A) Didelphys uterus INCORRECT
A didelphys uterus occurs as a result of complete nonfusion of the Müllerian duct, resulting in duplicated uterine horns, duplicated cervices, and occasionally the proximal vagina, which is often associated with a transverse or longitudinal vaginal septum.1,2 Fusion anomalies (didelphys and bicornuate) can be differentiated from resorption anomalies (septate and arcuate) on ultrasonography (US) by a deep fundal cleft (>1 cm).1 3D US is highly accurate in identifying Müllerian duct anomalies (MDAs), it correlates well with magnetic resonance imaging (MRI), and it is more cost effective and accessible.3 For the didelphys uterus, 3D US obtained coronal to the uterine fundus often demonstrates widely divergent uterine horns separated by a deep serosal fundal cleft (>1 cm) and noncommunicating endometrial cavities with 2 cervices.1 Identifying a duplicated vaginal canal can help differentiate a didelphys uterus from a bicornuate bicollis uterus.1

B) Unicornuate uterus INCORRECT
A unicornuate uterus results from arrested development of one Müllerian duct and normal development of the other. There are 4 different subtypes: no rudimentary horn, rudimentary horn without endometrial cavity, rudimentary horn with communicating endometrial cavity, and rudimentary horn with noncommunicating uterine cavity.1 Renal anomalies, especially renal agenesis when present, are usually ipsilateral to the rudimentary horn.1 On US, unicornuate uterus appears as a lobular oblong (banana-shaped) structure away from the midline. The rudimentary horn is often difficult to identify and, when seen, the presence or absence of endometrium is an important finding.1

C) Partial septate uterus CORRECT
A septate uterus is a result of a resorption anomaly. The septum may be partial (subseptate) or complete secondary to failure of resorption of the uterovaginal septum. A complete septum extends to the external cervical os and occasionally to the vagina.1 US demonstrates interruption of the myometrium by a hypoechoic fibrous septum and/or a muscular septum which is isoechoic to the myometrium and arises midline from the fundus.1 3D US often clearly delineates the septum and improves visualization of the external fundal contour.2 The external fundal contour in septate uterus is convex (little or no serosal fundal cleft) and the apex is greater than 5 mm above the interostial line compared with a bicornuate or didelphys uterus, where the apex is less than 5 mm from the interostial line.1,2 Additionally, the intercornual distance is less than 4 cm.4 A septate uterus with duplicated cervix can appear similar to a bicornuate bicollis uterus and can be correctly diagnosed by evaluating the external fundal contour.1

D) Arcuate uterus INCORRECT
The arcuate uterus also represents a resorption anomaly as a sequela of near-complete resorption of the uterovaginal septum.1 On 3D US, the fundal indentation in an arcuate uterus is well delineated and appears broad based with an obtuse angle at the most central point, which can help differentiate it from a septate uterus that has an acute angle.2,3 Both an arcuate and a septate uterus have a normal external (serosal) fundal contour.2,3 With an arcuate uterus, the length of the vertical measurement from the tubal ostia line, which is drawn horizontally between the tubal ostia to the fundal midline component of the endometrial cavity, is 1 cm or less. With a septate uterus, this vertical distance is greater than 1 cm.

A) Didelphys uterus INCORRECT
A didelphys uterus occurs as a result of complete nonfusion of the Müllerian duct, resulting in duplicated uterine horns, duplicated cervices, and occasionally the proximal vagina, which is often associated with a transverse or longitudinal vaginal septum.1,2 Fusion anomalies (didelphys and bicornuate) can be differentiated from resorption anomalies (septate and arcuate) on ultrasonography (US) by a deep fundal cleft (>1 cm).1 3D US is highly accurate in identifying Müllerian duct anomalies (MDAs), it correlates well with magnetic resonance imaging (MRI), and it is more cost effective and accessible.3 For the didelphys uterus, 3D US obtained coronal to the uterine fundus often demonstrates widely divergent uterine horns separated by a deep serosal fundal cleft (>1 cm) and noncommunicating endometrial cavities with 2 cervices.1 Identifying a duplicated vaginal canal can help differentiate a didelphys uterus from a bicornuate bicollis uterus.1

B) Unicornuate uterus INCORRECT
A unicornuate uterus results from arrested development of one Müllerian duct and normal development of the other. There are 4 different subtypes: no rudimentary horn, rudimentary horn without endometrial cavity, rudimentary horn with communicating endometrial cavity, and rudimentary horn with noncommunicating uterine cavity.1 Renal anomalies, especially renal agenesis when present, are usually ipsilateral to the rudimentary horn.1 On US, unicornuate uterus appears as a lobular oblong (banana-shaped) structure away from the midline. The rudimentary horn is often difficult to identify and, when seen, the presence or absence of endometrium is an important finding.1

C) Partial septate uterus CORRECT
A septate uterus is a result of a resorption anomaly. The septum may be partial (subseptate) or complete secondary to failure of resorption of the uterovaginal septum. A complete septum extends to the external cervical os and occasionally to the vagina.1 US demonstrates interruption of the myometrium by a hypoechoic fibrous septum and/or a muscular septum which is isoechoic to the myometrium and arises midline from the fundus.1 3D US often clearly delineates the septum and improves visualization of the external fundal contour.2 The external fundal contour in septate uterus is convex (little or no serosal fundal cleft) and the apex is greater than 5 mm above the interostial line compared with a bicornuate or didelphys uterus, where the apex is less than 5 mm from the interostial line.1,2 Additionally, the intercornual distance is less than 4 cm.4 A septate uterus with duplicated cervix can appear similar to a bicornuate bicollis uterus and can be correctly diagnosed by evaluating the external fundal contour.1

D) Arcuate uterus INCORRECT
The arcuate uterus also represents a resorption anomaly as a sequela of near-complete resorption of the uterovaginal septum.1 On 3D US, the fundal indentation in an arcuate uterus is well delineated and appears broad based with an obtuse angle at the most central point, which can help differentiate it from a septate uterus that has an acute angle.2,3 Both an arcuate and a septate uterus have a normal external (serosal) fundal contour.2,3 With an arcuate uterus, the length of the vertical measurement from the tubal ostia line, which is drawn horizontally between the tubal ostia to the fundal midline component of the endometrial cavity, is 1 cm or less. With a septate uterus, this vertical distance is greater than 1 cm.

- CambriaBehr SC, Courtier JL, Qayyum A. Imaging of MMS PGothicüCambriallerian duct anomalies. RadioGraphics. 2012;32(6):e23.
- Bermejo C, Martinez Ten P, Diaz D, et al. Three-dimensional ultrasound in the diagnosis of MMS PGothicüCambriallerian duct anomaly and concordance with MRI. Ultrasound Obstet Gynecol.Cambria 2010;35(5):593–601.
- Deutch TD, Abuhamed AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of MMS PGothicüCambriallerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- CambriaChandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. MMS PGothicüCambriallerian duct anomalies: from diagnosis to intervention.Cambria Br J Radiol. 2009;82(984):1034–1042.
- CambriaBehr SC, Courtier JL, Qayyum A. Imaging of MMS PGothicüCambriallerian duct anomalies. RadioGraphics. 2012;32(6):e23.
- Bermejo C, Martinez Ten P, Diaz D, et al. Three-dimensional ultrasound in the diagnosis of MMS PGothicüCambriallerian duct anomaly and concordance with MRI. Ultrasound Obstet Gynecol.Cambria 2010;35(5):593–601.
- Deutch TD, Abuhamed AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of MMS PGothicüCambriallerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- CambriaChandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. MMS PGothicüCambriallerian duct anomalies: from diagnosis to intervention.Cambria Br J Radiol. 2009;82(984):1034–1042.
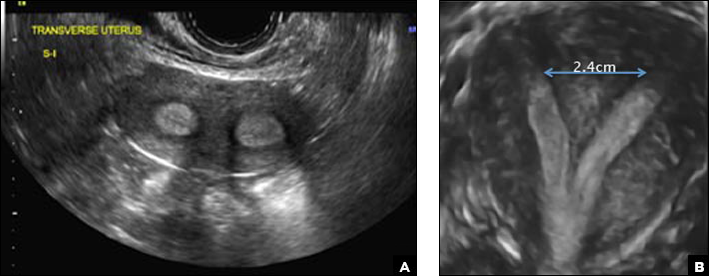
A 29-year-old woman presents to her ObGyn’s office with a history of multiple miscarriages. Transverse pelvic 2D ultrasonography of the uterus (A) and coronal 3D imaging (B) are performed.
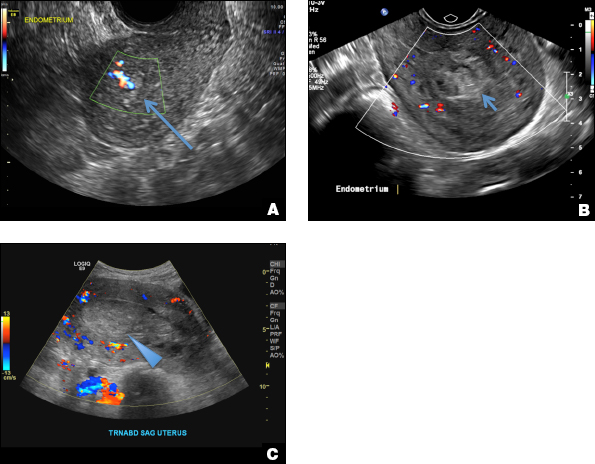
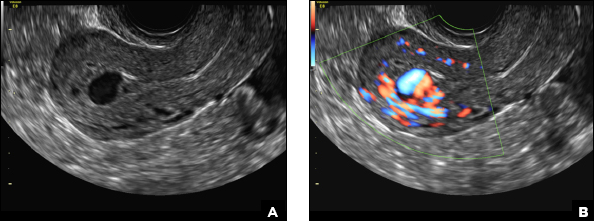
42-year-old woman with abnormal uterine bleeding
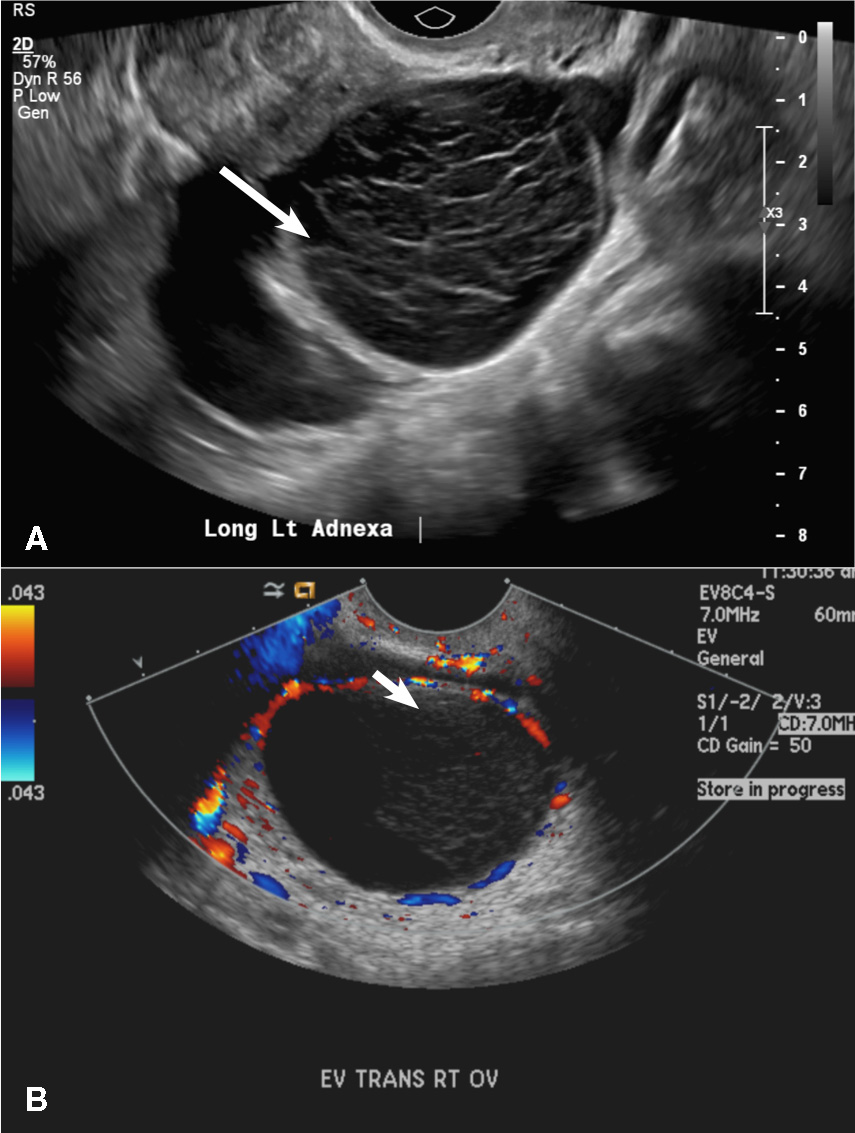
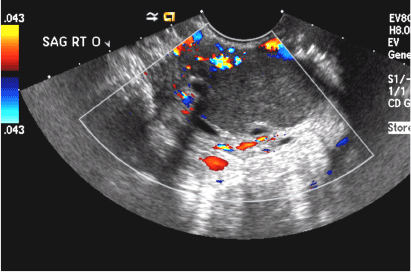
A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3
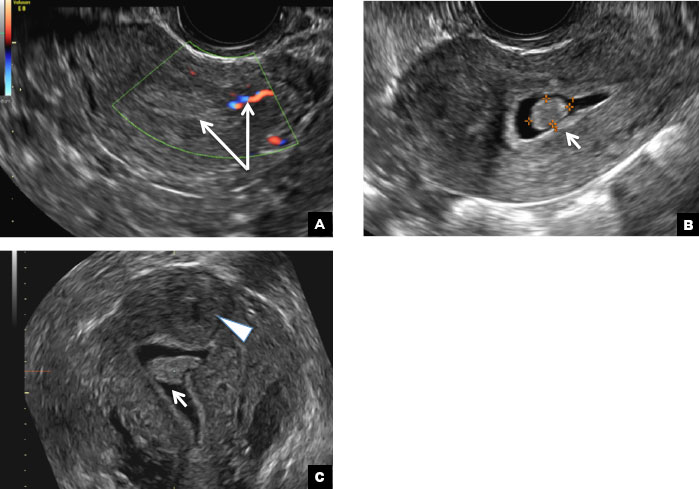
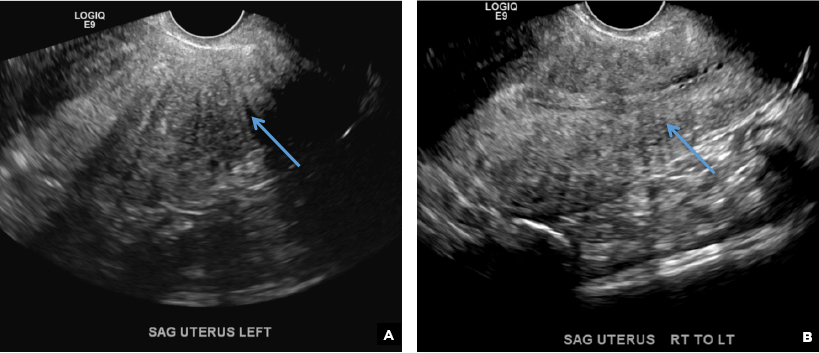
B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3
C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2
D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
A 42-year-old woman presents to her gynecologist's office with abnormal uterine bleeding. Pelvic ultrasonography of the uterus is performed with color Doppler (A) and subsequent sonohysterogram (SHG)/saline infusion sonohysterography (SIS) (B). Figures shown above.
More than one-third of tumors found on breast cancer screening represent overdiagnosis
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
32-year-old woman with pelvic pain and irregular menstrual periods
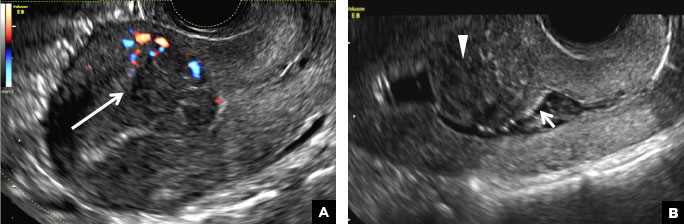
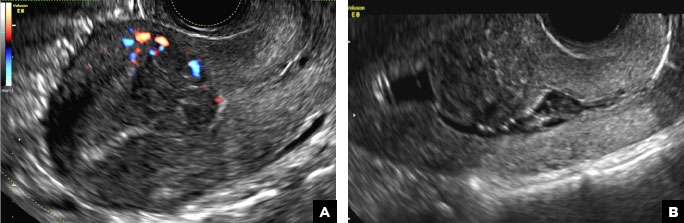
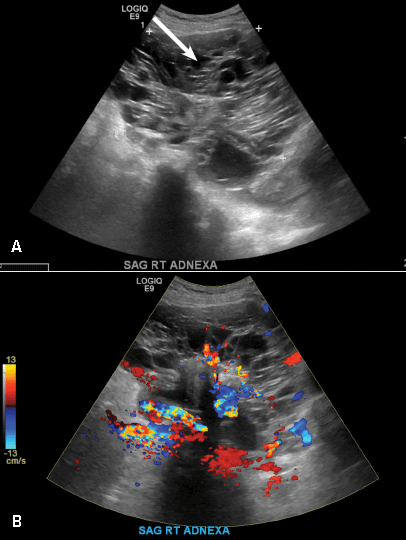
(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2
B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.
C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
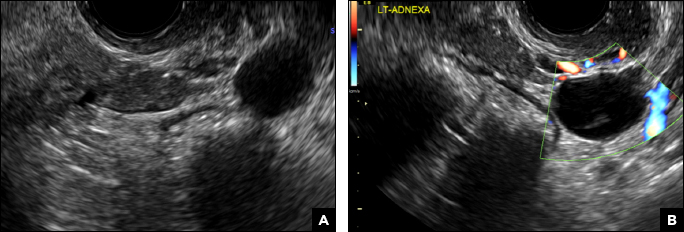
A 32-year-old women presents to her gynecologist’s office reporting pelvic pain and irregular menstrual periods. Results of a urine pregnancy test are negative. Pelvic ultrasonography is performed, with gray scale ( A ) and color Doppler ( B ) images of the left adnexa obtained. Figures shown above.
2-week left-sided pelvic pain
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.
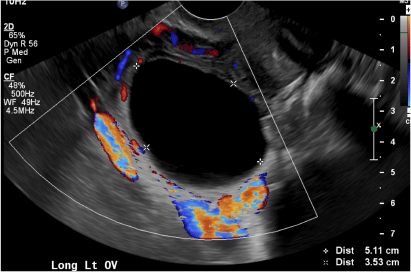
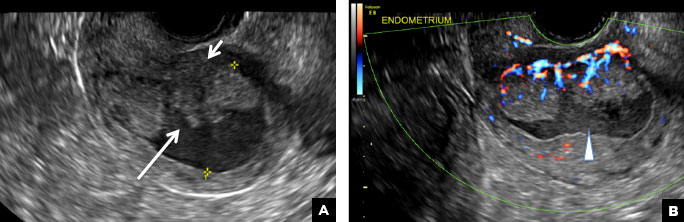
(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.
(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.
(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
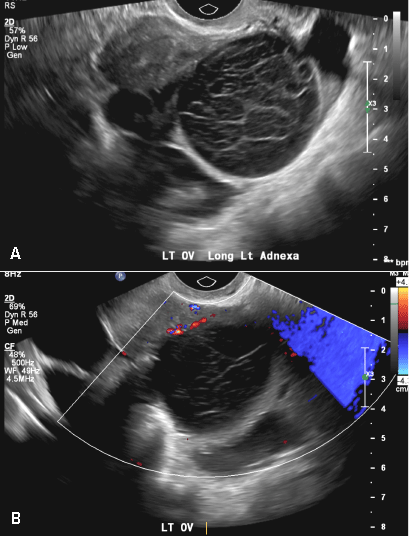
A 37-year-old woman presents to the emergency department reporting left-sided pelvic pain for 2 weeks duration. She has a negative urine pregnancy test. Pelvic ultrasonography of the left adnexa is performed with gray scale (A) and color Doppler images (B). Figures shown above.
Focus on treating genital atrophy symptoms
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
Benefit of self-administered vaginal lidocaine gel in IUD placement
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
In this article