User login
Managing menopausal symptoms in women with a BRCA mutation
This audiocast was recorded at the North American Menopause Society Annual Meeting held September 30 to October 3, 2015, in Las Vegas, Nevada
For more on this topic, read Dr. Kaunitz's August 2015 Cases in Menopause article, Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
This audiocast was recorded at the North American Menopause Society Annual Meeting held September 30 to October 3, 2015, in Las Vegas, Nevada
For more on this topic, read Dr. Kaunitz's August 2015 Cases in Menopause article, Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
This audiocast was recorded at the North American Menopause Society Annual Meeting held September 30 to October 3, 2015, in Las Vegas, Nevada
For more on this topic, read Dr. Kaunitz's August 2015 Cases in Menopause article, Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Imaging the suspected ovarian malignancy: 14 cases
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
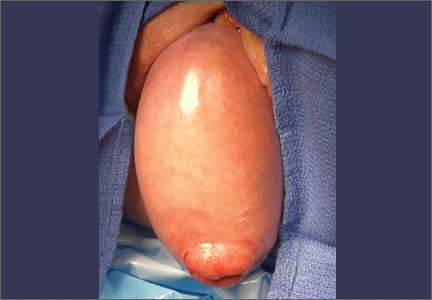
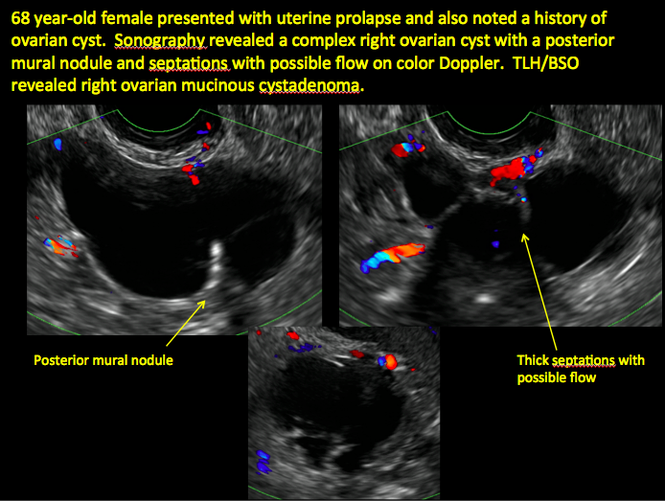
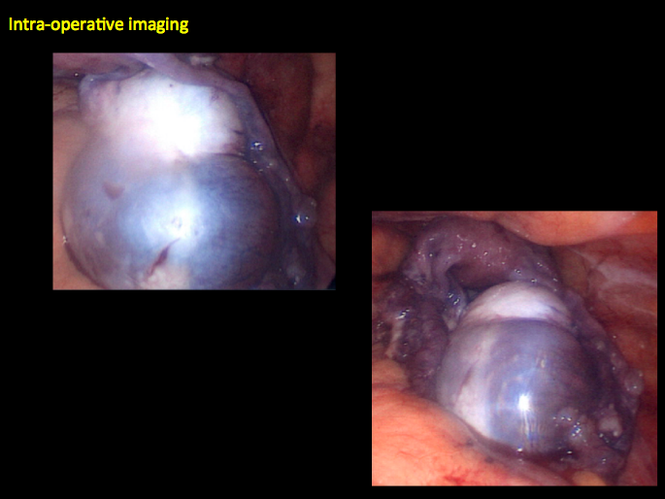
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst
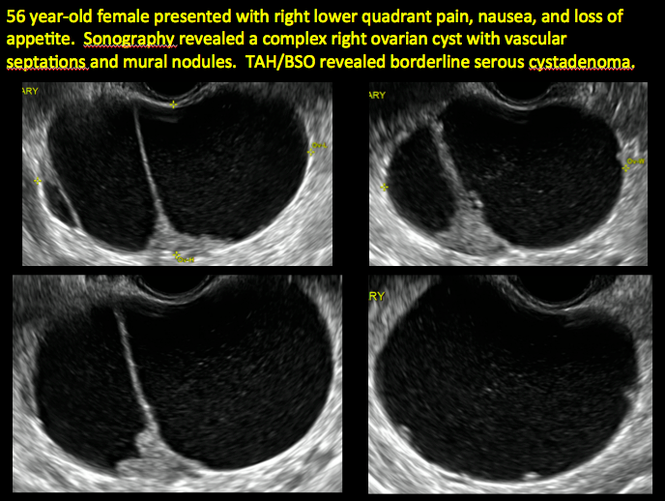
CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite

CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion

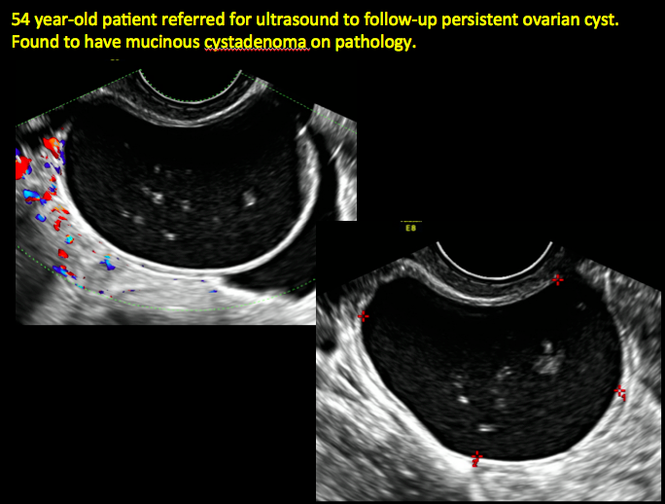
CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst
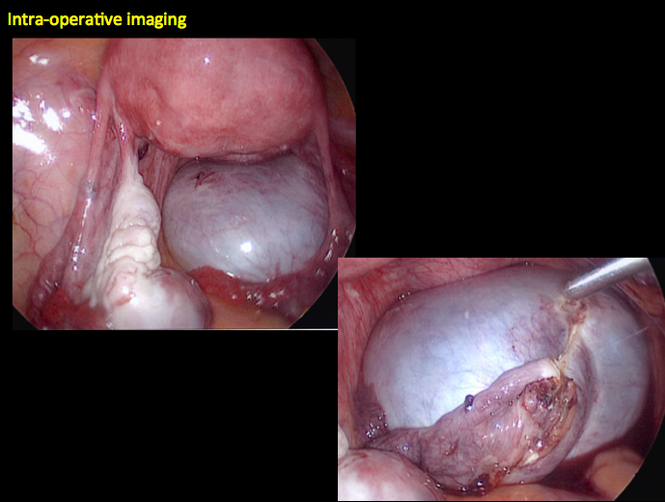
CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain

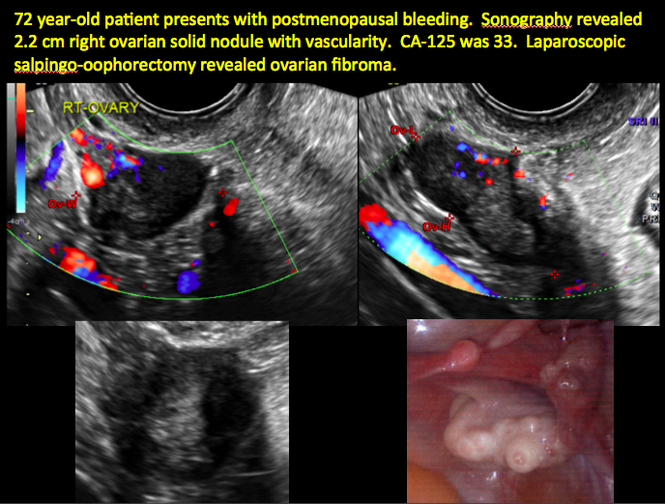
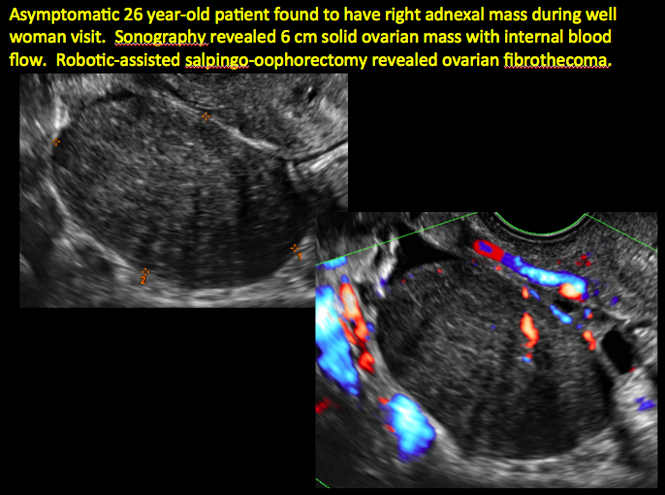
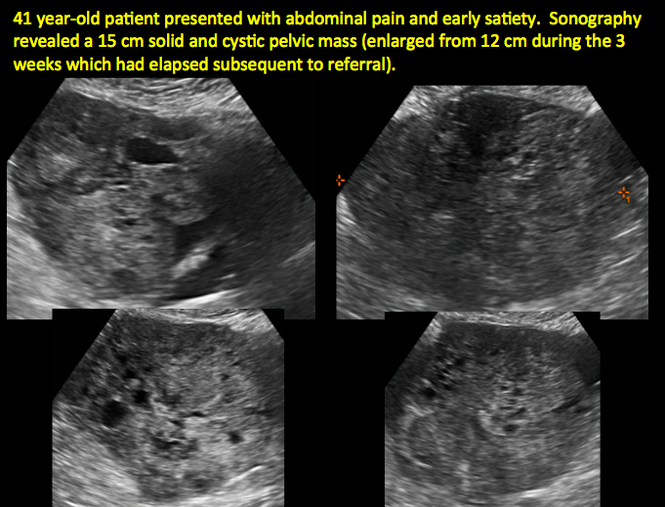
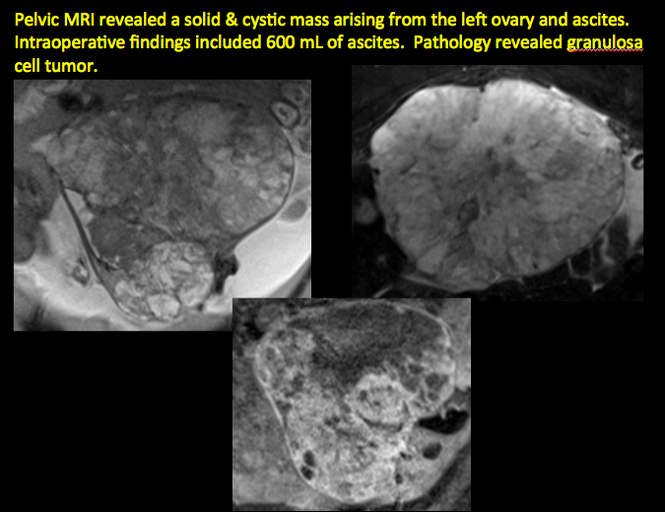
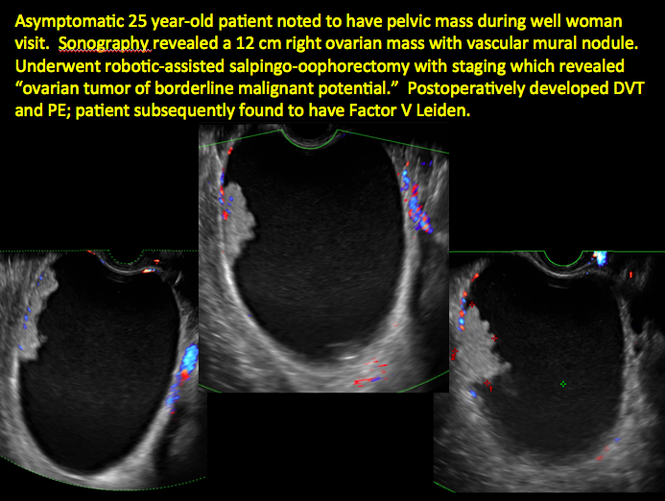
CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding
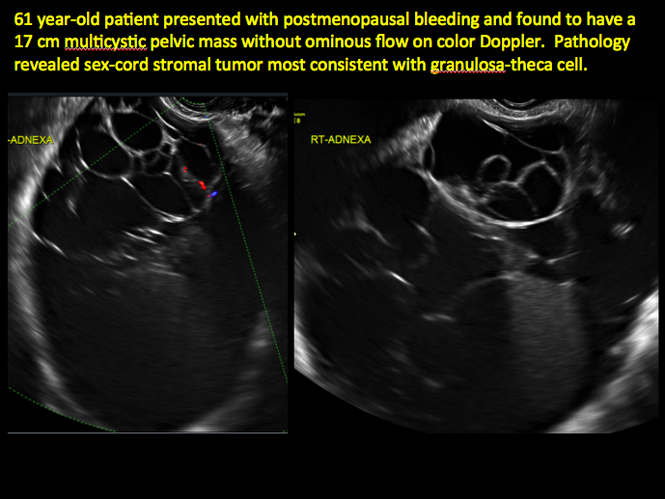
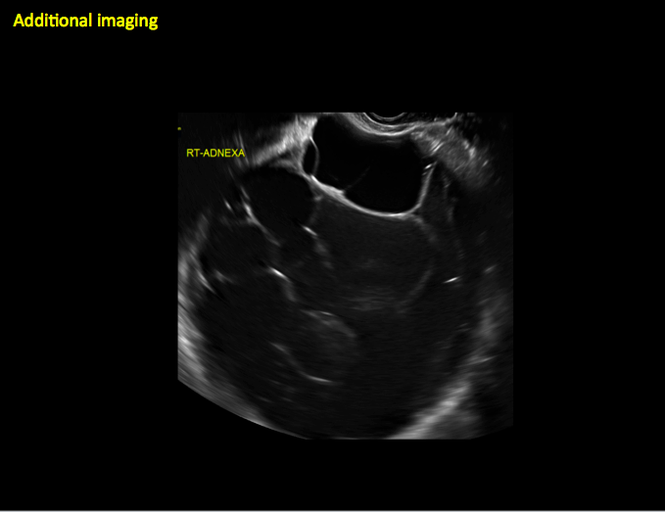
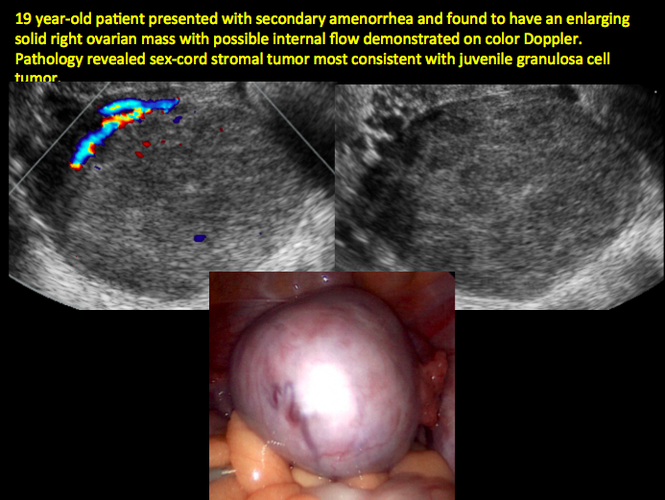
CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea
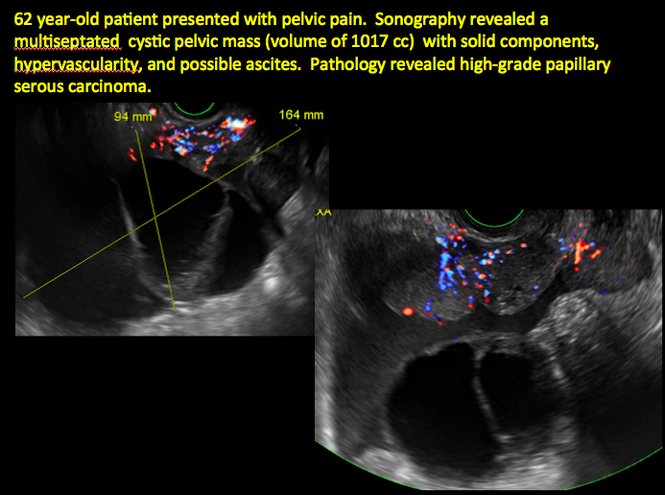
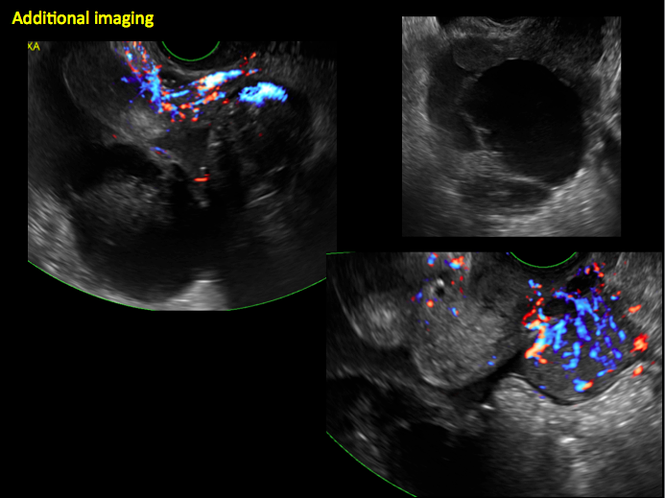
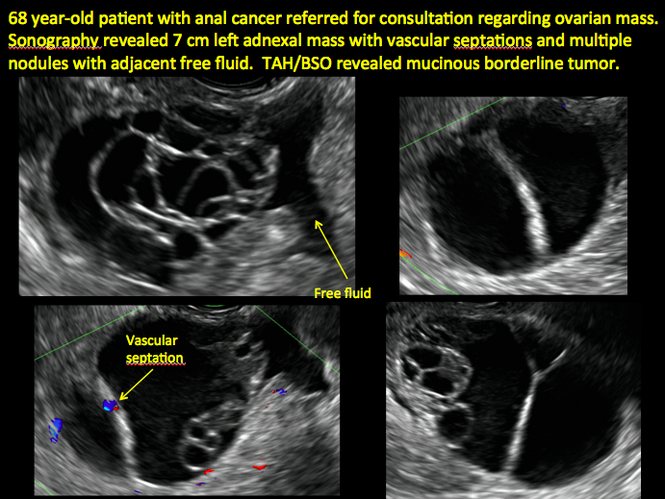
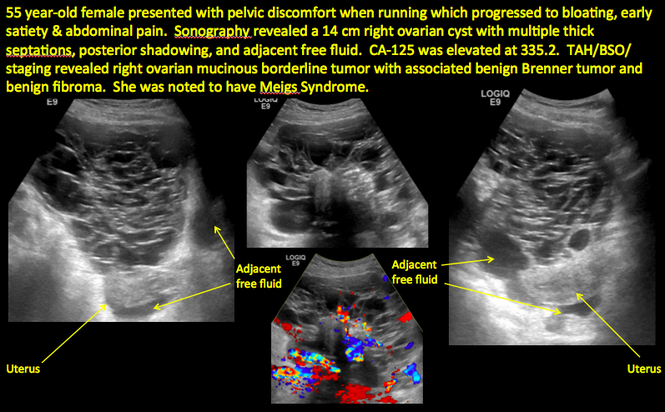
CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Which vaginal procedure is best for uterine prolapse?
More than one-third of women aged 45 years or older experience uterine prolapse, a condition that can impair physical, psychological, and sexual function. To compare vaginal vault suspension with hysterectomy, investigators at 4 large Dutch teaching hospitals from 2009 to 2012 randomly assigned women with uterine prolapse to sacrospinous hysteropexy (SSLF) or vaginal hysterectomy with uterosacral ligament suspension (ULS). The primary outcome was recurrent stage 2 or greater prolapse (within 1 cm or more of the hymenal ring) with bothersome bulge symptoms or repeat surgery for prolapse by 12 months follow-up.
Details of the trialOne hundred two women assigned to SSLF (median age, 62.7 years) and 100 assigned to hysterectomy with ULS (median age, 61.9 years) were analyzed for the primary outcome. The patients ranged in age from 33 to 85 years.
Surgical failure rates and adverse events were similarMean hospital stay was 3 days in both groups and the occurrence of urinary retention was likewise similar (15% for SSLF and 11% for hysterectomy with ULS). At 12 months, 0 and 4 women in the SSLF and hysterectomy with ULS groups, respectively, met the primary outcome. Study participants were considered a “surgical failure” if any type of prolapse with bothersome symptoms or repeat surgery or pessary use occurred. Failures occurred in approximately one-half of the women in both groups.
Rates of serious adverse events were low, and none were related to type of surgery. Nine women experienced buttock pain following SSLF hysteropexy, a known complication of this surgery. This pain resolved within 6 weeks in 8 of these women. In the remaining woman, persistent pain led to release of the hysteropexy suture and vaginal hysterectomy 4 months after her initial procedure.
What this evidence means for practice
Advantages of hysterectomy at the time of vaginal vault suspension include prevention of endometrial and cervical cancers as well as elimination of uterine bleeding. However, data from published surveys indicate that many US women with prolapse prefer to avoid hysterectomy if effective alternate surgeries are available.1
In the previously published 2014 Barber and colleagues’ OPTIMAL trial,1,2 the efficacy of vaginal hysterectomy with either SSLF or USL was equivalent (63.1% versus 64.5%, respectively). The success rates are lower for both procedures in this trial by Detollenaere and colleagues.
Both SSLF and ULS may result in life-altering buttock or leg pain, necessitating removal of the offending sutures; however, the ULS procedure offers a more anatomically correct result. Although the short follow-up interval represents a limitation, these trial results suggest that sacrospinous fixation without hysterectomy represents a reasonable option for women with bothersome uterine prolapse who would like to avoid hysterectomy.
—Meadow M. Good, DO, and Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Korbly N, Kassis N, Good MM, et al. Patient preference for uterine preservation in women with pelvic organ prolapse: a fellow’s pelvic network research study. Am J Obstet Gynecol. 2013;209(5):470.e1−e6.
- Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse. The OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
More than one-third of women aged 45 years or older experience uterine prolapse, a condition that can impair physical, psychological, and sexual function. To compare vaginal vault suspension with hysterectomy, investigators at 4 large Dutch teaching hospitals from 2009 to 2012 randomly assigned women with uterine prolapse to sacrospinous hysteropexy (SSLF) or vaginal hysterectomy with uterosacral ligament suspension (ULS). The primary outcome was recurrent stage 2 or greater prolapse (within 1 cm or more of the hymenal ring) with bothersome bulge symptoms or repeat surgery for prolapse by 12 months follow-up.
Details of the trialOne hundred two women assigned to SSLF (median age, 62.7 years) and 100 assigned to hysterectomy with ULS (median age, 61.9 years) were analyzed for the primary outcome. The patients ranged in age from 33 to 85 years.
Surgical failure rates and adverse events were similarMean hospital stay was 3 days in both groups and the occurrence of urinary retention was likewise similar (15% for SSLF and 11% for hysterectomy with ULS). At 12 months, 0 and 4 women in the SSLF and hysterectomy with ULS groups, respectively, met the primary outcome. Study participants were considered a “surgical failure” if any type of prolapse with bothersome symptoms or repeat surgery or pessary use occurred. Failures occurred in approximately one-half of the women in both groups.
Rates of serious adverse events were low, and none were related to type of surgery. Nine women experienced buttock pain following SSLF hysteropexy, a known complication of this surgery. This pain resolved within 6 weeks in 8 of these women. In the remaining woman, persistent pain led to release of the hysteropexy suture and vaginal hysterectomy 4 months after her initial procedure.
What this evidence means for practice
Advantages of hysterectomy at the time of vaginal vault suspension include prevention of endometrial and cervical cancers as well as elimination of uterine bleeding. However, data from published surveys indicate that many US women with prolapse prefer to avoid hysterectomy if effective alternate surgeries are available.1
In the previously published 2014 Barber and colleagues’ OPTIMAL trial,1,2 the efficacy of vaginal hysterectomy with either SSLF or USL was equivalent (63.1% versus 64.5%, respectively). The success rates are lower for both procedures in this trial by Detollenaere and colleagues.
Both SSLF and ULS may result in life-altering buttock or leg pain, necessitating removal of the offending sutures; however, the ULS procedure offers a more anatomically correct result. Although the short follow-up interval represents a limitation, these trial results suggest that sacrospinous fixation without hysterectomy represents a reasonable option for women with bothersome uterine prolapse who would like to avoid hysterectomy.
—Meadow M. Good, DO, and Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
More than one-third of women aged 45 years or older experience uterine prolapse, a condition that can impair physical, psychological, and sexual function. To compare vaginal vault suspension with hysterectomy, investigators at 4 large Dutch teaching hospitals from 2009 to 2012 randomly assigned women with uterine prolapse to sacrospinous hysteropexy (SSLF) or vaginal hysterectomy with uterosacral ligament suspension (ULS). The primary outcome was recurrent stage 2 or greater prolapse (within 1 cm or more of the hymenal ring) with bothersome bulge symptoms or repeat surgery for prolapse by 12 months follow-up.
Details of the trialOne hundred two women assigned to SSLF (median age, 62.7 years) and 100 assigned to hysterectomy with ULS (median age, 61.9 years) were analyzed for the primary outcome. The patients ranged in age from 33 to 85 years.
Surgical failure rates and adverse events were similarMean hospital stay was 3 days in both groups and the occurrence of urinary retention was likewise similar (15% for SSLF and 11% for hysterectomy with ULS). At 12 months, 0 and 4 women in the SSLF and hysterectomy with ULS groups, respectively, met the primary outcome. Study participants were considered a “surgical failure” if any type of prolapse with bothersome symptoms or repeat surgery or pessary use occurred. Failures occurred in approximately one-half of the women in both groups.
Rates of serious adverse events were low, and none were related to type of surgery. Nine women experienced buttock pain following SSLF hysteropexy, a known complication of this surgery. This pain resolved within 6 weeks in 8 of these women. In the remaining woman, persistent pain led to release of the hysteropexy suture and vaginal hysterectomy 4 months after her initial procedure.
What this evidence means for practice
Advantages of hysterectomy at the time of vaginal vault suspension include prevention of endometrial and cervical cancers as well as elimination of uterine bleeding. However, data from published surveys indicate that many US women with prolapse prefer to avoid hysterectomy if effective alternate surgeries are available.1
In the previously published 2014 Barber and colleagues’ OPTIMAL trial,1,2 the efficacy of vaginal hysterectomy with either SSLF or USL was equivalent (63.1% versus 64.5%, respectively). The success rates are lower for both procedures in this trial by Detollenaere and colleagues.
Both SSLF and ULS may result in life-altering buttock or leg pain, necessitating removal of the offending sutures; however, the ULS procedure offers a more anatomically correct result. Although the short follow-up interval represents a limitation, these trial results suggest that sacrospinous fixation without hysterectomy represents a reasonable option for women with bothersome uterine prolapse who would like to avoid hysterectomy.
—Meadow M. Good, DO, and Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Korbly N, Kassis N, Good MM, et al. Patient preference for uterine preservation in women with pelvic organ prolapse: a fellow’s pelvic network research study. Am J Obstet Gynecol. 2013;209(5):470.e1−e6.
- Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse. The OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Korbly N, Kassis N, Good MM, et al. Patient preference for uterine preservation in women with pelvic organ prolapse: a fellow’s pelvic network research study. Am J Obstet Gynecol. 2013;209(5):470.e1−e6.
- Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse. The OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
Does the injection of ketorolac prior to IUD placement reduce pain?
Although the use of intrauterine devices (IUDs) is increasing, these highly effective contraceptives remain underutilized in the United States, compared with other developed countries. Concerns about pain with insertion represent one barrier to use.
In a double-blind trial, Ngo and colleagues randomly assigned women presenting for first-time IUD placement from 2012 to 2014 to either:
- ketorolac, a potent nonsteroidal anti-inflammatory drug (NSAID) (1-mL gluteal intramuscular injection of 30 mg of ketorolac) or
- saline (1-mL saline intramuscular injection).
The injection was given 30 minutes prior to IUD placement.
Pain associated with injection of the study drug, speculum and tenaculum placement, uterine sounding, IUD placement, and postinsertion pain were measured using a visual analog scale from 0 cm (no pain) to 10 cm (worst pain possible).
Of 67 participants (mean age, approximately 27 years; white race, 33%; African-American race, 33%; median parity, 1), pain was similar between ketorolac and placebo arms for all parameters except postinsertion pain, which was 0 cm and 1.3 cm for ketorolac and placebo, respectively, 15 minutes after placement (P<.001).
Although approximately 75% of participants reported that pain from the injection was “not as bad” as the pain from IUD placement, about 1 in 5 indicated that injection pain was equivalent to pain from IUD placement. Regardless of study group allocation, more than 90% of participants reported being satisfied or very satisfied with IUD placement overall, and more than 75% said they would recommend IUD placement to a friend.
More than 90% of women were satisfied with IUD placement, regardless of study allocation
As Ngo and colleagues observe, the analgesic effect of ketorolac peaks 1 to 2 hours after injection. This observation may explain why pain reduction was noted only after IUD insertion. Although ketorolac is not expensive, logistic considerations may make its routine use prior to IUD placement unrealistic in many ambulatory settings. Further, the great majority of participants (>90%) reported being satisfied with their IUD placement experience overall, regardless of study allocation.
Earlier studies suggesting that preplacement oral NSAIDs are ineffective in reducing placement pain involved the administration of analgesia in the clinic less than 1 hour before IUD insertion. I agree with Ngo and colleagues that future trials of oral NSAIDs should focus on administration of the medication prior to arrival at the clinic.
What this evidence means for practice
Findings from this randomized controlled trial provide only limited support for injection of an NSAID prior to IUD placement.
--Andrew M. Kaunitz, MD
Although the use of intrauterine devices (IUDs) is increasing, these highly effective contraceptives remain underutilized in the United States, compared with other developed countries. Concerns about pain with insertion represent one barrier to use.
In a double-blind trial, Ngo and colleagues randomly assigned women presenting for first-time IUD placement from 2012 to 2014 to either:
- ketorolac, a potent nonsteroidal anti-inflammatory drug (NSAID) (1-mL gluteal intramuscular injection of 30 mg of ketorolac) or
- saline (1-mL saline intramuscular injection).
The injection was given 30 minutes prior to IUD placement.
Pain associated with injection of the study drug, speculum and tenaculum placement, uterine sounding, IUD placement, and postinsertion pain were measured using a visual analog scale from 0 cm (no pain) to 10 cm (worst pain possible).
Of 67 participants (mean age, approximately 27 years; white race, 33%; African-American race, 33%; median parity, 1), pain was similar between ketorolac and placebo arms for all parameters except postinsertion pain, which was 0 cm and 1.3 cm for ketorolac and placebo, respectively, 15 minutes after placement (P<.001).
Although approximately 75% of participants reported that pain from the injection was “not as bad” as the pain from IUD placement, about 1 in 5 indicated that injection pain was equivalent to pain from IUD placement. Regardless of study group allocation, more than 90% of participants reported being satisfied or very satisfied with IUD placement overall, and more than 75% said they would recommend IUD placement to a friend.
More than 90% of women were satisfied with IUD placement, regardless of study allocation
As Ngo and colleagues observe, the analgesic effect of ketorolac peaks 1 to 2 hours after injection. This observation may explain why pain reduction was noted only after IUD insertion. Although ketorolac is not expensive, logistic considerations may make its routine use prior to IUD placement unrealistic in many ambulatory settings. Further, the great majority of participants (>90%) reported being satisfied with their IUD placement experience overall, regardless of study allocation.
Earlier studies suggesting that preplacement oral NSAIDs are ineffective in reducing placement pain involved the administration of analgesia in the clinic less than 1 hour before IUD insertion. I agree with Ngo and colleagues that future trials of oral NSAIDs should focus on administration of the medication prior to arrival at the clinic.
What this evidence means for practice
Findings from this randomized controlled trial provide only limited support for injection of an NSAID prior to IUD placement.
--Andrew M. Kaunitz, MD
Although the use of intrauterine devices (IUDs) is increasing, these highly effective contraceptives remain underutilized in the United States, compared with other developed countries. Concerns about pain with insertion represent one barrier to use.
In a double-blind trial, Ngo and colleagues randomly assigned women presenting for first-time IUD placement from 2012 to 2014 to either:
- ketorolac, a potent nonsteroidal anti-inflammatory drug (NSAID) (1-mL gluteal intramuscular injection of 30 mg of ketorolac) or
- saline (1-mL saline intramuscular injection).
The injection was given 30 minutes prior to IUD placement.
Pain associated with injection of the study drug, speculum and tenaculum placement, uterine sounding, IUD placement, and postinsertion pain were measured using a visual analog scale from 0 cm (no pain) to 10 cm (worst pain possible).
Of 67 participants (mean age, approximately 27 years; white race, 33%; African-American race, 33%; median parity, 1), pain was similar between ketorolac and placebo arms for all parameters except postinsertion pain, which was 0 cm and 1.3 cm for ketorolac and placebo, respectively, 15 minutes after placement (P<.001).
Although approximately 75% of participants reported that pain from the injection was “not as bad” as the pain from IUD placement, about 1 in 5 indicated that injection pain was equivalent to pain from IUD placement. Regardless of study group allocation, more than 90% of participants reported being satisfied or very satisfied with IUD placement overall, and more than 75% said they would recommend IUD placement to a friend.
More than 90% of women were satisfied with IUD placement, regardless of study allocation
As Ngo and colleagues observe, the analgesic effect of ketorolac peaks 1 to 2 hours after injection. This observation may explain why pain reduction was noted only after IUD insertion. Although ketorolac is not expensive, logistic considerations may make its routine use prior to IUD placement unrealistic in many ambulatory settings. Further, the great majority of participants (>90%) reported being satisfied with their IUD placement experience overall, regardless of study allocation.
Earlier studies suggesting that preplacement oral NSAIDs are ineffective in reducing placement pain involved the administration of analgesia in the clinic less than 1 hour before IUD insertion. I agree with Ngo and colleagues that future trials of oral NSAIDs should focus on administration of the medication prior to arrival at the clinic.
What this evidence means for practice
Findings from this randomized controlled trial provide only limited support for injection of an NSAID prior to IUD placement.
--Andrew M. Kaunitz, MD
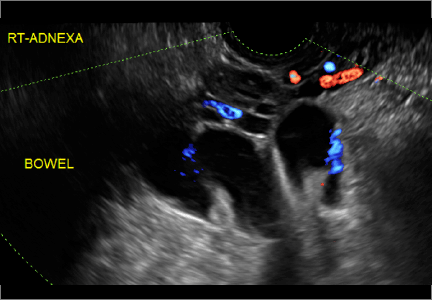
“Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts
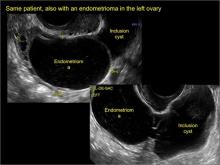
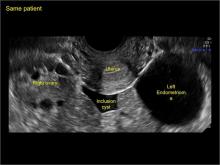
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
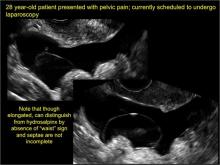
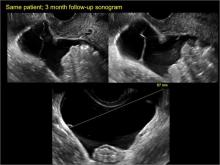
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
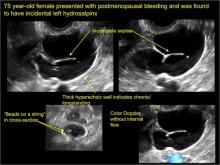
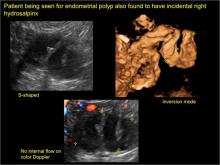
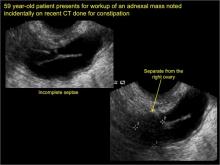
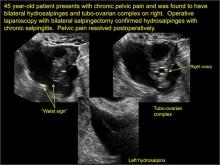
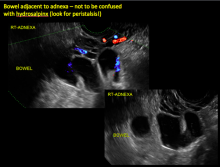
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
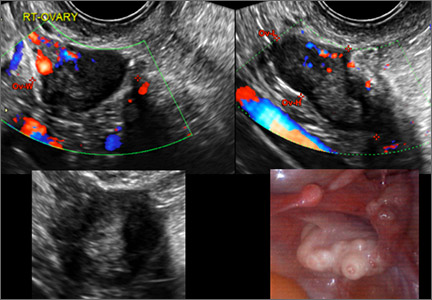
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
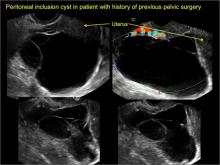
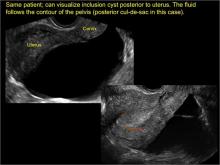
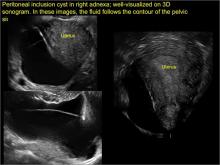
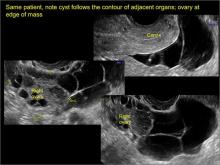
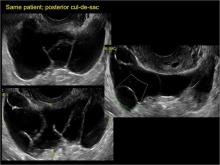
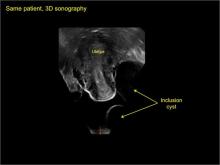
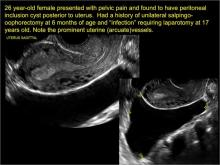
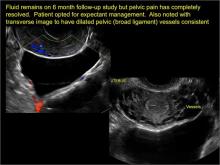
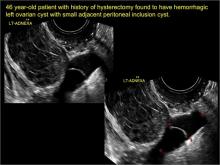
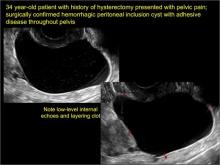
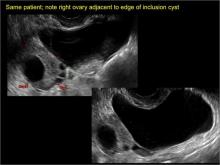
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
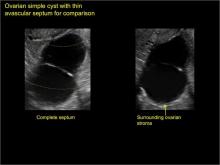
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
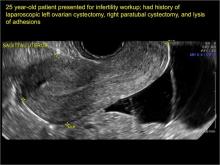
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
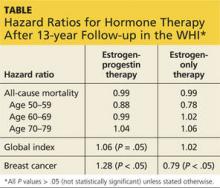
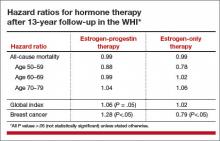
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
In This Article
- Angelina Jolie describes her surgeries
- Hormone therapy for previvors with intact breasts?
- When a patient refuses hormone therapy
Update on Menopause
“Critical Window” HT Timing Is Revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to receive conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, research published in the Journal of the American Medical Association in October 2013 details findings from a 13-year follow-up of WHI HT clinical trial participants and better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95-1.45) and 0.94 in the ET arm (95% CI, 0.78-1.14). In both arms, women given HT had reduced risk for vasomotor symptoms, hip fractures, and diabetes, and increased risk for stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 at baseline, the risk for cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk for breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11-1.48). In contrast, in the ET arm, a significantly reduced risk for breast cancer materialized (HR, 0.79; 95% CI, 0.65-0.97) (see Table).
To put into perspective the elevated risk for breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming one glass of wine daily and lower than the HR noted with two glasses daily.1
Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk for adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women ages 50 to 59 at baseline. In the EPT arm, the risk for MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risk for stroke, VTE, and gallbladder disease.
EPT increased the risk for breast cancer in all age-groups. However, the lower absolute risks for adverse events, and generally more favorable HRs for many outcomes, in younger women resulted in substantially lower rates of adverse events attributable to HT in the younger age-group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar).
REFERENCE
1. Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884-190.
Continue for ACOG Guidance on menopausal symptoms >>
ACOG Guidance on Menopausal Symptoms
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202-216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.1 Along with many of the clinicians reading this update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
“The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk-benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, the American College of Obstetricians and Gynecologists recommends against routine discontinuation of systemic estrogen at age 65 years. As with younger women, use of HT and estrogen therapy should be individualized, based on each woman’s risk-benefit ratio and clinical presentation.”
Three new options for menopausal HT
The ACOG Practice Bulletin describes three formulations for the treatment of menopausal symptoms that have recently become available:
• In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with the selective estrogen receptor modulator (SERM) bazedoxifene (20 mg).
• The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
• Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week, I encounter patients who have recently visited clinicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
“Because of a lack of FDA oversight, most compounded preparations have not undergone any rigorous clinical testing for either safety or efficacy, so the purity, potency, and quality of compounded preparations are a concern. In addition, both underdosage and overdosage are possible because of variable bioavailability and bioactivity. Evidence is lacking to support superiority claims of compounded bioidentical hormones over conventional menopausal HT…. Conventional HT is preferred, given the available data.”
REFERENCE
1. Geriatrics Care Online: Beers Pocket Card. www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed July 21, 2015.
Continue for Testosterone improves parameters of sexual function >>
Testosterone Improves Parameters of Sexual Function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612-623.
No formulation of testosterone is approved by the FDA for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.1
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0, 6.0, 12.5, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were five to six times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the two highest doses of testosterone.
REFERENCE
1. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
Continue to find out if HT is safe in statin users
Is HT Safe in Statin Users?
Berglind IA, Andersen M, Citarella A, Liet al. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376. Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publication of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women ages 40 to 74 who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of four years after beginning statins, until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks for mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk for CVD, compared with other estrogens1).
REFERENCE
1. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
“Critical Window” HT Timing Is Revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to receive conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, research published in the Journal of the American Medical Association in October 2013 details findings from a 13-year follow-up of WHI HT clinical trial participants and better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95-1.45) and 0.94 in the ET arm (95% CI, 0.78-1.14). In both arms, women given HT had reduced risk for vasomotor symptoms, hip fractures, and diabetes, and increased risk for stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 at baseline, the risk for cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk for breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11-1.48). In contrast, in the ET arm, a significantly reduced risk for breast cancer materialized (HR, 0.79; 95% CI, 0.65-0.97) (see Table).
To put into perspective the elevated risk for breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming one glass of wine daily and lower than the HR noted with two glasses daily.1
Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk for adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women ages 50 to 59 at baseline. In the EPT arm, the risk for MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risk for stroke, VTE, and gallbladder disease.
EPT increased the risk for breast cancer in all age-groups. However, the lower absolute risks for adverse events, and generally more favorable HRs for many outcomes, in younger women resulted in substantially lower rates of adverse events attributable to HT in the younger age-group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar).
REFERENCE
1. Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884-190.
Continue for ACOG Guidance on menopausal symptoms >>
ACOG Guidance on Menopausal Symptoms
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202-216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.1 Along with many of the clinicians reading this update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
“The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk-benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, the American College of Obstetricians and Gynecologists recommends against routine discontinuation of systemic estrogen at age 65 years. As with younger women, use of HT and estrogen therapy should be individualized, based on each woman’s risk-benefit ratio and clinical presentation.”
Three new options for menopausal HT
The ACOG Practice Bulletin describes three formulations for the treatment of menopausal symptoms that have recently become available:
• In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with the selective estrogen receptor modulator (SERM) bazedoxifene (20 mg).
• The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
• Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week, I encounter patients who have recently visited clinicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
“Because of a lack of FDA oversight, most compounded preparations have not undergone any rigorous clinical testing for either safety or efficacy, so the purity, potency, and quality of compounded preparations are a concern. In addition, both underdosage and overdosage are possible because of variable bioavailability and bioactivity. Evidence is lacking to support superiority claims of compounded bioidentical hormones over conventional menopausal HT…. Conventional HT is preferred, given the available data.”
REFERENCE
1. Geriatrics Care Online: Beers Pocket Card. www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed July 21, 2015.
Continue for Testosterone improves parameters of sexual function >>
Testosterone Improves Parameters of Sexual Function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612-623.
No formulation of testosterone is approved by the FDA for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.1
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0, 6.0, 12.5, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were five to six times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the two highest doses of testosterone.
REFERENCE
1. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
Continue to find out if HT is safe in statin users
Is HT Safe in Statin Users?
Berglind IA, Andersen M, Citarella A, Liet al. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376. Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publication of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women ages 40 to 74 who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of four years after beginning statins, until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks for mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk for CVD, compared with other estrogens1).
REFERENCE
1. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
“Critical Window” HT Timing Is Revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to receive conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, research published in the Journal of the American Medical Association in October 2013 details findings from a 13-year follow-up of WHI HT clinical trial participants and better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95-1.45) and 0.94 in the ET arm (95% CI, 0.78-1.14). In both arms, women given HT had reduced risk for vasomotor symptoms, hip fractures, and diabetes, and increased risk for stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 at baseline, the risk for cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk for breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11-1.48). In contrast, in the ET arm, a significantly reduced risk for breast cancer materialized (HR, 0.79; 95% CI, 0.65-0.97) (see Table).
To put into perspective the elevated risk for breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming one glass of wine daily and lower than the HR noted with two glasses daily.1
Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk for adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women ages 50 to 59 at baseline. In the EPT arm, the risk for MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risk for stroke, VTE, and gallbladder disease.
EPT increased the risk for breast cancer in all age-groups. However, the lower absolute risks for adverse events, and generally more favorable HRs for many outcomes, in younger women resulted in substantially lower rates of adverse events attributable to HT in the younger age-group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar).
REFERENCE
1. Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884-190.
Continue for ACOG Guidance on menopausal symptoms >>
ACOG Guidance on Menopausal Symptoms
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202-216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.1 Along with many of the clinicians reading this update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
“The decision to continue HT should be individualized and be based on a woman’s symptoms and the risk-benefit ratio, regardless of age. Because some women aged 65 years and older may continue to need systemic HT for the management of vasomotor symptoms, the American College of Obstetricians and Gynecologists recommends against routine discontinuation of systemic estrogen at age 65 years. As with younger women, use of HT and estrogen therapy should be individualized, based on each woman’s risk-benefit ratio and clinical presentation.”
Three new options for menopausal HT
The ACOG Practice Bulletin describes three formulations for the treatment of menopausal symptoms that have recently become available:
• In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with the selective estrogen receptor modulator (SERM) bazedoxifene (20 mg).
• The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
• Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week, I encounter patients who have recently visited clinicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
“Because of a lack of FDA oversight, most compounded preparations have not undergone any rigorous clinical testing for either safety or efficacy, so the purity, potency, and quality of compounded preparations are a concern. In addition, both underdosage and overdosage are possible because of variable bioavailability and bioactivity. Evidence is lacking to support superiority claims of compounded bioidentical hormones over conventional menopausal HT…. Conventional HT is preferred, given the available data.”
REFERENCE
1. Geriatrics Care Online: Beers Pocket Card. www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed July 21, 2015.
Continue for Testosterone improves parameters of sexual function >>
Testosterone Improves Parameters of Sexual Function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612-623.
No formulation of testosterone is approved by the FDA for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.1
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0, 6.0, 12.5, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were five to six times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the two highest doses of testosterone.
REFERENCE
1. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
Continue to find out if HT is safe in statin users
Is HT Safe in Statin Users?
Berglind IA, Andersen M, Citarella A, Liet al. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376. Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publication of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women ages 40 to 74 who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of four years after beginning statins, until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks for mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk for CVD, compared with other estrogens1).
REFERENCE
1. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
Does adjuvant oophorectomy improve survival in BRCA1 or BRCA2 mutation carriers with breast cancer?
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
Atypical hyperplasia of the breast: Cancer risk-reduction strategies
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
In this Article
- Dr. Samiian: How I counsel patients about their management options
- Mammography shows microcalcifications
2015 Update on menopause
As new options for managing menopausal symptoms emerge, so do data on their efficacy and safety. In this article, I highlight the following publications:
- long-term follow-up data from the Women’s Health Initiative (WHI) on the benefits and risks of hormone therapy (HT)
- a randomized trial of testosterone enanthate to improve sexual function among hysterectomized women
- guidance from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms, including advice on individualization of therapy for older women
- a Swedish study on concomitant use of HT and statins.
After long-term follow-up of WHI participants, a “critical window” of HT timing is revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, an October 2013 publication from the Journal of the American Medical Association, which details 13-year follow-up of WHI HT clinical trial participants, better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95–1.45) and 0.94 in the ET arm (95% CI, 0.78–1.14). In both arms, women given HT had reduced risks of vasomotor symptoms, hip fractures, and diabetes, and increased risks of stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 years at baseline, the risk of cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk of breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11–1.48). In contrast, in the ET arm, a significantly reduced risk of breast cancer materialized (HR, 0.79; 95% CI, 0.65–0.97) (TABLE).
To put into perspective the elevated risk of breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming 1 glass of wine daily and lower than the HR noted with 2 glasses daily.1 Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk of adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women aged 50 to 59 years at baseline. In the EPT arm, the risk of MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risks of stroke, VTE, and gallbladder disease.
EPT increased the risk of breast cancer in all age groups. However, the lower absolute risks of adverse events in younger women, together with the generally more favorable HRs for many outcomes in the younger women, resulted in substantially lower rates of adverse events attributable to HT in the younger age group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar below).
What this EVIDENCE means for practice
Long-term follow-up of women who participated in the WHI clarifies the benefit-risk profile of systemic HT, underscoring that the benefit-risk ratio is greatest in younger menopausal women.
Because the safety of HT is greater in women nearer the onset of menopause, as well as in those at lower baseline risk of cardiovascular disease (CVD), individualized risk assessment may improve the benefit-risk profile and safety of HT. One approach to decision-making for women with bothersome menopausal symptoms is the MenoPro app, a free mobile app from the North American Menopause Society, with modes for both clinicians and patients.
Further evidence that HT is safe when initiated soon after menopause
Tuomikoski P, Lyytinen H, Korhonen P, et al. Coronary heart disease mortality and hormone therapy before and after the Women’s Health Initiative. Obstet Gynecol. 2014;124(5):947–953.
In Finland, all deaths are recorded in a national register, in which particular attention is paid to accurately classifying those thought to result from coronary heart disease (CHD). In addition, since 1994, all HT users have been included in a national health insurance database, enabling detailed assessment of HT use and coronary artery disease. Investigators assessed CHD mortality from 1995 to 2009 in more than 290,000 HT users, comparing them with the background population matched for year and age.
Use of HT was associated with reductions in the CHD mortality rate of 18% to 29% (for ≤1 year of use) and 43% to 54% (for 1–8 years of use). Similar trends were noted for EPT and ET. The HT-associated protection against CHD mortality was more pronounced in users younger than 60.
Tuomikoski and colleagues concluded, and I concur, that the observational nature of their data does not allow us to recommend HT specifically to prevent CHD. Nonetheless, these findings, along with long-term follow-up data from the WHI, make the case that, for menopausal women who are younger than 60 or within 10 years of the onset of menopause, clinicians may consider initiating HT to treat bothersome vasomotor symptoms, a safe strategy with respect to CHD.
—Andrew M. Kaunitz, MD
In hysterectomized women, supraphysiologic doses of testosterone improve parameters of sexual function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612–623.
No formulation of testosterone is approved by the US Food and Drug Administration (FDA) for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.2
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0 mg, 6.0 mg, 12.5 mg, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were 5 to 6 times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the 2 highest doses of testosterone.
What this EVIDENCE means for practice
Although this well-executed study was small and short-term, it confirms that, in menopausal women receiving estrogen, testosterone can enhance parameters of sexuality. It is unfortunate that the dose needed to achieve this benefit results in markedly supraphysiologic serum testosterone levels.
One important caveat raised by this trial: It did not specifically recruit participants with low sexual desire. Therefore, it remains unknown whether lower doses of testosterone might provide benefits in women with low baseline libido. Regrettably, no randomized trials have addressed the long-term benefits and risks of use of testosterone among menopausal women.
ACOG offers valuable guidance on management of menopausal symptoms
ACOG Practice Bulletin No. 141: management of menopausal symptoms. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(1):202–216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65 years
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.3 Along with many of the clinicians reading this Update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65 years. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
Three new options for menopausal HT
The ACOG Practice Bulletin describes 3 formulations for the treatment of menopausal symptoms that have recently become available:
- In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with 20 mg of the selective estrogen receptor modulator (SERM) bazedoxifene.
- The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
- Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week I encounter patients who have recently visited physicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
What this EVIDENCE means for practice
ACOG’s Practice Bulletin provides useful guidance for clinicians regarding treatment of menopausal symptoms. Besides clarifying that systemic HT should not be arbitrarily discontinued at age 65 and that FDA-approved HT is preferable to compounded HT, this publication details newer (including nonhormonal) formulations for treating menopausal symptoms as well as traditional HT formulations, including useful dosing information.
Is menopausal HT safe in statin users?
Berglind IA, Andersen M, Citarella A, Linder M, Sundström A, Kieler H. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376.
Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publications of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women 40 to 74 years old who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of 4 years after beginning statins until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62 years, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks of mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk of CVD, compared with other estrogens4).
What this EVIDENCE means for practice
This important study provides strong evidence that, for menopausal women with bothersome vasomotor symptoms or an elevated risk for osteoporosis, concomitant use of statins should not be considered a contraindication to HT.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1190.
2. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
3. Geriatrics Care Online: Beers Pocket Card. http://www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed May 16, 2015.
4. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
As new options for managing menopausal symptoms emerge, so do data on their efficacy and safety. In this article, I highlight the following publications:
- long-term follow-up data from the Women’s Health Initiative (WHI) on the benefits and risks of hormone therapy (HT)
- a randomized trial of testosterone enanthate to improve sexual function among hysterectomized women
- guidance from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms, including advice on individualization of therapy for older women
- a Swedish study on concomitant use of HT and statins.
After long-term follow-up of WHI participants, a “critical window” of HT timing is revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, an October 2013 publication from the Journal of the American Medical Association, which details 13-year follow-up of WHI HT clinical trial participants, better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95–1.45) and 0.94 in the ET arm (95% CI, 0.78–1.14). In both arms, women given HT had reduced risks of vasomotor symptoms, hip fractures, and diabetes, and increased risks of stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 years at baseline, the risk of cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk of breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11–1.48). In contrast, in the ET arm, a significantly reduced risk of breast cancer materialized (HR, 0.79; 95% CI, 0.65–0.97) (TABLE).
To put into perspective the elevated risk of breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming 1 glass of wine daily and lower than the HR noted with 2 glasses daily.1 Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk of adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women aged 50 to 59 years at baseline. In the EPT arm, the risk of MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risks of stroke, VTE, and gallbladder disease.
EPT increased the risk of breast cancer in all age groups. However, the lower absolute risks of adverse events in younger women, together with the generally more favorable HRs for many outcomes in the younger women, resulted in substantially lower rates of adverse events attributable to HT in the younger age group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar below).
What this EVIDENCE means for practice
Long-term follow-up of women who participated in the WHI clarifies the benefit-risk profile of systemic HT, underscoring that the benefit-risk ratio is greatest in younger menopausal women.
Because the safety of HT is greater in women nearer the onset of menopause, as well as in those at lower baseline risk of cardiovascular disease (CVD), individualized risk assessment may improve the benefit-risk profile and safety of HT. One approach to decision-making for women with bothersome menopausal symptoms is the MenoPro app, a free mobile app from the North American Menopause Society, with modes for both clinicians and patients.
Further evidence that HT is safe when initiated soon after menopause
Tuomikoski P, Lyytinen H, Korhonen P, et al. Coronary heart disease mortality and hormone therapy before and after the Women’s Health Initiative. Obstet Gynecol. 2014;124(5):947–953.
In Finland, all deaths are recorded in a national register, in which particular attention is paid to accurately classifying those thought to result from coronary heart disease (CHD). In addition, since 1994, all HT users have been included in a national health insurance database, enabling detailed assessment of HT use and coronary artery disease. Investigators assessed CHD mortality from 1995 to 2009 in more than 290,000 HT users, comparing them with the background population matched for year and age.
Use of HT was associated with reductions in the CHD mortality rate of 18% to 29% (for ≤1 year of use) and 43% to 54% (for 1–8 years of use). Similar trends were noted for EPT and ET. The HT-associated protection against CHD mortality was more pronounced in users younger than 60.
Tuomikoski and colleagues concluded, and I concur, that the observational nature of their data does not allow us to recommend HT specifically to prevent CHD. Nonetheless, these findings, along with long-term follow-up data from the WHI, make the case that, for menopausal women who are younger than 60 or within 10 years of the onset of menopause, clinicians may consider initiating HT to treat bothersome vasomotor symptoms, a safe strategy with respect to CHD.
—Andrew M. Kaunitz, MD
In hysterectomized women, supraphysiologic doses of testosterone improve parameters of sexual function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612–623.
No formulation of testosterone is approved by the US Food and Drug Administration (FDA) for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.2
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0 mg, 6.0 mg, 12.5 mg, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were 5 to 6 times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the 2 highest doses of testosterone.
What this EVIDENCE means for practice
Although this well-executed study was small and short-term, it confirms that, in menopausal women receiving estrogen, testosterone can enhance parameters of sexuality. It is unfortunate that the dose needed to achieve this benefit results in markedly supraphysiologic serum testosterone levels.
One important caveat raised by this trial: It did not specifically recruit participants with low sexual desire. Therefore, it remains unknown whether lower doses of testosterone might provide benefits in women with low baseline libido. Regrettably, no randomized trials have addressed the long-term benefits and risks of use of testosterone among menopausal women.
ACOG offers valuable guidance on management of menopausal symptoms
ACOG Practice Bulletin No. 141: management of menopausal symptoms. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(1):202–216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65 years
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.3 Along with many of the clinicians reading this Update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65 years. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
Three new options for menopausal HT
The ACOG Practice Bulletin describes 3 formulations for the treatment of menopausal symptoms that have recently become available:
- In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with 20 mg of the selective estrogen receptor modulator (SERM) bazedoxifene.
- The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
- Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week I encounter patients who have recently visited physicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
What this EVIDENCE means for practice
ACOG’s Practice Bulletin provides useful guidance for clinicians regarding treatment of menopausal symptoms. Besides clarifying that systemic HT should not be arbitrarily discontinued at age 65 and that FDA-approved HT is preferable to compounded HT, this publication details newer (including nonhormonal) formulations for treating menopausal symptoms as well as traditional HT formulations, including useful dosing information.
Is menopausal HT safe in statin users?
Berglind IA, Andersen M, Citarella A, Linder M, Sundström A, Kieler H. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376.
Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publications of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women 40 to 74 years old who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of 4 years after beginning statins until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62 years, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks of mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk of CVD, compared with other estrogens4).
What this EVIDENCE means for practice
This important study provides strong evidence that, for menopausal women with bothersome vasomotor symptoms or an elevated risk for osteoporosis, concomitant use of statins should not be considered a contraindication to HT.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
As new options for managing menopausal symptoms emerge, so do data on their efficacy and safety. In this article, I highlight the following publications:
- long-term follow-up data from the Women’s Health Initiative (WHI) on the benefits and risks of hormone therapy (HT)
- a randomized trial of testosterone enanthate to improve sexual function among hysterectomized women
- guidance from the American College of Obstetricians and Gynecologists (ACOG) on the management of menopausal symptoms, including advice on individualization of therapy for older women
- a Swedish study on concomitant use of HT and statins.
After long-term follow-up of WHI participants, a “critical window” of HT timing is revealed
Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
After the initial 2002 publication of findings from the WHI trial of women with an intact uterus who were randomized to conjugated equine estrogens and medroxyprogesterone acetate or placebo, prominent news headlines claimed that HT causes myocardial infarction (MI) and breast cancer. As a result, millions of women worldwide stopped taking HT. A second impact of the report: Many clinicians became reluctant to prescribe HT.
Although it generated far less media attention, an October 2013 publication from the Journal of the American Medical Association, which details 13-year follow-up of WHI HT clinical trial participants, better informs clinicians and our patients about HT’s safety profile.
During the WHI intervention phase, absolute risks were modest
Although HT was associated with a multifaceted pattern of benefits and risks in both the estrogen-progestin therapy (EPT) and estrogen-only therapy (ET) arms of the WHI, absolute risks, as reflected in an increase or decrease in the number of cases per 10,000 women treated per year, were modest.
For example, the hazard ratio (HR) for coronary heart disease (CHD) during the intervention phase, during which participants were given HT or placebo (mean 5.2 years for EPT and 6.8 years for ET) was 1.18 in the EPT arm (95% confidence interval [CI], 0.95–1.45) and 0.94 in the ET arm (95% CI, 0.78–1.14). In both arms, women given HT had reduced risks of vasomotor symptoms, hip fractures, and diabetes, and increased risks of stroke, venous thromboembolism (VTE), and gallbladder disease, compared with women receiving placebo.
The results for breast cancer differed markedly between arms. During the intervention period, an elevated risk was observed with EPT while a borderline reduced risk was observed with ET.
Among participants older than 65 years at baseline, the risk of cognitive decline was increased in the EPT arm but not in the ET arm.
Post intervention, most risks and benefits attenuated
An elevation in the risk of breast cancer persisted in the EPT arm (cumulative HR over 13 years, 1.28; 95% CI, 1.11–1.48). In contrast, in the ET arm, a significantly reduced risk of breast cancer materialized (HR, 0.79; 95% CI, 0.65–0.97) (TABLE).
To put into perspective the elevated risk of breast cancer observed among women randomly allocated to EPT, the attributable risk is less than 1 additional case of breast cancer diagnosed per 1,000 EPT users annually. Another way to frame this elevated risk: An HR of 1.28 is slightly higher than the HR conferred by consuming 1 glass of wine daily and lower than the HR noted with 2 glasses daily.1 Overall, results tended to be more favorable for ET than for EPT. Neither type of HT affected overall mortality rates.
Age differences come to the fore
The WHI findings demonstrate a lower absolute risk of adverse events with HT in younger versus older participants. In addition, age and time since menopause appeared to affect many of the HRs observed in the trial. In the ET arm, more favorable results for all-cause mortality, MI, colorectal cancer, and the global index (CHD, invasive breast cancer, pulmonary embolism, colorectal cancer, and endometrial cancer) were observed in women aged 50 to 59 years at baseline. In the EPT arm, the risk of MI was elevated only in women more than 10 years past the onset of menopause at baseline. Both HT regimens, however, were associated with increased risks of stroke, VTE, and gallbladder disease.
EPT increased the risk of breast cancer in all age groups. However, the lower absolute risks of adverse events in younger women, together with the generally more favorable HRs for many outcomes in the younger women, resulted in substantially lower rates of adverse events attributable to HT in the younger age group, compared with older women.
As far as CHD is concerned, the impact of age (or time since menopause) on the vascular response to HT in women and in nonhuman models has generated support for a “critical window” or timing hypothesis, which postulates that estrogen reduces the development of early stages of atherosclerosis while causing plaque destabilization and other adverse effects when advanced atherosclerotic lesions are present. Recent studies from Scandinavia provide additional support for this hypothesis (see the sidebar below).
What this EVIDENCE means for practice
Long-term follow-up of women who participated in the WHI clarifies the benefit-risk profile of systemic HT, underscoring that the benefit-risk ratio is greatest in younger menopausal women.
Because the safety of HT is greater in women nearer the onset of menopause, as well as in those at lower baseline risk of cardiovascular disease (CVD), individualized risk assessment may improve the benefit-risk profile and safety of HT. One approach to decision-making for women with bothersome menopausal symptoms is the MenoPro app, a free mobile app from the North American Menopause Society, with modes for both clinicians and patients.
Further evidence that HT is safe when initiated soon after menopause
Tuomikoski P, Lyytinen H, Korhonen P, et al. Coronary heart disease mortality and hormone therapy before and after the Women’s Health Initiative. Obstet Gynecol. 2014;124(5):947–953.
In Finland, all deaths are recorded in a national register, in which particular attention is paid to accurately classifying those thought to result from coronary heart disease (CHD). In addition, since 1994, all HT users have been included in a national health insurance database, enabling detailed assessment of HT use and coronary artery disease. Investigators assessed CHD mortality from 1995 to 2009 in more than 290,000 HT users, comparing them with the background population matched for year and age.
Use of HT was associated with reductions in the CHD mortality rate of 18% to 29% (for ≤1 year of use) and 43% to 54% (for 1–8 years of use). Similar trends were noted for EPT and ET. The HT-associated protection against CHD mortality was more pronounced in users younger than 60.
Tuomikoski and colleagues concluded, and I concur, that the observational nature of their data does not allow us to recommend HT specifically to prevent CHD. Nonetheless, these findings, along with long-term follow-up data from the WHI, make the case that, for menopausal women who are younger than 60 or within 10 years of the onset of menopause, clinicians may consider initiating HT to treat bothersome vasomotor symptoms, a safe strategy with respect to CHD.
—Andrew M. Kaunitz, MD
In hysterectomized women, supraphysiologic doses of testosterone improve parameters of sexual function
Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance, and physical function in a randomized trial. Menopause. 2014;21(6):612–623.
No formulation of testosterone is approved by the US Food and Drug Administration (FDA) for use in women. Nonetheless, in the United States, many menopausal women hoping to boost their sexual desire are prescribed, off-label, testosterone formulations indicated for use in men, as well as compounded formulations.2
Investigators randomly allocated women who had undergone hysterectomy to 12 weeks of transdermal estradiol followed by 24 weekly intramuscular injections of placebo or testosterone enanthate at doses of 3.0 mg, 6.0 mg, 12.5 mg, or 25.0 mg while continuing estrogen. At the outset of the trial, all women had serum free testosterone levels below the range for healthy premenopausal women.
Among the 62 women who received testosterone, serum testosterone levels increased in a dose-related fashion. Among those allocated to the highest dose, serum total testosterone levels at 24 weeks were 5 to 6 times higher than values in healthy premenopausal women. Compared with women who received placebo, those who received the highest testosterone dose had better measures of sexual desire, arousal, and frequency of sexual activity. Excess hair growth was significantly more common in women who received the 2 highest doses of testosterone.
What this EVIDENCE means for practice
Although this well-executed study was small and short-term, it confirms that, in menopausal women receiving estrogen, testosterone can enhance parameters of sexuality. It is unfortunate that the dose needed to achieve this benefit results in markedly supraphysiologic serum testosterone levels.
One important caveat raised by this trial: It did not specifically recruit participants with low sexual desire. Therefore, it remains unknown whether lower doses of testosterone might provide benefits in women with low baseline libido. Regrettably, no randomized trials have addressed the long-term benefits and risks of use of testosterone among menopausal women.
ACOG offers valuable guidance on management of menopausal symptoms
ACOG Practice Bulletin No. 141: management of menopausal symptoms. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(1):202–216.
Despite findings from new studies, optimal management of menopausal symptoms remains controversial. In January 2014, ACOG issued guidance regarding conventional systemic and vaginal HT, recently approved treatments, and compounded HT.
For the management of vasomotor symptoms, ACOG indicated that systemic HT (including oral and transdermal routes), alone or combined with a progestin, is the most effective treatment for bothersome menopausal vasomotor symptoms. The ACOG Practice Bulletin also pointed out that systemic EPT increases the risk for VTE and breast cancer and that, compared with oral estrogen, transdermal estrogen may carry a lower risk for VTE.
Some insurers deny coverage of HT for women older than 65 years
A classification of medications from the American Geriatrics Society known as “the Beers List” [the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults] includes oral and transdermal estrogen, with or without a progestin.3 Along with many of the clinicians reading this Update, I routinely receive notices from insurance companies that, based on the Beers List, they will no longer provide reimbursement for systemic HT in patients who are older than 65 years. In this regard, I believe that one of the most important components of ACOG’s Practice Bulletin is the following text:
Three new options for menopausal HT
The ACOG Practice Bulletin describes 3 formulations for the treatment of menopausal symptoms that have recently become available:
- In women with a uterus and with bothersome vasomotor symptoms, an alternative to EPT is oral tablets combining conjugated equine estrogen (0.45 mg) with 20 mg of the selective estrogen receptor modulator (SERM) bazedoxifene.
- The oral SERM ospemifene (60 mg) is effective for relief of dyspareunia associated with vulvovaginal atrophy (also known as genitourinary syndrome of menopause).
- Paroxetine mesylate (7.5 mg) is the only FDA-approved nonhormonal formulation for management of vasomotor symptoms and is dosed lower than regimens used to treat psychiatric conditions.
Steer patients clear of compounded formulations
Every week I encounter patients who have recently visited physicians who prescribe and sell compounded bioidentical hormones. In addressing this issue, ACOG provides a useful service to women and their clinicians:
What this EVIDENCE means for practice
ACOG’s Practice Bulletin provides useful guidance for clinicians regarding treatment of menopausal symptoms. Besides clarifying that systemic HT should not be arbitrarily discontinued at age 65 and that FDA-approved HT is preferable to compounded HT, this publication details newer (including nonhormonal) formulations for treating menopausal symptoms as well as traditional HT formulations, including useful dosing information.
Is menopausal HT safe in statin users?
Berglind IA, Andersen M, Citarella A, Linder M, Sundström A, Kieler H. Hormone therapy and risk of cardiovascular outcomes and mortality in women treated with statins. Menopause. 2015;22(4):369–376.
Hodis HN, Mack WJ. Hormone therapy and risk of all-cause mortality in women treated with statins [comment]. Menopause. 2015;22(4):363–364.
Since the initial publications of findings from the WHI, clinicians have been cautioned not to prescribe menopausal HT in women at elevated risk for CVD. In this study from Sweden, investigators enrolled women 40 to 74 years old who initiated statin use between 2006 and 2007 due to known CVD (secondary prevention) or in the absence of known CVD (primary prevention). Women were followed for a mean of 4 years after beginning statins until the end of 2011.
Of 40,958 statin users, 7% used HT (mean age of HT users and nonusers was 61 and 62 years, respectively). Overall, 70% of statin use was for primary prevention. Deaths from CVD occurred in 5 and 18 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.38). All-cause mortality occurred in 33 and 87 patients per 10,000 person-years among HT users and nonusers, respectively (HR, 0.53). These reduced risks of mortality noted in women who used concomitant statins achieved statistical significance. Whether statins were used for primary or secondary prevention, the incidence of cardiovascular events was similar in HT users and nonusers.
Why these findings diverge from those of the WHI
The findings of this large prospective cohort study are consistent with findings from other large observational studies—though they diverge from WHI findings. As Berglind and colleagues note, few WHI participants used statins at baseline. Also in contrast with the WHI, in which all HT was based on conjugated estrogen, all HT users in this Swedish study used oral or transdermal estradiol, as conjugated estrogen is not available in Sweden (and appears to be associated with an elevated risk of CVD, compared with other estrogens4).
What this EVIDENCE means for practice
This important study provides strong evidence that, for menopausal women with bothersome vasomotor symptoms or an elevated risk for osteoporosis, concomitant use of statins should not be considered a contraindication to HT.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1190.
2. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
3. Geriatrics Care Online: Beers Pocket Card. http://www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed May 16, 2015.
4. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
1. Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1190.
2. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477–486.
3. Geriatrics Care Online: Beers Pocket Card. http://www.americangeriatrics.org/files/documents/beers/PrintableBeersPocketCard.pdf. Accessed May 16, 2015.
4. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
In This Article
- Testosterone for low desire?
- ACOG on the management of menopausal symptoms
- Is concomitant use of hormone therapy and statins a good idea?