User login
Pretrial screening panels: Do they reduce frivolous claims?
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.
So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
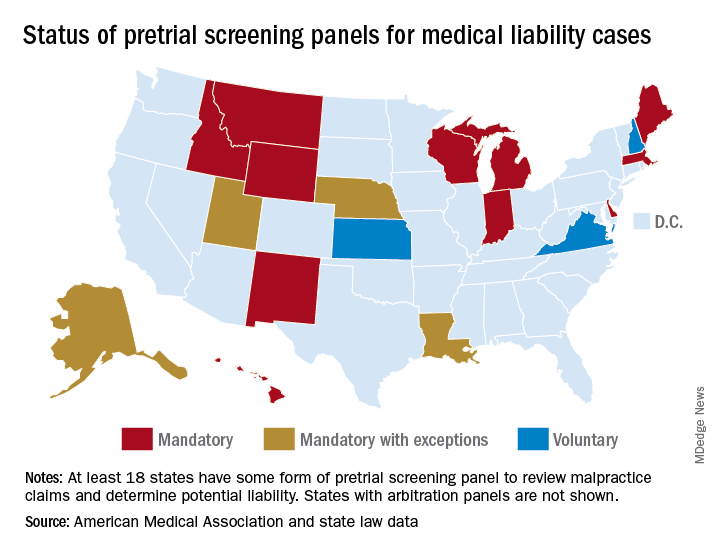
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”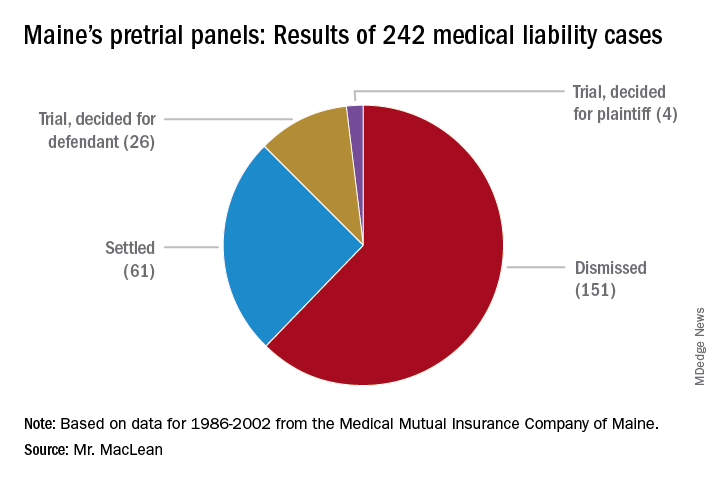
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
Courts temporarily block Title X changes
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
agallegos@mdedge.com
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
agallegos@mdedge.com
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
agallegos@mdedge.com
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Report: Part B funds stable, hospital trust running out
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”
Malpractice: Diagnostic errors top allegation involving children
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.
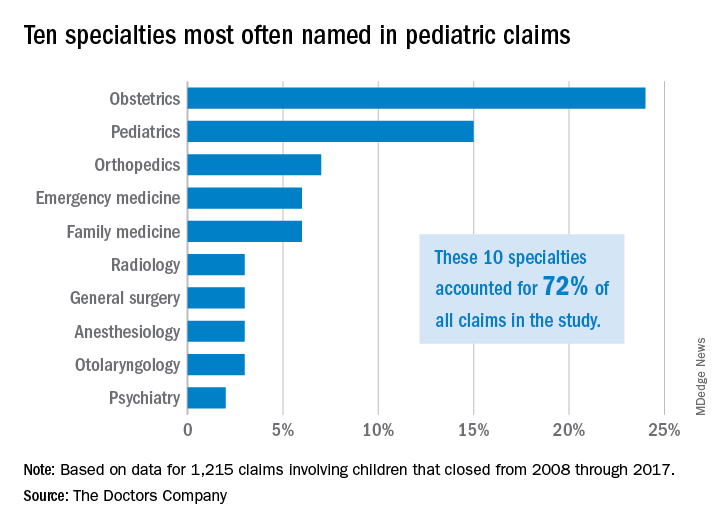
Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
CDC clarifies opioid prescribing guidelines in cancer, sickle cell disease
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Malpractice: More lawsuits does not equal more relocations
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Psychiatrists sue ABPN over its MOC process
Two psychiatrists are suing the American Board of Psychiatry and Neurology (ABPN) over its maintenance of certification program, contending that the board has created a monopoly over the MOC market and that its process violates antitrust laws.
The lawsuit, filed March 6 in U.S. District Court for the Northern District of Illinois, alleges that the board is illegally tying its initial certification product to its MOC product, and that ABPN is using anticompetitive actions to prevent and limit competition from new MOC providers. The suit is filed as a class action on behalf of all psychiatrists and neurologists required by ABPN to buy MOC to maintain their certifications. The plaintiffs seek damages and injunctive relief arising from the alleged violations.
In an interview, ABPN President and CEO Larry R. Faulkner, MD, said the legal challenge has no merit.
“The ABPN intends to defend itself vigorously and anticipates that, like other similar cases previously filed against the American Board of Medical Specialties and other member boards, this lawsuit will be dismissed.”
Plaintiff Emily Lazarou, MD, an Odessa, Fla.-based psychiatrist, did not return messages seeking comment. The other plaintiff, Aafaque Akhter, MD, a psychiatrist based in Norton, Mass., declined to comment at the advice of his attorneys. In a LinkedIn post, Dr. Akhter wrote that he filed the class action to put an end to MOC.
“This moneymaking operation has to be abolished,” he wrote in the post. “MOC has to go.
The 35-page complaint against the ABPN contends that the organization’s antitrust and monopolistic actions have resulted in overly burdensome conditions for physicians forced to buy MOC, and that the board’s requirements constrain the supply of psychiatrists and neurologists, thereby harming competition. The ABPN has made tens of millions of dollars in MOC revenue from psychiatrists and neurologists, the suit alleges, while physicians face countless hours away from their practice and families in order to meet MOC demands, to the physicians’ financial detriment. The challenge also asserts that ABPN’s grandfather clause – which exempts from MOC physicians who received initial certifications prior to Oct. 1, 1994 – discriminates against younger physicians, women, and persons of color, all of whom are underrepresented in the group of psychiatrists and neurologists grandfathered by ABPN.
The lawsuit details how MOC has personally and professionally affected the plaintiffs. In 2017, Dr. Lazarou, a forensic psychiatrist who also practices telepsychiatry, asked the ABPN in advance for a private room during her upcoming 10-year MOC examination. At the time, Dr. Lazarou was nursing her twin newborns and needed the private room to pump, according to the lawsuit. The suit alleges that ABPN would not make an accommodation for Dr. Lazarou to pump at the testing site; however, as a professional courtesy, board officials allowed her to travel to a test center farther away that had private rooms. Because the distance required Dr. Lazarou to be away from her newborns for an extended period, she did not take the MOC examination, and her ABPN certification lapsed, according to the suit. Because of the lapse, she is no longer able to practice telepsychiatry, which has resulted in a loss of income and to the detriment of patients who need telepsychiatric care, she claims in the lawsuit.
Dr. Akhter, who practices addiction medicine, was informed in August 2018 that he was selected for a random audit of his ABPN MOC activities. During the audit, officials informed Dr. Akhter that the continuing medical education (CME) requirement for completion of his 3-year continuance maintenance of certification (C-MOC) cycle had not been met, according to the suit. As part of the 3-year C-MOC cycle, physicians are required to complete 90 CME credits, including 24 credits for self-assessment (SA) activities. ABPN defines SA activities as a specific type of CME activity that help physicians recognize their current knowledge base in order to identify specific topics for gaining further knowledge. Although Dr. Akhter had obtained a subspecialty certification from the American Board of Preventive Medicine, including completion of 60 CME credits that included SA activities, the board determined that those efforts did not fulfill Dr. Akhter’s SA CME requirements. The board denied his request to recognize the 60 CME credits as SA CME credits and also refused to give him SA CME credits for obtaining his subspecialty certification, the lawsuit says. He now is listed on the ABPN website as “Not Meeting MOC Requirements.”
The ABPN has not yet responded to the lawsuit. Defendants generally have 30 days from the time a challenge is filed to respond, unless they request an extension. Court documents show that an attorney for ABPN entered his appearance on March 20.
The ABPN lawsuit comes on the heels of a similar class action filed last year by a group of internists against the American Board of Internal Medicine (ABIM) over its MOC process. That lawsuit, filed Dec. 6 in Pennsylvania district court, contends that the ABIM is charging inflated monopoly prices for maintaining certification and that the organization is violating antitrust laws. In a motion filed March 18, attorneys for the ABIM asked the court to dismiss the suit for failure to state a valid claim. A third lawsuit that levies similar allegations against the Board of Radiology was filed in late February. All three lawsuits are being funded by the advocacy organization Practicing Physicians of America, through a GoFundMe campaign.
Richard Rosin, MD, a geriatric psychiatrist based in Vancouver, said in an interview that he welcomes and applauds the lawsuit against ABPN. He hopes that the legal challenge will put a spotlight on the board’s activities and compel administrators to answer questions about the MOC process that have gone unanswered in the past.
“If it is successful, I think the impact will be dramatic,” said Dr. Rosin, an outspoken critic of the ABPN’s MOC process. “They’ll essentially have to disband the [MOC] program.”
Paul G. Mathew, MD, a Boston-based neurologist, said he was not surprised by the lawsuit. Dissatisfaction about MOC continues to rise, and resistance grows as more diplomates learn about the financial misdoings and monopolistic nature of the board, said Dr. Mathew, director of legislative affairs for the National Board of Physicians and Surgeons, an alternative board that provides continuing certification for physicians.
“At the end of the day, I hope that the case wins, because diplomates have been forced to expend their time, effort, and money on a product that has scant evidence that it improves practice,” Dr. Mathew said in an interview. “Unnecessary administrative burdens of this nature that do not improve patient care contribute to physician burnout, which is a nationwide epidemic.”
Dr. Mathew hopes to see monetary damages awarded as well as injunctive relief that would enable competition in the MOC market, he said. Such relief would prevent insurance companies and other stakeholders from only recognizing and requiring recertification with ABPN.*
“This would allow physicians the flexibility to recertify with boards like the National Board of Physicians and Surgeons,” he said.
The ABPN, meanwhile, defended its recertification, calling it a credential that is valued by patients, families, and medical organizations as an indicator that physicians have pursued “a meaningful program of lifelong learning sufficient to maintain the competence to provide quality patient care.” In recent years, the board has made improvements to its MOC requirements based on constructive feedback from diplomates and consistent with the evolving standards of the American Board of Medical Specialties, Dr. Faulkner said in the interview.
“Relevant options have been provided for the documentation of self-assessment and performance improvement, and they have been well received by diplomates,” he said. “The ABPN is also in the process of implementing an optional, article-based Pilot Project as an alternative to its secure continuing certification examination. Almost 15,000 ABPN diplomates have enrolled to date in the Pilot Project, and initial feedback from those diplomates has also been very favorable.”
*Correction, 4/5/19: Due to an editing error, an earlier version of this article omitted the word "only" from this sentence.
Two psychiatrists are suing the American Board of Psychiatry and Neurology (ABPN) over its maintenance of certification program, contending that the board has created a monopoly over the MOC market and that its process violates antitrust laws.
The lawsuit, filed March 6 in U.S. District Court for the Northern District of Illinois, alleges that the board is illegally tying its initial certification product to its MOC product, and that ABPN is using anticompetitive actions to prevent and limit competition from new MOC providers. The suit is filed as a class action on behalf of all psychiatrists and neurologists required by ABPN to buy MOC to maintain their certifications. The plaintiffs seek damages and injunctive relief arising from the alleged violations.
In an interview, ABPN President and CEO Larry R. Faulkner, MD, said the legal challenge has no merit.
“The ABPN intends to defend itself vigorously and anticipates that, like other similar cases previously filed against the American Board of Medical Specialties and other member boards, this lawsuit will be dismissed.”
Plaintiff Emily Lazarou, MD, an Odessa, Fla.-based psychiatrist, did not return messages seeking comment. The other plaintiff, Aafaque Akhter, MD, a psychiatrist based in Norton, Mass., declined to comment at the advice of his attorneys. In a LinkedIn post, Dr. Akhter wrote that he filed the class action to put an end to MOC.
“This moneymaking operation has to be abolished,” he wrote in the post. “MOC has to go.
The 35-page complaint against the ABPN contends that the organization’s antitrust and monopolistic actions have resulted in overly burdensome conditions for physicians forced to buy MOC, and that the board’s requirements constrain the supply of psychiatrists and neurologists, thereby harming competition. The ABPN has made tens of millions of dollars in MOC revenue from psychiatrists and neurologists, the suit alleges, while physicians face countless hours away from their practice and families in order to meet MOC demands, to the physicians’ financial detriment. The challenge also asserts that ABPN’s grandfather clause – which exempts from MOC physicians who received initial certifications prior to Oct. 1, 1994 – discriminates against younger physicians, women, and persons of color, all of whom are underrepresented in the group of psychiatrists and neurologists grandfathered by ABPN.
The lawsuit details how MOC has personally and professionally affected the plaintiffs. In 2017, Dr. Lazarou, a forensic psychiatrist who also practices telepsychiatry, asked the ABPN in advance for a private room during her upcoming 10-year MOC examination. At the time, Dr. Lazarou was nursing her twin newborns and needed the private room to pump, according to the lawsuit. The suit alleges that ABPN would not make an accommodation for Dr. Lazarou to pump at the testing site; however, as a professional courtesy, board officials allowed her to travel to a test center farther away that had private rooms. Because the distance required Dr. Lazarou to be away from her newborns for an extended period, she did not take the MOC examination, and her ABPN certification lapsed, according to the suit. Because of the lapse, she is no longer able to practice telepsychiatry, which has resulted in a loss of income and to the detriment of patients who need telepsychiatric care, she claims in the lawsuit.
Dr. Akhter, who practices addiction medicine, was informed in August 2018 that he was selected for a random audit of his ABPN MOC activities. During the audit, officials informed Dr. Akhter that the continuing medical education (CME) requirement for completion of his 3-year continuance maintenance of certification (C-MOC) cycle had not been met, according to the suit. As part of the 3-year C-MOC cycle, physicians are required to complete 90 CME credits, including 24 credits for self-assessment (SA) activities. ABPN defines SA activities as a specific type of CME activity that help physicians recognize their current knowledge base in order to identify specific topics for gaining further knowledge. Although Dr. Akhter had obtained a subspecialty certification from the American Board of Preventive Medicine, including completion of 60 CME credits that included SA activities, the board determined that those efforts did not fulfill Dr. Akhter’s SA CME requirements. The board denied his request to recognize the 60 CME credits as SA CME credits and also refused to give him SA CME credits for obtaining his subspecialty certification, the lawsuit says. He now is listed on the ABPN website as “Not Meeting MOC Requirements.”
The ABPN has not yet responded to the lawsuit. Defendants generally have 30 days from the time a challenge is filed to respond, unless they request an extension. Court documents show that an attorney for ABPN entered his appearance on March 20.
The ABPN lawsuit comes on the heels of a similar class action filed last year by a group of internists against the American Board of Internal Medicine (ABIM) over its MOC process. That lawsuit, filed Dec. 6 in Pennsylvania district court, contends that the ABIM is charging inflated monopoly prices for maintaining certification and that the organization is violating antitrust laws. In a motion filed March 18, attorneys for the ABIM asked the court to dismiss the suit for failure to state a valid claim. A third lawsuit that levies similar allegations against the Board of Radiology was filed in late February. All three lawsuits are being funded by the advocacy organization Practicing Physicians of America, through a GoFundMe campaign.
Richard Rosin, MD, a geriatric psychiatrist based in Vancouver, said in an interview that he welcomes and applauds the lawsuit against ABPN. He hopes that the legal challenge will put a spotlight on the board’s activities and compel administrators to answer questions about the MOC process that have gone unanswered in the past.
“If it is successful, I think the impact will be dramatic,” said Dr. Rosin, an outspoken critic of the ABPN’s MOC process. “They’ll essentially have to disband the [MOC] program.”
Paul G. Mathew, MD, a Boston-based neurologist, said he was not surprised by the lawsuit. Dissatisfaction about MOC continues to rise, and resistance grows as more diplomates learn about the financial misdoings and monopolistic nature of the board, said Dr. Mathew, director of legislative affairs for the National Board of Physicians and Surgeons, an alternative board that provides continuing certification for physicians.
“At the end of the day, I hope that the case wins, because diplomates have been forced to expend their time, effort, and money on a product that has scant evidence that it improves practice,” Dr. Mathew said in an interview. “Unnecessary administrative burdens of this nature that do not improve patient care contribute to physician burnout, which is a nationwide epidemic.”
Dr. Mathew hopes to see monetary damages awarded as well as injunctive relief that would enable competition in the MOC market, he said. Such relief would prevent insurance companies and other stakeholders from only recognizing and requiring recertification with ABPN.*
“This would allow physicians the flexibility to recertify with boards like the National Board of Physicians and Surgeons,” he said.
The ABPN, meanwhile, defended its recertification, calling it a credential that is valued by patients, families, and medical organizations as an indicator that physicians have pursued “a meaningful program of lifelong learning sufficient to maintain the competence to provide quality patient care.” In recent years, the board has made improvements to its MOC requirements based on constructive feedback from diplomates and consistent with the evolving standards of the American Board of Medical Specialties, Dr. Faulkner said in the interview.
“Relevant options have been provided for the documentation of self-assessment and performance improvement, and they have been well received by diplomates,” he said. “The ABPN is also in the process of implementing an optional, article-based Pilot Project as an alternative to its secure continuing certification examination. Almost 15,000 ABPN diplomates have enrolled to date in the Pilot Project, and initial feedback from those diplomates has also been very favorable.”
*Correction, 4/5/19: Due to an editing error, an earlier version of this article omitted the word "only" from this sentence.
Two psychiatrists are suing the American Board of Psychiatry and Neurology (ABPN) over its maintenance of certification program, contending that the board has created a monopoly over the MOC market and that its process violates antitrust laws.
The lawsuit, filed March 6 in U.S. District Court for the Northern District of Illinois, alleges that the board is illegally tying its initial certification product to its MOC product, and that ABPN is using anticompetitive actions to prevent and limit competition from new MOC providers. The suit is filed as a class action on behalf of all psychiatrists and neurologists required by ABPN to buy MOC to maintain their certifications. The plaintiffs seek damages and injunctive relief arising from the alleged violations.
In an interview, ABPN President and CEO Larry R. Faulkner, MD, said the legal challenge has no merit.
“The ABPN intends to defend itself vigorously and anticipates that, like other similar cases previously filed against the American Board of Medical Specialties and other member boards, this lawsuit will be dismissed.”
Plaintiff Emily Lazarou, MD, an Odessa, Fla.-based psychiatrist, did not return messages seeking comment. The other plaintiff, Aafaque Akhter, MD, a psychiatrist based in Norton, Mass., declined to comment at the advice of his attorneys. In a LinkedIn post, Dr. Akhter wrote that he filed the class action to put an end to MOC.
“This moneymaking operation has to be abolished,” he wrote in the post. “MOC has to go.
The 35-page complaint against the ABPN contends that the organization’s antitrust and monopolistic actions have resulted in overly burdensome conditions for physicians forced to buy MOC, and that the board’s requirements constrain the supply of psychiatrists and neurologists, thereby harming competition. The ABPN has made tens of millions of dollars in MOC revenue from psychiatrists and neurologists, the suit alleges, while physicians face countless hours away from their practice and families in order to meet MOC demands, to the physicians’ financial detriment. The challenge also asserts that ABPN’s grandfather clause – which exempts from MOC physicians who received initial certifications prior to Oct. 1, 1994 – discriminates against younger physicians, women, and persons of color, all of whom are underrepresented in the group of psychiatrists and neurologists grandfathered by ABPN.
The lawsuit details how MOC has personally and professionally affected the plaintiffs. In 2017, Dr. Lazarou, a forensic psychiatrist who also practices telepsychiatry, asked the ABPN in advance for a private room during her upcoming 10-year MOC examination. At the time, Dr. Lazarou was nursing her twin newborns and needed the private room to pump, according to the lawsuit. The suit alleges that ABPN would not make an accommodation for Dr. Lazarou to pump at the testing site; however, as a professional courtesy, board officials allowed her to travel to a test center farther away that had private rooms. Because the distance required Dr. Lazarou to be away from her newborns for an extended period, she did not take the MOC examination, and her ABPN certification lapsed, according to the suit. Because of the lapse, she is no longer able to practice telepsychiatry, which has resulted in a loss of income and to the detriment of patients who need telepsychiatric care, she claims in the lawsuit.
Dr. Akhter, who practices addiction medicine, was informed in August 2018 that he was selected for a random audit of his ABPN MOC activities. During the audit, officials informed Dr. Akhter that the continuing medical education (CME) requirement for completion of his 3-year continuance maintenance of certification (C-MOC) cycle had not been met, according to the suit. As part of the 3-year C-MOC cycle, physicians are required to complete 90 CME credits, including 24 credits for self-assessment (SA) activities. ABPN defines SA activities as a specific type of CME activity that help physicians recognize their current knowledge base in order to identify specific topics for gaining further knowledge. Although Dr. Akhter had obtained a subspecialty certification from the American Board of Preventive Medicine, including completion of 60 CME credits that included SA activities, the board determined that those efforts did not fulfill Dr. Akhter’s SA CME requirements. The board denied his request to recognize the 60 CME credits as SA CME credits and also refused to give him SA CME credits for obtaining his subspecialty certification, the lawsuit says. He now is listed on the ABPN website as “Not Meeting MOC Requirements.”
The ABPN has not yet responded to the lawsuit. Defendants generally have 30 days from the time a challenge is filed to respond, unless they request an extension. Court documents show that an attorney for ABPN entered his appearance on March 20.
The ABPN lawsuit comes on the heels of a similar class action filed last year by a group of internists against the American Board of Internal Medicine (ABIM) over its MOC process. That lawsuit, filed Dec. 6 in Pennsylvania district court, contends that the ABIM is charging inflated monopoly prices for maintaining certification and that the organization is violating antitrust laws. In a motion filed March 18, attorneys for the ABIM asked the court to dismiss the suit for failure to state a valid claim. A third lawsuit that levies similar allegations against the Board of Radiology was filed in late February. All three lawsuits are being funded by the advocacy organization Practicing Physicians of America, through a GoFundMe campaign.
Richard Rosin, MD, a geriatric psychiatrist based in Vancouver, said in an interview that he welcomes and applauds the lawsuit against ABPN. He hopes that the legal challenge will put a spotlight on the board’s activities and compel administrators to answer questions about the MOC process that have gone unanswered in the past.
“If it is successful, I think the impact will be dramatic,” said Dr. Rosin, an outspoken critic of the ABPN’s MOC process. “They’ll essentially have to disband the [MOC] program.”
Paul G. Mathew, MD, a Boston-based neurologist, said he was not surprised by the lawsuit. Dissatisfaction about MOC continues to rise, and resistance grows as more diplomates learn about the financial misdoings and monopolistic nature of the board, said Dr. Mathew, director of legislative affairs for the National Board of Physicians and Surgeons, an alternative board that provides continuing certification for physicians.
“At the end of the day, I hope that the case wins, because diplomates have been forced to expend their time, effort, and money on a product that has scant evidence that it improves practice,” Dr. Mathew said in an interview. “Unnecessary administrative burdens of this nature that do not improve patient care contribute to physician burnout, which is a nationwide epidemic.”
Dr. Mathew hopes to see monetary damages awarded as well as injunctive relief that would enable competition in the MOC market, he said. Such relief would prevent insurance companies and other stakeholders from only recognizing and requiring recertification with ABPN.*
“This would allow physicians the flexibility to recertify with boards like the National Board of Physicians and Surgeons,” he said.
The ABPN, meanwhile, defended its recertification, calling it a credential that is valued by patients, families, and medical organizations as an indicator that physicians have pursued “a meaningful program of lifelong learning sufficient to maintain the competence to provide quality patient care.” In recent years, the board has made improvements to its MOC requirements based on constructive feedback from diplomates and consistent with the evolving standards of the American Board of Medical Specialties, Dr. Faulkner said in the interview.
“Relevant options have been provided for the documentation of self-assessment and performance improvement, and they have been well received by diplomates,” he said. “The ABPN is also in the process of implementing an optional, article-based Pilot Project as an alternative to its secure continuing certification examination. Almost 15,000 ABPN diplomates have enrolled to date in the Pilot Project, and initial feedback from those diplomates has also been very favorable.”
*Correction, 4/5/19: Due to an editing error, an earlier version of this article omitted the word "only" from this sentence.
Session encourages action on improving gender equity
How to develop and employ strategies to advance gender equality in hospital medicine was the focus of Tuesday’s Quick Talk “Lead In: Advancing Gender Equity in Hospital Medicine.”
Attendees heard the importance of taking action now to improve gender equality in hospital medicine to better the climate for current and future physicians. The session’s aim was to be informative and serve as a call-to-action, said presenter Vineet Arora, MD, MAPP, MPM, a professor of medicine and assistant dean for scholarship and discovery at the University of Chicago.
“Without deliberate focus and attention, it will take 200 years to close the gender equity gap worldwide,” Dr. Arora said in an interview. “This is a call to action for us to not only help current women but also future generations to come. To make a dent, gender inequity needs to be treated like a never event, much like how we have approached patient safety for the past 20 years, for us to change the culture and make actual progress.”
During the presentation, Dr. Arora discussed various strategies that hospital medicine program leaders can utilize to recognize and empower women in the workplace and that center on making women seen, heard, and known among work teams and leadership. For example, prominent women leaders can be displayed through photos in the building to draw recognition. Supporting and expanding on ideas made by women with appropriate attribution also is key, Dr. Arora noted. She discussed a strategy used by women staffers in the Obama administration during meetings. When one woman staffer made a key point, another woman staffer would repeat the point and give credit to its author, which compelled men in the room to acknowledge the idea while preventing them from claiming the idea as their own later.
Dr. Arora stressed the importance of sponsoring women in their career endeavors, supporting women who are successful, and recognizing gender bias in yourself. She noted a recent study that found when female residents struggled, they received discordant feedback on autonomy and assertiveness.
The take-home message for attendees is that today is the right time to make changes and improve gender equality, said Dr. Arora, who is a founding member of TIME’S UP Healthcare, an organization that addresses gender inequalities and sexual harassment.
“I hope attendees will take immediate action to address gender equity, including create sponsorship programs, make sure women are not only seen but also heard, and address any implicit bias that may be hampering advancement of women,” she said.
How to develop and employ strategies to advance gender equality in hospital medicine was the focus of Tuesday’s Quick Talk “Lead In: Advancing Gender Equity in Hospital Medicine.”
Attendees heard the importance of taking action now to improve gender equality in hospital medicine to better the climate for current and future physicians. The session’s aim was to be informative and serve as a call-to-action, said presenter Vineet Arora, MD, MAPP, MPM, a professor of medicine and assistant dean for scholarship and discovery at the University of Chicago.
“Without deliberate focus and attention, it will take 200 years to close the gender equity gap worldwide,” Dr. Arora said in an interview. “This is a call to action for us to not only help current women but also future generations to come. To make a dent, gender inequity needs to be treated like a never event, much like how we have approached patient safety for the past 20 years, for us to change the culture and make actual progress.”
During the presentation, Dr. Arora discussed various strategies that hospital medicine program leaders can utilize to recognize and empower women in the workplace and that center on making women seen, heard, and known among work teams and leadership. For example, prominent women leaders can be displayed through photos in the building to draw recognition. Supporting and expanding on ideas made by women with appropriate attribution also is key, Dr. Arora noted. She discussed a strategy used by women staffers in the Obama administration during meetings. When one woman staffer made a key point, another woman staffer would repeat the point and give credit to its author, which compelled men in the room to acknowledge the idea while preventing them from claiming the idea as their own later.
Dr. Arora stressed the importance of sponsoring women in their career endeavors, supporting women who are successful, and recognizing gender bias in yourself. She noted a recent study that found when female residents struggled, they received discordant feedback on autonomy and assertiveness.
The take-home message for attendees is that today is the right time to make changes and improve gender equality, said Dr. Arora, who is a founding member of TIME’S UP Healthcare, an organization that addresses gender inequalities and sexual harassment.
“I hope attendees will take immediate action to address gender equity, including create sponsorship programs, make sure women are not only seen but also heard, and address any implicit bias that may be hampering advancement of women,” she said.
How to develop and employ strategies to advance gender equality in hospital medicine was the focus of Tuesday’s Quick Talk “Lead In: Advancing Gender Equity in Hospital Medicine.”
Attendees heard the importance of taking action now to improve gender equality in hospital medicine to better the climate for current and future physicians. The session’s aim was to be informative and serve as a call-to-action, said presenter Vineet Arora, MD, MAPP, MPM, a professor of medicine and assistant dean for scholarship and discovery at the University of Chicago.
“Without deliberate focus and attention, it will take 200 years to close the gender equity gap worldwide,” Dr. Arora said in an interview. “This is a call to action for us to not only help current women but also future generations to come. To make a dent, gender inequity needs to be treated like a never event, much like how we have approached patient safety for the past 20 years, for us to change the culture and make actual progress.”
During the presentation, Dr. Arora discussed various strategies that hospital medicine program leaders can utilize to recognize and empower women in the workplace and that center on making women seen, heard, and known among work teams and leadership. For example, prominent women leaders can be displayed through photos in the building to draw recognition. Supporting and expanding on ideas made by women with appropriate attribution also is key, Dr. Arora noted. She discussed a strategy used by women staffers in the Obama administration during meetings. When one woman staffer made a key point, another woman staffer would repeat the point and give credit to its author, which compelled men in the room to acknowledge the idea while preventing them from claiming the idea as their own later.
Dr. Arora stressed the importance of sponsoring women in their career endeavors, supporting women who are successful, and recognizing gender bias in yourself. She noted a recent study that found when female residents struggled, they received discordant feedback on autonomy and assertiveness.
The take-home message for attendees is that today is the right time to make changes and improve gender equality, said Dr. Arora, who is a founding member of TIME’S UP Healthcare, an organization that addresses gender inequalities and sexual harassment.
“I hope attendees will take immediate action to address gender equity, including create sponsorship programs, make sure women are not only seen but also heard, and address any implicit bias that may be hampering advancement of women,” she said.
ABIM contests class-action lawsuit, asks judge to dismiss
Attorneys for the American Board of Internal Medicine have asked Judge Robert Kelly of the United States District Court for the Eastern District of Pennsylvania to dismiss a class-action lawsuit against the board’s maintenance of certification (MOC) program.
The legal challenge, filed Dec. 6, 2018, claims that ABIM charges inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification as a condition of employment.
The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their certification. On Jan. 23 of this year, the suit was amended to include racketeering and unjust enrichment claims.
In a motion filed March 18, attorneys for ABIM asserted that the plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Richard J. Baron, MD, ABIM president and CEO said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing.
More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by Practicing Physicians of America to pay for the plaintiffs’ legal costs.
Attorneys for the American Board of Internal Medicine have asked Judge Robert Kelly of the United States District Court for the Eastern District of Pennsylvania to dismiss a class-action lawsuit against the board’s maintenance of certification (MOC) program.
The legal challenge, filed Dec. 6, 2018, claims that ABIM charges inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification as a condition of employment.
The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their certification. On Jan. 23 of this year, the suit was amended to include racketeering and unjust enrichment claims.
In a motion filed March 18, attorneys for ABIM asserted that the plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Richard J. Baron, MD, ABIM president and CEO said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing.
More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by Practicing Physicians of America to pay for the plaintiffs’ legal costs.
Attorneys for the American Board of Internal Medicine have asked Judge Robert Kelly of the United States District Court for the Eastern District of Pennsylvania to dismiss a class-action lawsuit against the board’s maintenance of certification (MOC) program.
The legal challenge, filed Dec. 6, 2018, claims that ABIM charges inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification as a condition of employment.
The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their certification. On Jan. 23 of this year, the suit was amended to include racketeering and unjust enrichment claims.
In a motion filed March 18, attorneys for ABIM asserted that the plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Richard J. Baron, MD, ABIM president and CEO said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing.
More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by Practicing Physicians of America to pay for the plaintiffs’ legal costs.



















