User login
Anticoagulation Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving treatment options for preventing stroke, acute coronary events, deep vein thrombosis, and pulmonary embolism in at-risk patients. The Anticoagulation Hub is powered by Frontline Medical Communications.
ACC, AHA update performance measures for lipid management
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Treat high LDL cholesterol in CKD, even with inflammation
SAN DIEGO – Among patients with chronic kidney disease, LDL cholesterol level was positively associated with risk of atherosclerotic vascular events, regardless of baseline inflammation, according to a large, randomized study.
In addition, lowering LDL cholesterol with ezetimibe/simvastatin was similarly effective in the presence or absence of inflammation.
“There has been substantial interest in the relationship between LDL cholesterol and outcomes in the general population, and in particular among people with kidney disease,” study author Dr. Richard Haynes of the Nuffield Department of Population Health at the University of Oxford (England) said in an interview in advance of the meeting.
“Previous studies have found a paradoxical increased risk of death at low cholesterol levels, which may be due to another disease process – such as inflammation – both lowering cholesterol and increasing the risk of death, thereby creating a false association,” he explained.
Dr. Haynes and his associates set out to determine the relevance of LDL cholesterol and C-reactive protein (CRP) to cardiovascular risk among 9,270 patients with chronic kidney disease who were enrolled in the Study of Heart and Renal Protection (SHARP), in which they received either ezetimibe/simvastatin or placebo.
The researchers used Cox regression to analyze the hazard ratio for all atherosclerotic vascular events over a period of 4.9 years. The effect of ezetimibe/simvastatin on major atherosclerotic events was estimated in the presence and absence of inflammation, which was defined as a CRP of 3 mg/L. The mean age of SHARP participants was 62 years, and 63% were men.
Dr. Ben Storey, a colleague of Dr. Haynes at Oxford, reported the study results at the meeting sponsored by the American Society of Nephrology.
Among all patients, usual LDL was positively associated with the risk of atherosclerotic vascular events (hazard ratio, 1.34). Compared with patients who had low CRP, those with high CRP were at higher risk, but the relationship between LDL-C and atherosclerotic vascular event risk was similar in both groups (HR, 1.32 and 1.41, respectively).
Ezetimibe/simvastatin was similarly effective at reducing major atherosclerotic events in patients with low and high CRP (risk ratio, 0.84 and 0.83, respectively). Sensitivity analyses yielded similar results.
“The observational data from SHARP show that LDL cholesterol is positively associated with the risk of atherosclerotic vascular events, irrespective of baseline inflammation,” Dr. Storey concluded. “The randomized evidence showed that lowering LDL cholesterol was similarly effective in the presence or absence of inflammation, but CRP is associated with vascular risk in [chronic kidney disease].”
The findings’ clinical implications are that inflammation, “while it is relevant to patients’ outcomes, does not modify the beneficial effects of lowering LDL cholesterol,” according to Dr. Haynes, “So, clinicians should not be put off from prescribing statin-based, cholesterol-lowering therapy by the presence – or absence – of inflammation.”
Merck funded SHARP, but the University of Oxford conducted and analyzed the study independently. The Australian National Health and Medical Research Council, the British Heart Foundation, Cancer Research UK, and the UK Medical Research Council provided additional support.
SAN DIEGO – Among patients with chronic kidney disease, LDL cholesterol level was positively associated with risk of atherosclerotic vascular events, regardless of baseline inflammation, according to a large, randomized study.
In addition, lowering LDL cholesterol with ezetimibe/simvastatin was similarly effective in the presence or absence of inflammation.
“There has been substantial interest in the relationship between LDL cholesterol and outcomes in the general population, and in particular among people with kidney disease,” study author Dr. Richard Haynes of the Nuffield Department of Population Health at the University of Oxford (England) said in an interview in advance of the meeting.
“Previous studies have found a paradoxical increased risk of death at low cholesterol levels, which may be due to another disease process – such as inflammation – both lowering cholesterol and increasing the risk of death, thereby creating a false association,” he explained.
Dr. Haynes and his associates set out to determine the relevance of LDL cholesterol and C-reactive protein (CRP) to cardiovascular risk among 9,270 patients with chronic kidney disease who were enrolled in the Study of Heart and Renal Protection (SHARP), in which they received either ezetimibe/simvastatin or placebo.
The researchers used Cox regression to analyze the hazard ratio for all atherosclerotic vascular events over a period of 4.9 years. The effect of ezetimibe/simvastatin on major atherosclerotic events was estimated in the presence and absence of inflammation, which was defined as a CRP of 3 mg/L. The mean age of SHARP participants was 62 years, and 63% were men.
Dr. Ben Storey, a colleague of Dr. Haynes at Oxford, reported the study results at the meeting sponsored by the American Society of Nephrology.
Among all patients, usual LDL was positively associated with the risk of atherosclerotic vascular events (hazard ratio, 1.34). Compared with patients who had low CRP, those with high CRP were at higher risk, but the relationship between LDL-C and atherosclerotic vascular event risk was similar in both groups (HR, 1.32 and 1.41, respectively).
Ezetimibe/simvastatin was similarly effective at reducing major atherosclerotic events in patients with low and high CRP (risk ratio, 0.84 and 0.83, respectively). Sensitivity analyses yielded similar results.
“The observational data from SHARP show that LDL cholesterol is positively associated with the risk of atherosclerotic vascular events, irrespective of baseline inflammation,” Dr. Storey concluded. “The randomized evidence showed that lowering LDL cholesterol was similarly effective in the presence or absence of inflammation, but CRP is associated with vascular risk in [chronic kidney disease].”
The findings’ clinical implications are that inflammation, “while it is relevant to patients’ outcomes, does not modify the beneficial effects of lowering LDL cholesterol,” according to Dr. Haynes, “So, clinicians should not be put off from prescribing statin-based, cholesterol-lowering therapy by the presence – or absence – of inflammation.”
Merck funded SHARP, but the University of Oxford conducted and analyzed the study independently. The Australian National Health and Medical Research Council, the British Heart Foundation, Cancer Research UK, and the UK Medical Research Council provided additional support.
SAN DIEGO – Among patients with chronic kidney disease, LDL cholesterol level was positively associated with risk of atherosclerotic vascular events, regardless of baseline inflammation, according to a large, randomized study.
In addition, lowering LDL cholesterol with ezetimibe/simvastatin was similarly effective in the presence or absence of inflammation.
“There has been substantial interest in the relationship between LDL cholesterol and outcomes in the general population, and in particular among people with kidney disease,” study author Dr. Richard Haynes of the Nuffield Department of Population Health at the University of Oxford (England) said in an interview in advance of the meeting.
“Previous studies have found a paradoxical increased risk of death at low cholesterol levels, which may be due to another disease process – such as inflammation – both lowering cholesterol and increasing the risk of death, thereby creating a false association,” he explained.
Dr. Haynes and his associates set out to determine the relevance of LDL cholesterol and C-reactive protein (CRP) to cardiovascular risk among 9,270 patients with chronic kidney disease who were enrolled in the Study of Heart and Renal Protection (SHARP), in which they received either ezetimibe/simvastatin or placebo.
The researchers used Cox regression to analyze the hazard ratio for all atherosclerotic vascular events over a period of 4.9 years. The effect of ezetimibe/simvastatin on major atherosclerotic events was estimated in the presence and absence of inflammation, which was defined as a CRP of 3 mg/L. The mean age of SHARP participants was 62 years, and 63% were men.
Dr. Ben Storey, a colleague of Dr. Haynes at Oxford, reported the study results at the meeting sponsored by the American Society of Nephrology.
Among all patients, usual LDL was positively associated with the risk of atherosclerotic vascular events (hazard ratio, 1.34). Compared with patients who had low CRP, those with high CRP were at higher risk, but the relationship between LDL-C and atherosclerotic vascular event risk was similar in both groups (HR, 1.32 and 1.41, respectively).
Ezetimibe/simvastatin was similarly effective at reducing major atherosclerotic events in patients with low and high CRP (risk ratio, 0.84 and 0.83, respectively). Sensitivity analyses yielded similar results.
“The observational data from SHARP show that LDL cholesterol is positively associated with the risk of atherosclerotic vascular events, irrespective of baseline inflammation,” Dr. Storey concluded. “The randomized evidence showed that lowering LDL cholesterol was similarly effective in the presence or absence of inflammation, but CRP is associated with vascular risk in [chronic kidney disease].”
The findings’ clinical implications are that inflammation, “while it is relevant to patients’ outcomes, does not modify the beneficial effects of lowering LDL cholesterol,” according to Dr. Haynes, “So, clinicians should not be put off from prescribing statin-based, cholesterol-lowering therapy by the presence – or absence – of inflammation.”
Merck funded SHARP, but the University of Oxford conducted and analyzed the study independently. The Australian National Health and Medical Research Council, the British Heart Foundation, Cancer Research UK, and the UK Medical Research Council provided additional support.
AT KIDNEY WEEK 2015
Key clinical point: LDL cholesterol level is positively associated with the risk of atherosclerotic vascular events in patients with chronic kidney disease, regardless of baseline inflammation.
Major finding: Among all patients, usual LDL cholesterol was positively associated with the risk of atherosclerotic vascular events (HR, 1.34).
Data source: Investigators attempted to determine the relevance of LDL cholesterol and C-reactive protein to cardiovascular risk among 9,270 patients with chronic kidney disease who were enrolled in SHARP.
Disclosures: Merck funded SHARP, but the University of Oxford conducted and analyzed the study independently. The Australian National Health and Medical Research Council, the British Heart Foundation, Cancer Research UK, and the UK Medical Research Council provided additional support.
Study characterizes intracerebral hemorrhage with new oral anticoagulants
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.
 |
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.
 |
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.
 |
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
FROM JAMA NEUROLOGY
Key clinical point: Intracerebral hemorrhage related to new oral anticoagulants frequently involves hematoma expansion and doesn’t appear to respond to prothrombin complex concentrate.
Major finding: Mortality was 28%, 65% of survivors had unfavorable outcomes, and substantial hematoma expansion occurred in 38% of patients.
Data source: A prospective, multicenter, observational study involving 61 patients treated during a 3-year period in Germany.
Disclosures: The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
AHA: Ezetimibe reduces ischemic stroke risk
ORLANDO – The combination of ezetimibe/simvastatin significantly reduced the risk of nonhemorrhagic stroke compared with simvastatin alone, with a particularly striking benefit seen in patients with prior history of stroke, in a new analysis from the landmark IMPROVE-IT trial.
“We believe these data support the use of intensive lipid lowering therapy, which includes ezetimibe to prevent ischemic stroke,” Dr. Stephen D. Wiviott said in reporting the findings at the American Heart Association scientific sessions.
He presented a prespecified secondary analysis from IMPROVE-IT, a double-blind study in which 18,144 patients on background optimal medical management were randomized post–acute coronary syndrome to simvastatin/ezetimibe at 40/10 mg/day (Vytorin) or simvastatin (Zocor) at 40 mg/day. At a median of 6 years of follow-up, the primary composite cardiovascular outcome was significantly reduced by 6% in the dual-therapy group compared with statin monotherapy, with a number-needed-to-treat (NNT) of 50, as previously reported (N Engl J Med. 2015 Jun 18;372[25]:2387-97).
The impetus for the prespecified stroke analysis was that up until IMPROVE-IT, no LDL cholesterol–lowering therapy other than statins had ever been shown to protect against stroke. The Cholesterol Trialists’ Collaboration meta-analysis, involving roughly 173,000 subjects, previously showed that statin therapy reduces ischemic stroke risk by 20% per 1 mmol/L of LDL lowering (Lancet. 2012 Aug 11;380[9841]:581-90). The question was, could add-on ezetimibe decrease stroke risk even further?
Stroke occurred in 641 patients during follow-up. As adjudicated by independent neurologists, 82% of the strokes were nonhemorrhagic, 16% were hemorrhagic, and 2% were unknown. The 14% relative risk reduction in overall stroke with simvastatin/ezetimibe compared with simvastatin, with rates of 4.2% versus 4.8%, just missed achieving statistical significance (P = .052). A significant 21% reduction in nonhemorrhagic strokes was seen with dual therapy, where the incidence during follow-up was 3.4%, compared with 4.1% with simvastatin alone, but this effect was dampened by a numeric albeit statistically nonsignificant absolute 0.2% increase in hemorrhagic strokes in the simvastatin/ezetimibe group.
Far more impressive was the stroke-prevention benefit of simvastatin/ezetimibe among the 1,071 subjects with prior stroke or TIA at baseline. Their nonhemorrhagic stroke rate during follow-up was 10.2% with simvastatin/ezetimibe versus 18.8% with simvastatin alone, for a 40% relative risk reduction favoring dual lipid-lowering therapy and an NNT of about 20. Again, there was no significant difference in hemorrhagic stroke between the two treatment arms, noted Dr. Wiviott of Brigham and Womens Hospital, Boston.
The stroke-prevention benefit achieved by adding ezetimibe to simvastatin was seen regardless of patient age, gender, renal function, baseline LDL cholesterol level, or other prespecified subcategories.
IMPROVE-IT was sponsored by Merck. Dr. Wiviott reported receiving research grants from Merck, AstraZeneca, and Eisai and serving as a consultant to nine pharmaceutical companies.
ORLANDO – The combination of ezetimibe/simvastatin significantly reduced the risk of nonhemorrhagic stroke compared with simvastatin alone, with a particularly striking benefit seen in patients with prior history of stroke, in a new analysis from the landmark IMPROVE-IT trial.
“We believe these data support the use of intensive lipid lowering therapy, which includes ezetimibe to prevent ischemic stroke,” Dr. Stephen D. Wiviott said in reporting the findings at the American Heart Association scientific sessions.
He presented a prespecified secondary analysis from IMPROVE-IT, a double-blind study in which 18,144 patients on background optimal medical management were randomized post–acute coronary syndrome to simvastatin/ezetimibe at 40/10 mg/day (Vytorin) or simvastatin (Zocor) at 40 mg/day. At a median of 6 years of follow-up, the primary composite cardiovascular outcome was significantly reduced by 6% in the dual-therapy group compared with statin monotherapy, with a number-needed-to-treat (NNT) of 50, as previously reported (N Engl J Med. 2015 Jun 18;372[25]:2387-97).
The impetus for the prespecified stroke analysis was that up until IMPROVE-IT, no LDL cholesterol–lowering therapy other than statins had ever been shown to protect against stroke. The Cholesterol Trialists’ Collaboration meta-analysis, involving roughly 173,000 subjects, previously showed that statin therapy reduces ischemic stroke risk by 20% per 1 mmol/L of LDL lowering (Lancet. 2012 Aug 11;380[9841]:581-90). The question was, could add-on ezetimibe decrease stroke risk even further?
Stroke occurred in 641 patients during follow-up. As adjudicated by independent neurologists, 82% of the strokes were nonhemorrhagic, 16% were hemorrhagic, and 2% were unknown. The 14% relative risk reduction in overall stroke with simvastatin/ezetimibe compared with simvastatin, with rates of 4.2% versus 4.8%, just missed achieving statistical significance (P = .052). A significant 21% reduction in nonhemorrhagic strokes was seen with dual therapy, where the incidence during follow-up was 3.4%, compared with 4.1% with simvastatin alone, but this effect was dampened by a numeric albeit statistically nonsignificant absolute 0.2% increase in hemorrhagic strokes in the simvastatin/ezetimibe group.
Far more impressive was the stroke-prevention benefit of simvastatin/ezetimibe among the 1,071 subjects with prior stroke or TIA at baseline. Their nonhemorrhagic stroke rate during follow-up was 10.2% with simvastatin/ezetimibe versus 18.8% with simvastatin alone, for a 40% relative risk reduction favoring dual lipid-lowering therapy and an NNT of about 20. Again, there was no significant difference in hemorrhagic stroke between the two treatment arms, noted Dr. Wiviott of Brigham and Womens Hospital, Boston.
The stroke-prevention benefit achieved by adding ezetimibe to simvastatin was seen regardless of patient age, gender, renal function, baseline LDL cholesterol level, or other prespecified subcategories.
IMPROVE-IT was sponsored by Merck. Dr. Wiviott reported receiving research grants from Merck, AstraZeneca, and Eisai and serving as a consultant to nine pharmaceutical companies.
ORLANDO – The combination of ezetimibe/simvastatin significantly reduced the risk of nonhemorrhagic stroke compared with simvastatin alone, with a particularly striking benefit seen in patients with prior history of stroke, in a new analysis from the landmark IMPROVE-IT trial.
“We believe these data support the use of intensive lipid lowering therapy, which includes ezetimibe to prevent ischemic stroke,” Dr. Stephen D. Wiviott said in reporting the findings at the American Heart Association scientific sessions.
He presented a prespecified secondary analysis from IMPROVE-IT, a double-blind study in which 18,144 patients on background optimal medical management were randomized post–acute coronary syndrome to simvastatin/ezetimibe at 40/10 mg/day (Vytorin) or simvastatin (Zocor) at 40 mg/day. At a median of 6 years of follow-up, the primary composite cardiovascular outcome was significantly reduced by 6% in the dual-therapy group compared with statin monotherapy, with a number-needed-to-treat (NNT) of 50, as previously reported (N Engl J Med. 2015 Jun 18;372[25]:2387-97).
The impetus for the prespecified stroke analysis was that up until IMPROVE-IT, no LDL cholesterol–lowering therapy other than statins had ever been shown to protect against stroke. The Cholesterol Trialists’ Collaboration meta-analysis, involving roughly 173,000 subjects, previously showed that statin therapy reduces ischemic stroke risk by 20% per 1 mmol/L of LDL lowering (Lancet. 2012 Aug 11;380[9841]:581-90). The question was, could add-on ezetimibe decrease stroke risk even further?
Stroke occurred in 641 patients during follow-up. As adjudicated by independent neurologists, 82% of the strokes were nonhemorrhagic, 16% were hemorrhagic, and 2% were unknown. The 14% relative risk reduction in overall stroke with simvastatin/ezetimibe compared with simvastatin, with rates of 4.2% versus 4.8%, just missed achieving statistical significance (P = .052). A significant 21% reduction in nonhemorrhagic strokes was seen with dual therapy, where the incidence during follow-up was 3.4%, compared with 4.1% with simvastatin alone, but this effect was dampened by a numeric albeit statistically nonsignificant absolute 0.2% increase in hemorrhagic strokes in the simvastatin/ezetimibe group.
Far more impressive was the stroke-prevention benefit of simvastatin/ezetimibe among the 1,071 subjects with prior stroke or TIA at baseline. Their nonhemorrhagic stroke rate during follow-up was 10.2% with simvastatin/ezetimibe versus 18.8% with simvastatin alone, for a 40% relative risk reduction favoring dual lipid-lowering therapy and an NNT of about 20. Again, there was no significant difference in hemorrhagic stroke between the two treatment arms, noted Dr. Wiviott of Brigham and Womens Hospital, Boston.
The stroke-prevention benefit achieved by adding ezetimibe to simvastatin was seen regardless of patient age, gender, renal function, baseline LDL cholesterol level, or other prespecified subcategories.
IMPROVE-IT was sponsored by Merck. Dr. Wiviott reported receiving research grants from Merck, AstraZeneca, and Eisai and serving as a consultant to nine pharmaceutical companies.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Intensive lipid-lowering therapy that incorporates ezetimibe provides enhanced protection against ischemic stroke.
Major finding: In patients with a baseline history of stroke who were on simvastatin/ezetimibe after an acute coronary syndrome, the risk of nonhemorrhagic stroke during 6 years of follow-up was reduced by 40%, compared with lipid-lowering via simvastatin alone.
Data source: A prespecified secondary analysis of stroke incidence during a median 6 years of follow-up in the double-blind, randomized, 18,144-patient IMPROVE-IT trial.
Disclosures: Merck sponsored the study. The presenter reported receiving a research grant from Merck and serving as a consultant to numerous pharmaceutical companies.
VIDEO: ASH highlights five Choosing Wisely initiatives
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
Lixisenatide not cardioprotective in type 2 diabetes
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding lixisenatide to usual care failed to prevent major cardiovascular events in patients with type 2 diabetes who had a recent ACS.
Major finding: Death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina occurred in 13.4% of participants receiving lixisenatide and 13.2% of those receiving placebo.
Data source: An international randomized double-blind placebo-controlled trial involving 6,068 patients followed for a median of 2 years.
Disclosures: Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
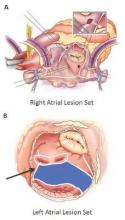
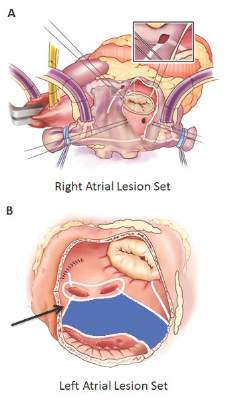
Surgical ablation endures at 5 years
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Key clinical point: Outcomes with the Cox-Maze IV procedure for surgical ablation are superior to catheter ablation and other forms of surgical ablation for atrial fibrillation for up to 5 years duration.
Major finding: Seventy-three percent of the study population was free from atrial tachyarrhythmias and 55% were free from anticoagulation at 5 years.
Data source: Prospective analysis of 576 consecutive patients with atrial fibrillation who had Cox-Maze IV procedure or a left-sized Cox-Maze IV procedure from 2002 to 2014 at a single institution
Disclosures: The National Institutes of Health provided grants for the study. Coauthor Dr. Ralph J. Damiano Jr. disclosed research grants and educational funding from AtriCure and Edwards LifeSciences. The other authors had no disclosures.
Mechanical thrombectomy improves stroke outcomes
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Mechanical thrombectomy and usual care are associated with significantly better functional outcomes after acute ischemic stroke than is usual care alone.
Major finding: Patients undergoing mechanical thrombectomy had a 67% greater likelihood of excellent functional outcome than patients having usual care alone.
Data source: Meta-analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients.
Disclosures: One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
AHA: DAPT prevents migraines after atrial-septal defect closure
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Three months of dual antiplatelet therapy surpassed aspirin monotherapy for preventing new-onset migraine headaches following transcatheter atrial-septal defect closure.
Major finding: Patients on DAPT had 0.4 migraine days per month, compared with a 1.4-day per month rate with aspirin monotherapy.
Data source: CANOA, a randomized trial with 171 patients run at six Canadian centers.
Disclosures: CANOA was investigator initiated. It received partial funding with unrestricted grants from Sanofi and St. Jude Medical. St. Jude markets the AMPLATZER device. Dr. Josep Rodés-Cabau had no disclosures. Dr. Abbott had no disclosures.
FDA okays prophylactic Pradaxa for VTE in hip replacement
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight