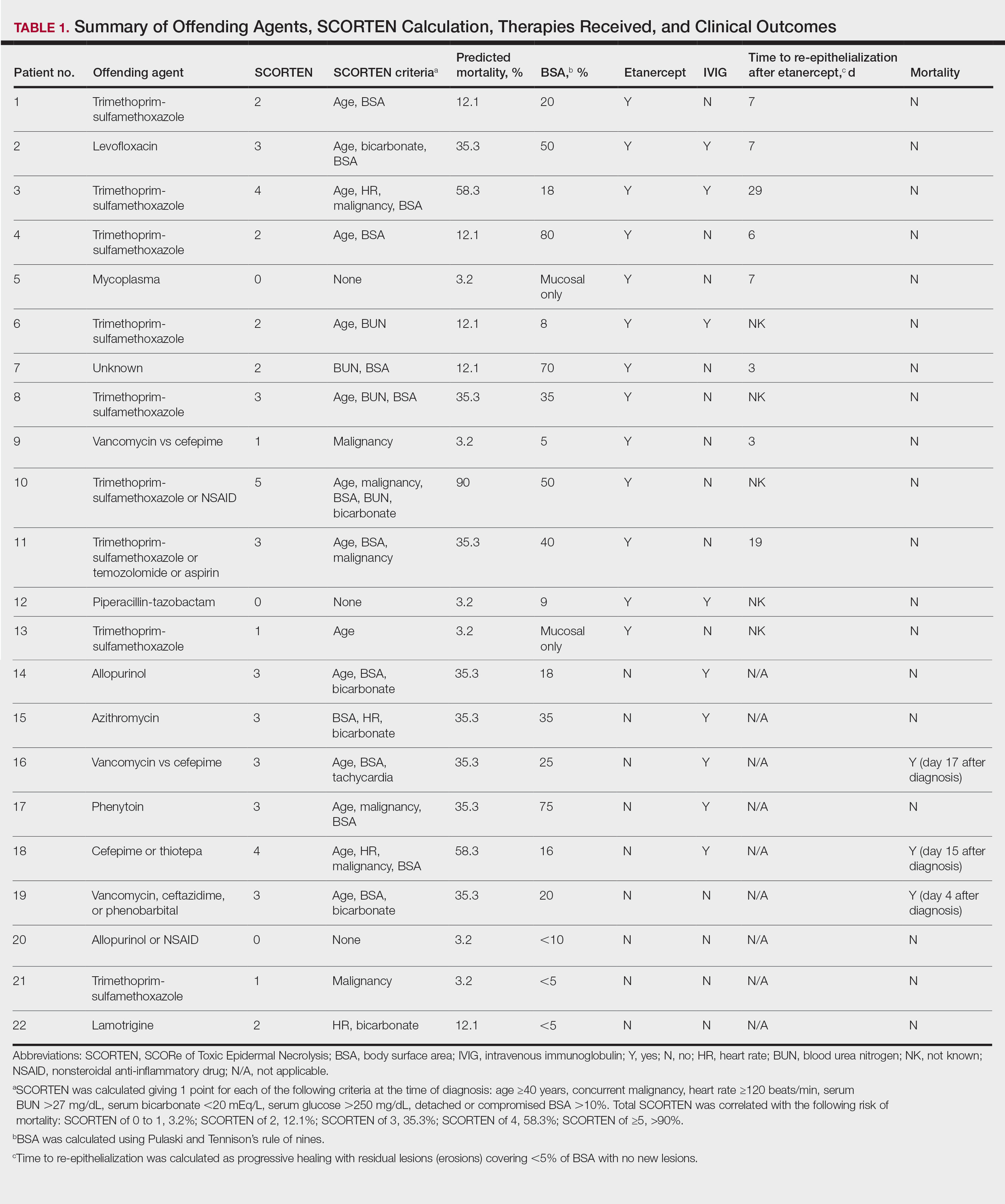
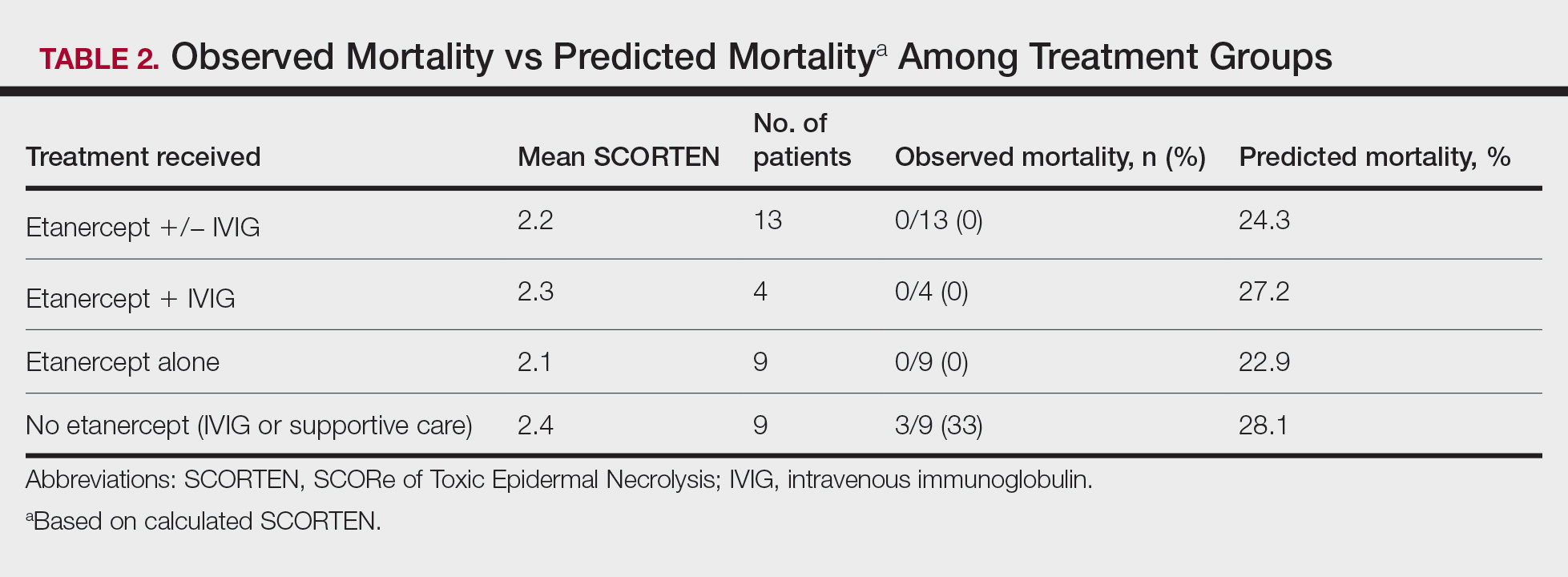
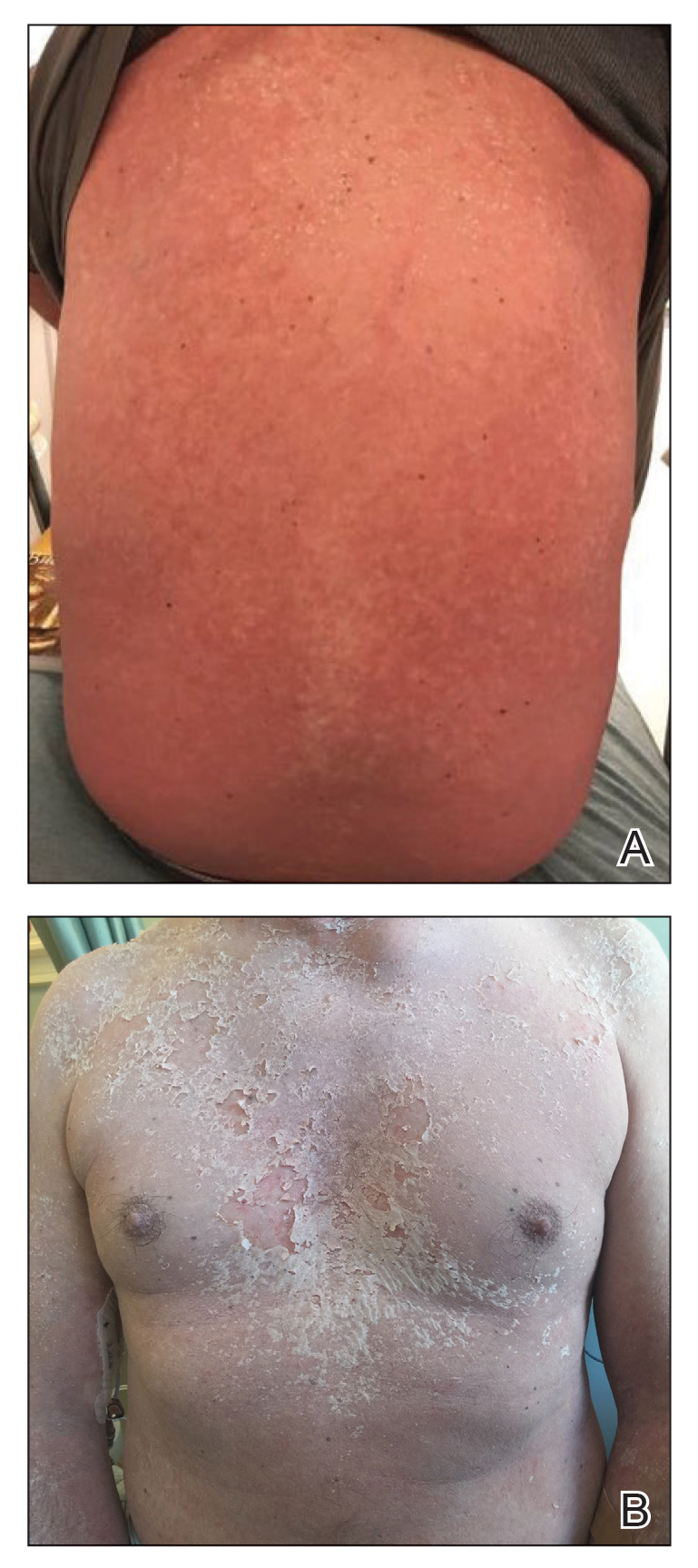
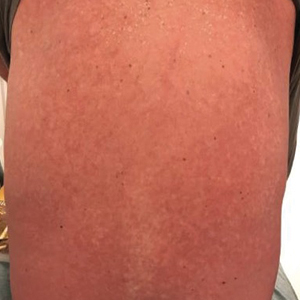
User login
An Algorithm for Managing Spitting Sutures
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
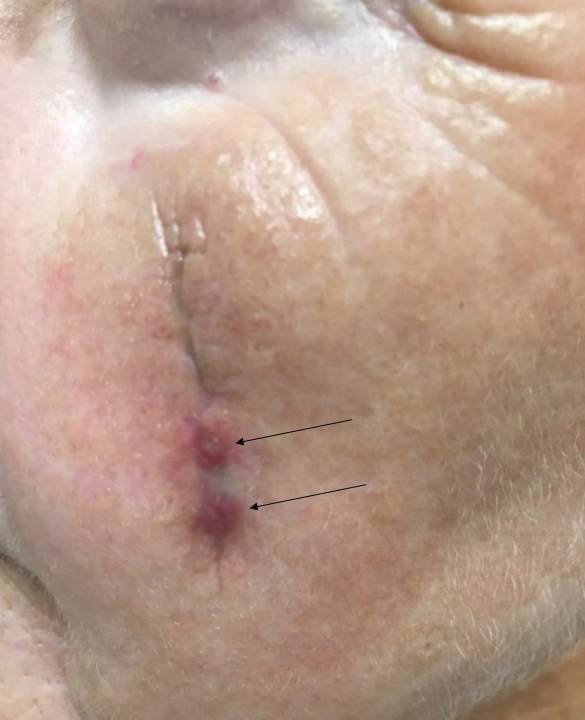
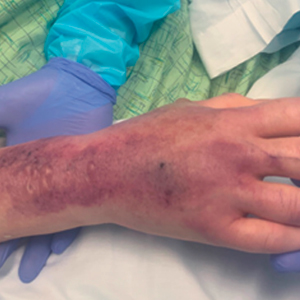
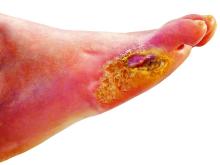
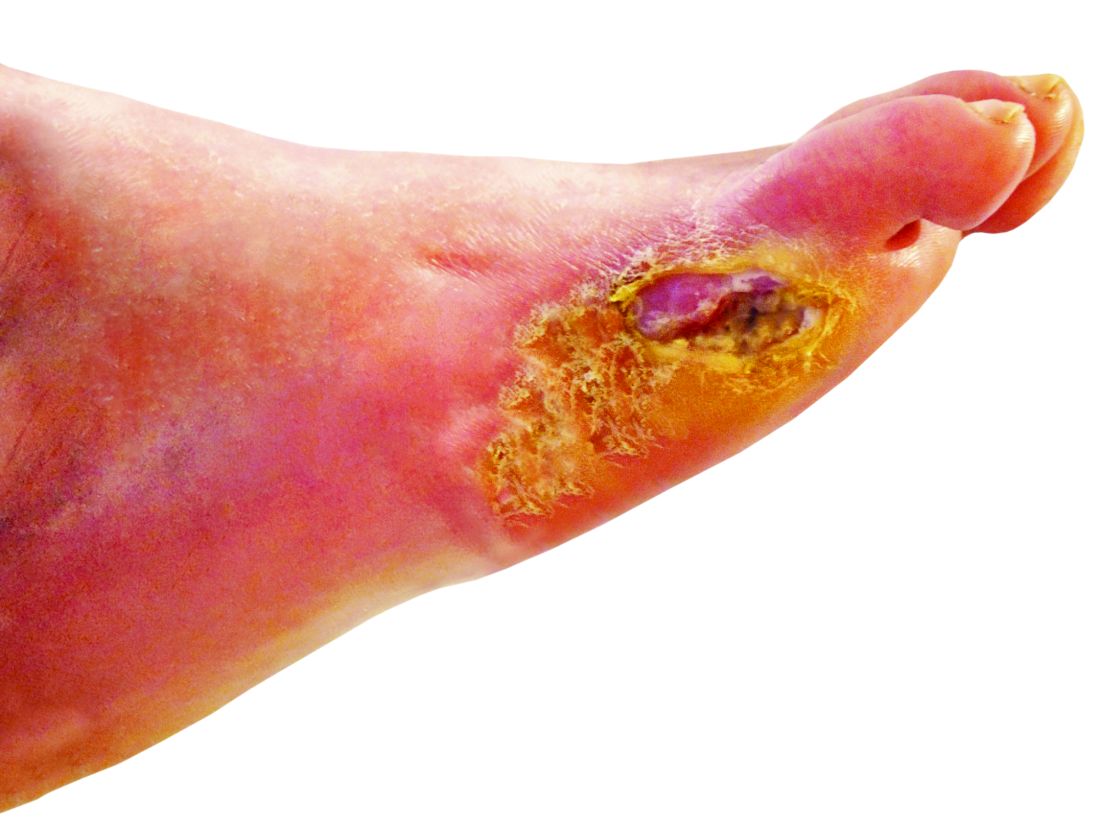
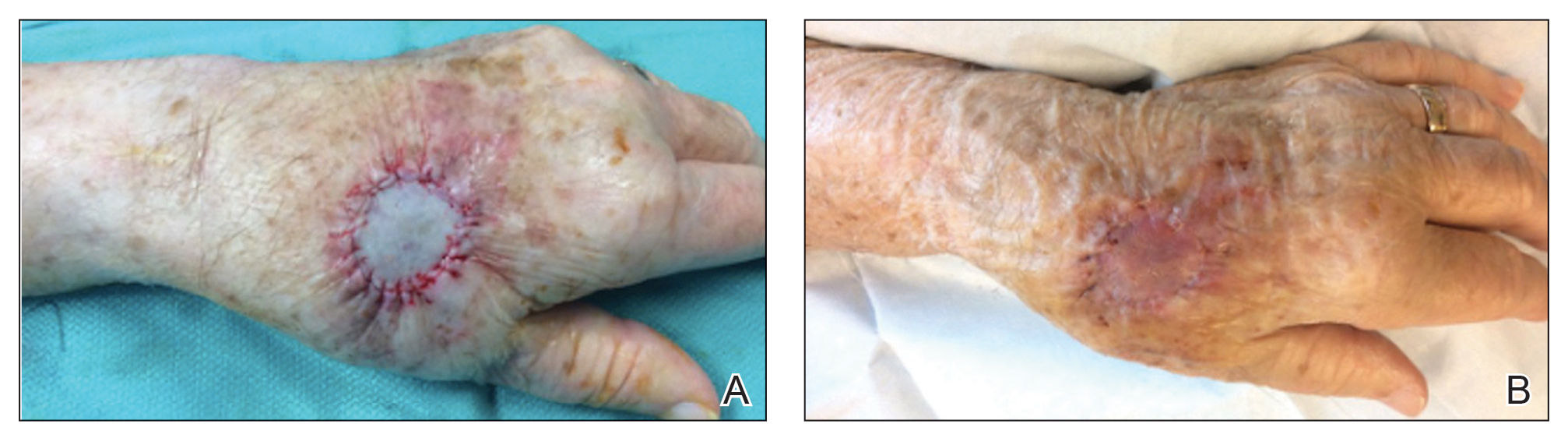
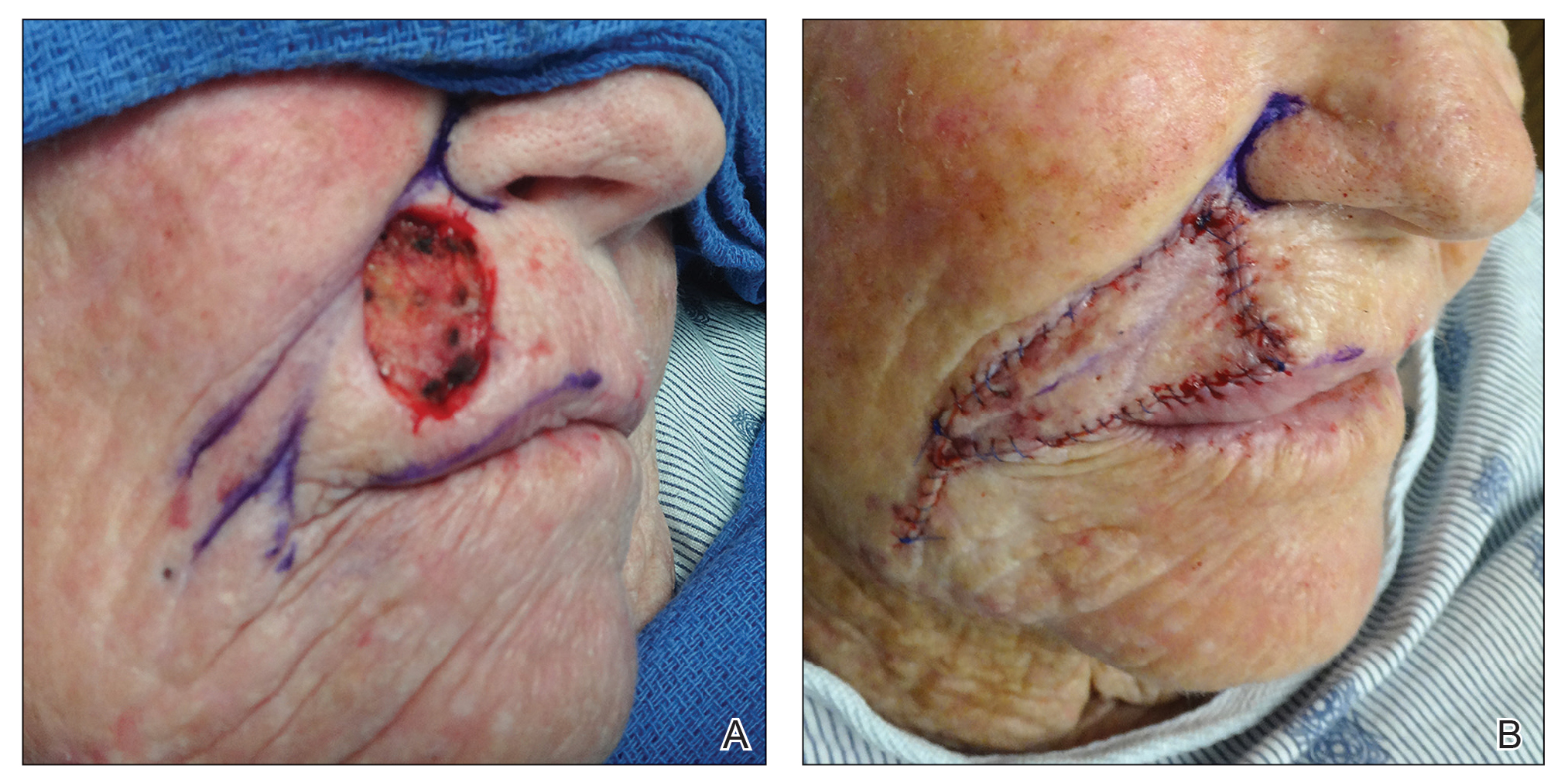
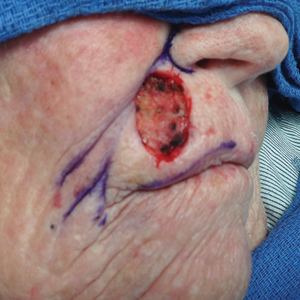
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
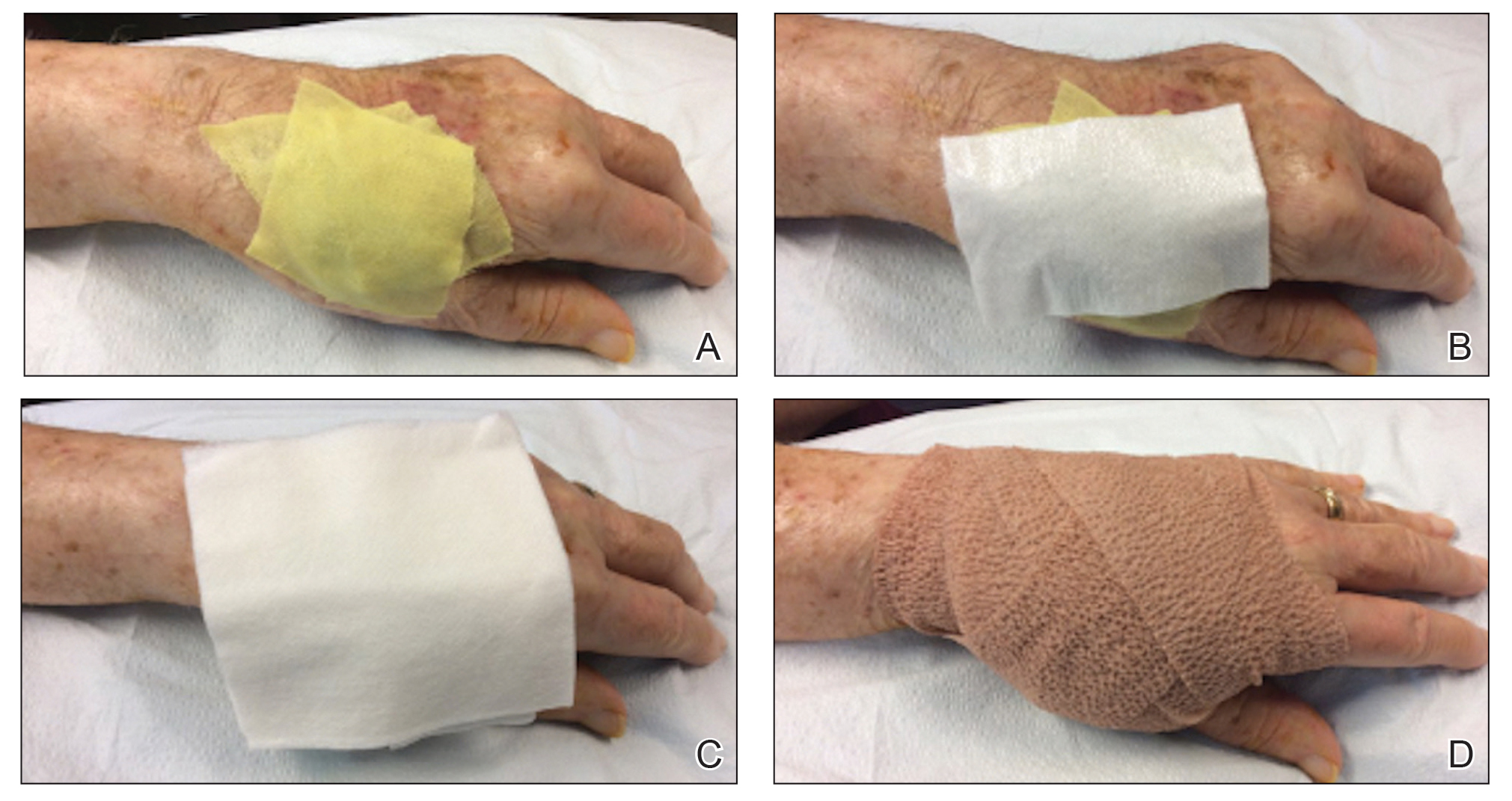
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
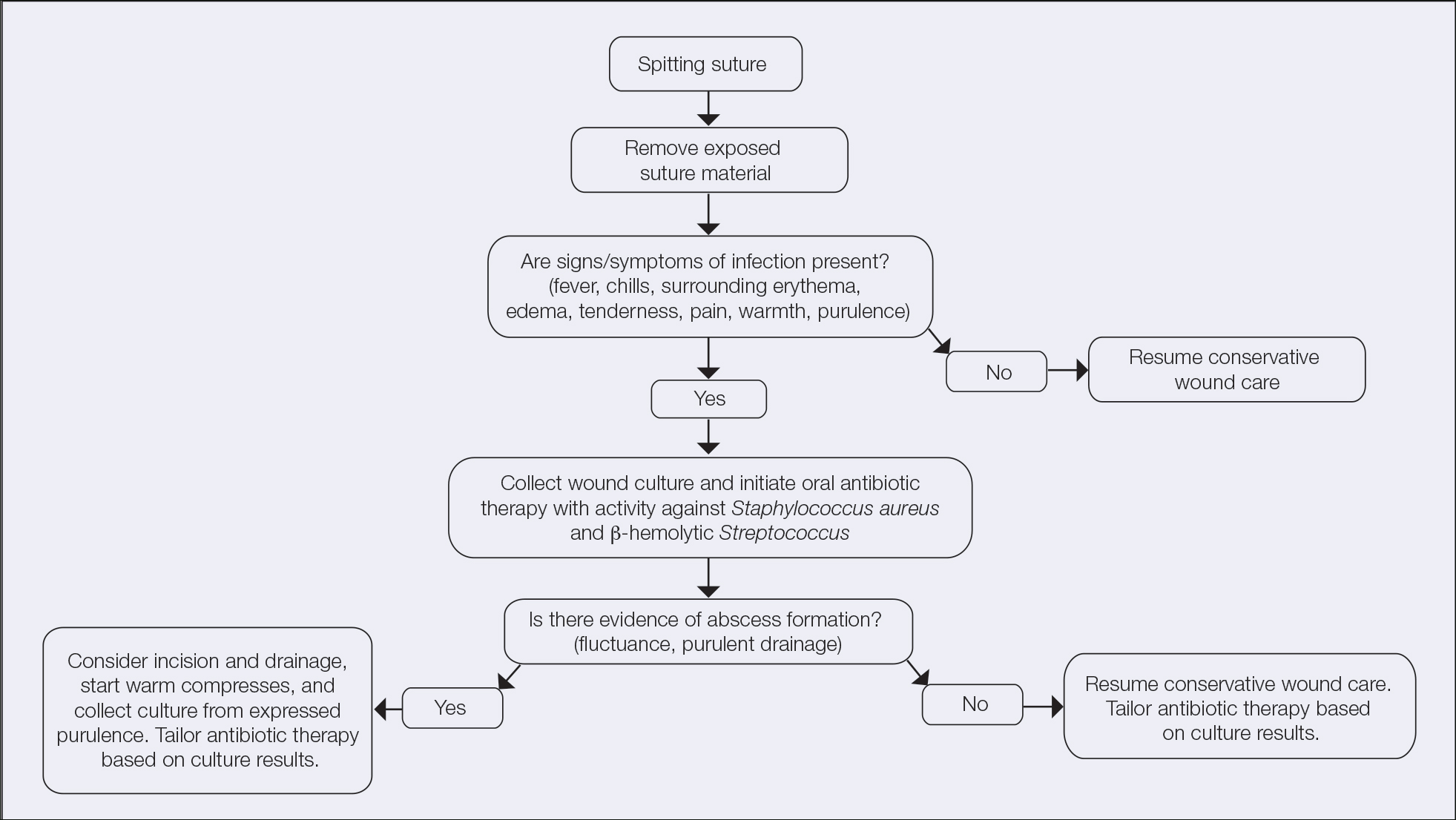
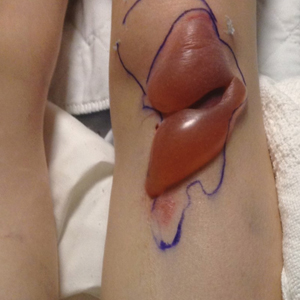
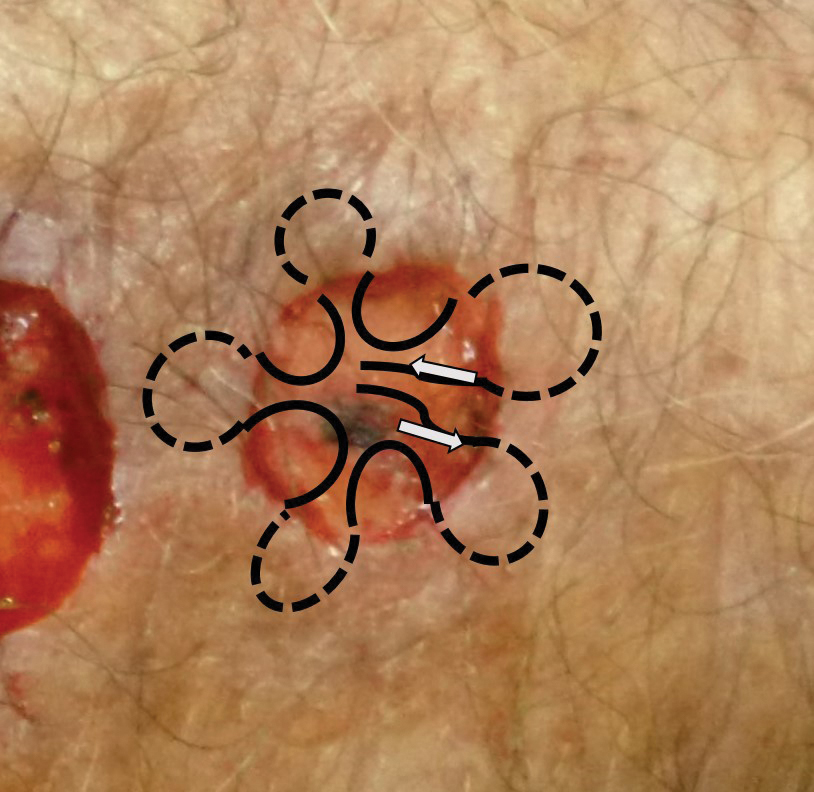
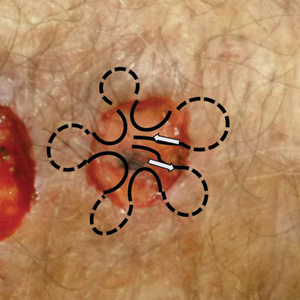
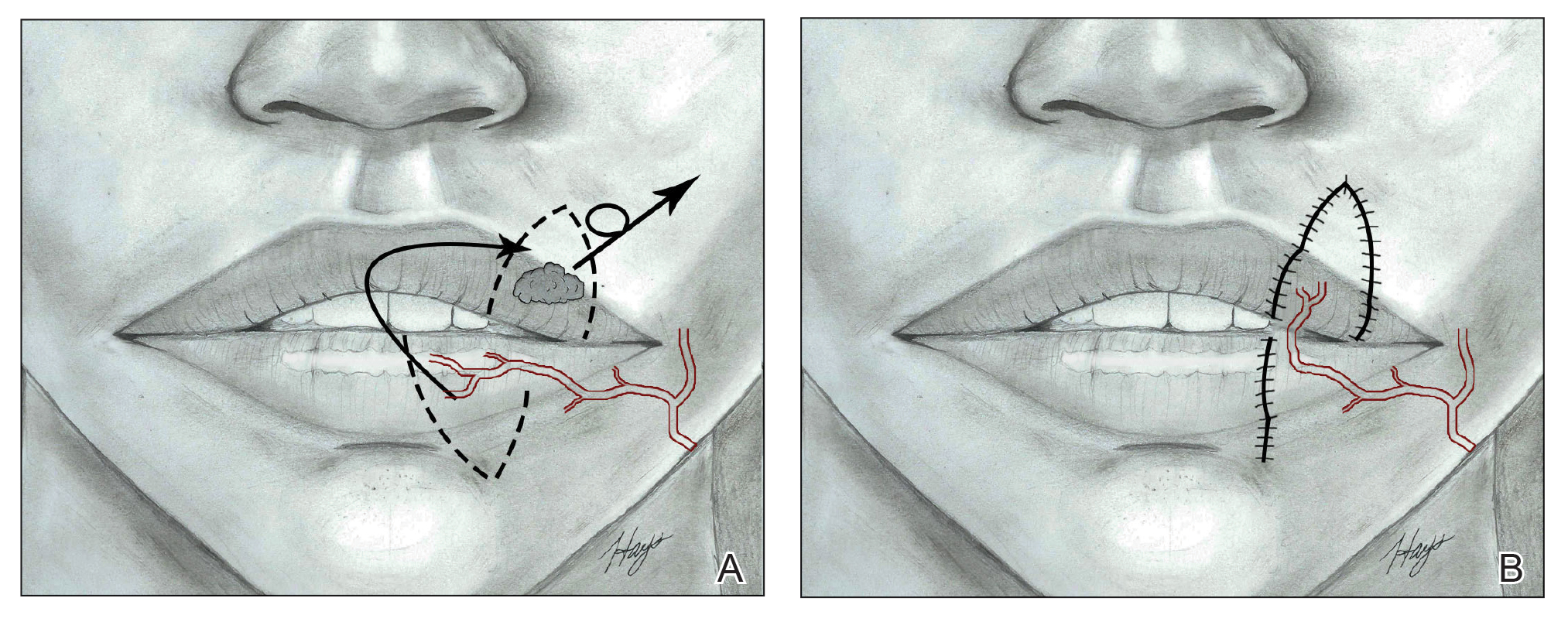
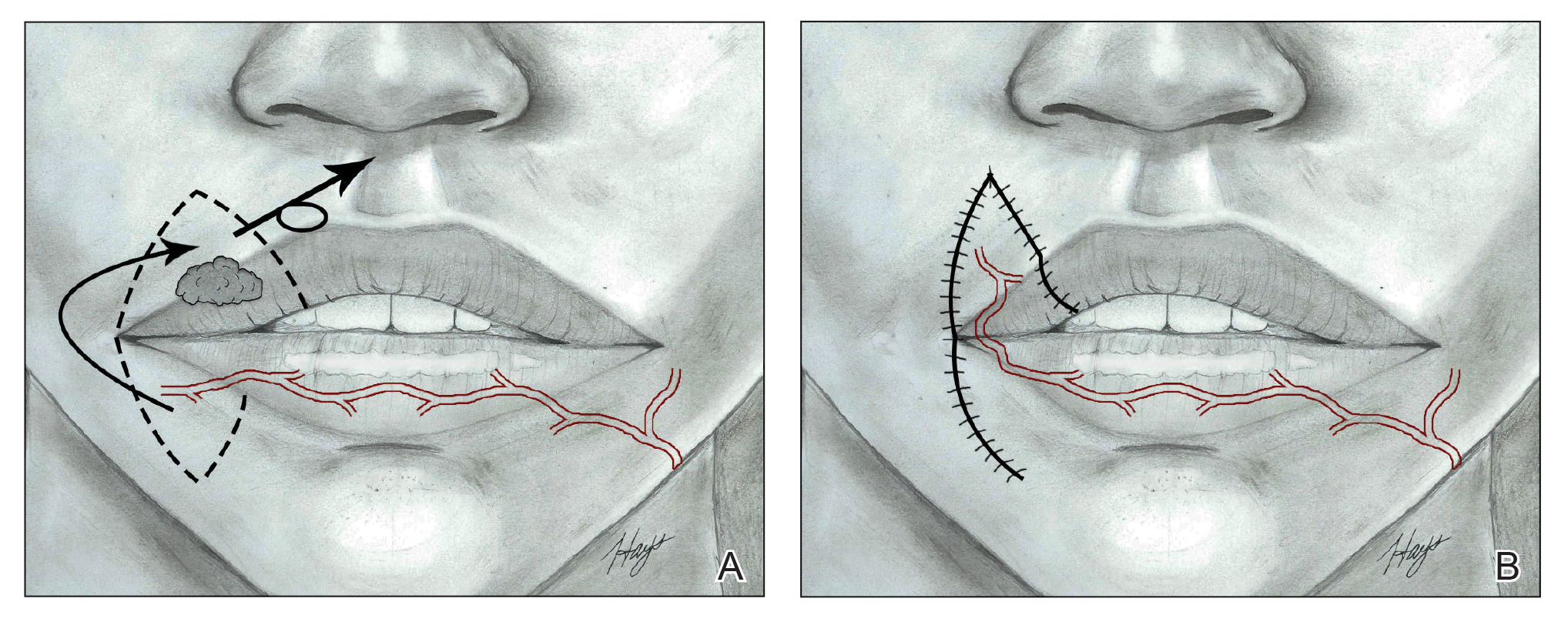
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
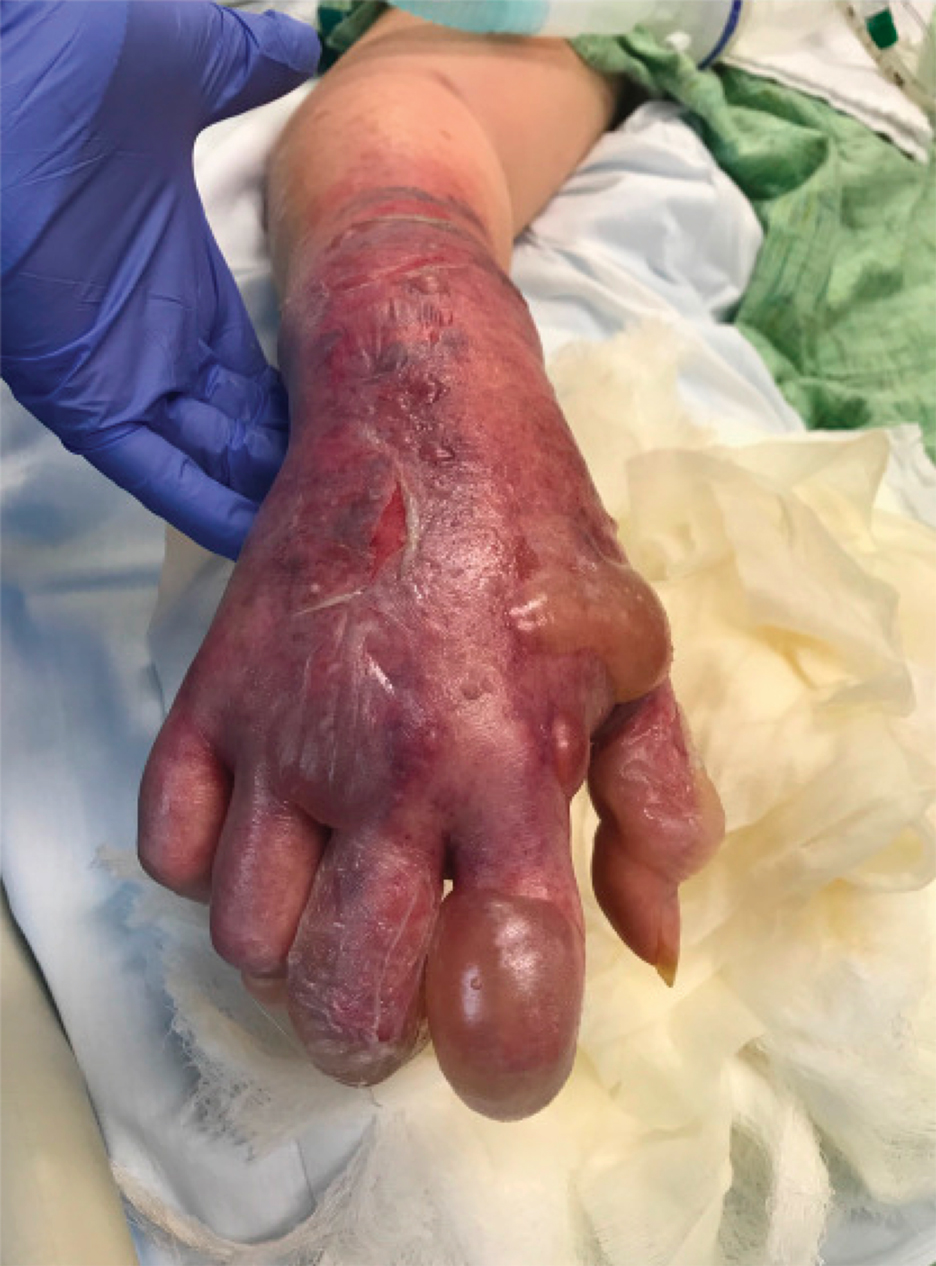
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
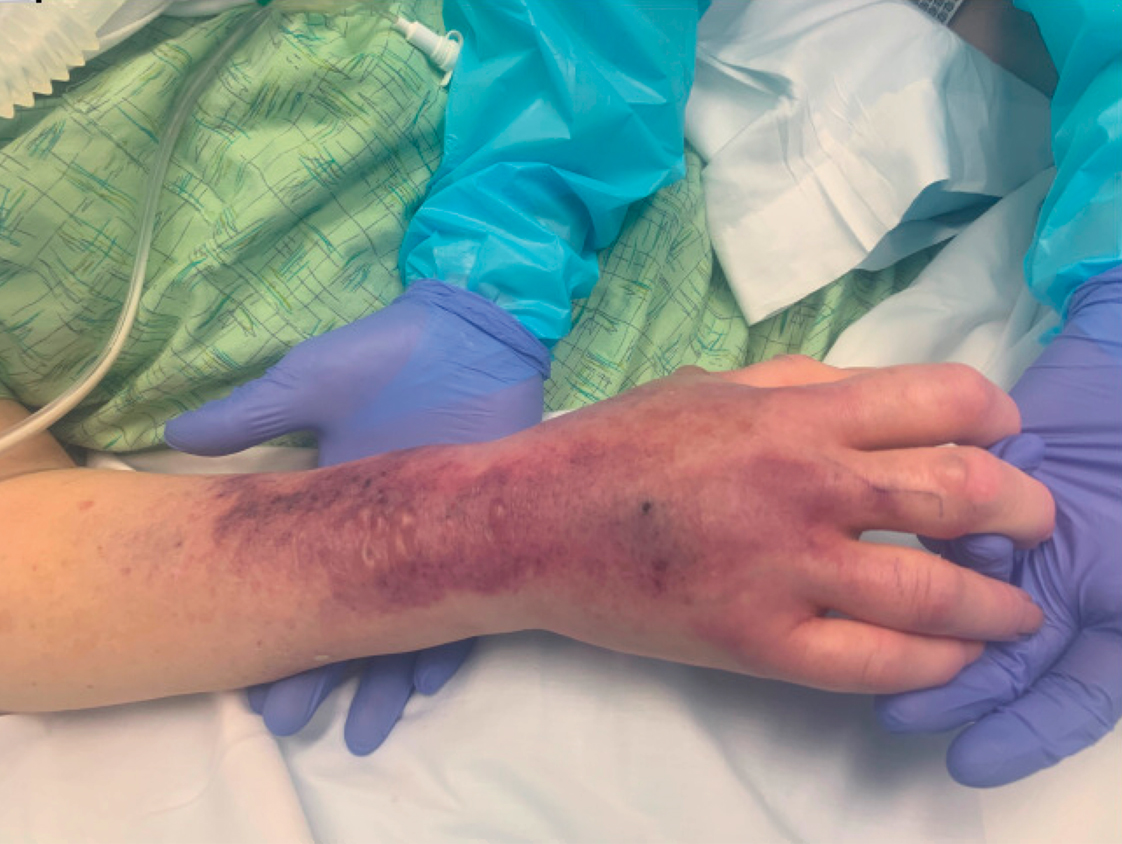
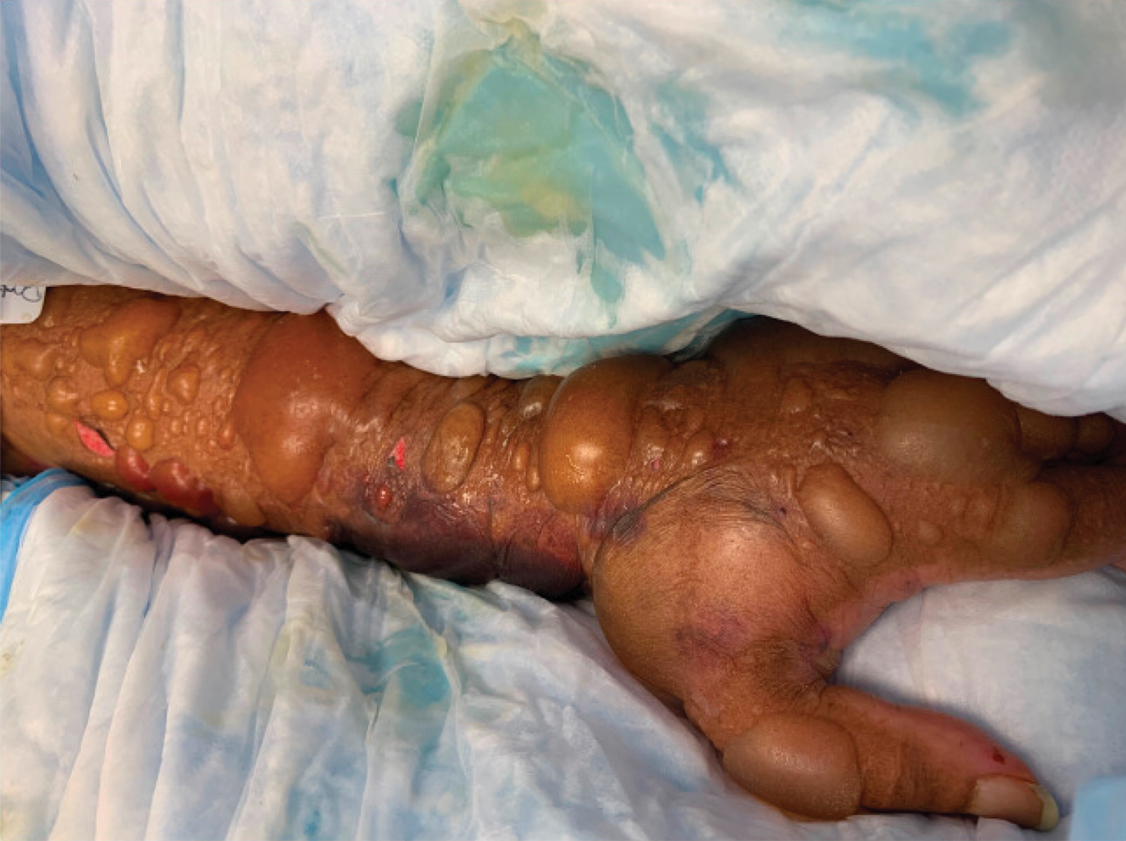
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.
Synthetic snake venom to the rescue? Potential uses in skin health and rejuvenation
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
Cutaneous Complications Associated With Intraosseous Access Placement
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Practice Points
- Intraosseous (IO) access provides rapid vascular access for the delivery of fluids, drugs, and blood products in emergent situations.
- Bullae are potential complications from IO line placement.
Efficacy of Etanercept in the Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.
Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.
Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.
Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.
Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.
Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.
Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Practice Points
- Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening dermatologic emergencies without a universally accepted treatment.
- Results of this study support the use of single-dose subcutaneous etanercept 50 mg as a potentially lifesaving therapy for patients with SJS/TEN.
Type 1 diabetes amputation rates fall in Sweden, rise in U.S.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
FROM ADA 2020
Wound Healing on the Dorsal Hands: An Intrapatient Comparison of Primary Closure, Purse-String Closure, and Secondary Intention
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
The cutaneous benefits of bee venom, Part II: Acupuncture, wound healing, and various potential indications
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
24-7 Dressing Technique to Optimize Wound Healing After Mohs Micrographic Surgery
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Upper Lip Anatomy, Mechanics of Local Flaps, and Considerations for Reconstruction
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.