User login
Atopic Dermatitis and Sleep Disturbances
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Daycare Providers’ Little Helper
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Obesity Drug Zepbound Approved for Obstructive Sleep Apnea
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
Smart Mattress to Reduce SUDEP?
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AES 2024
Sleep Apnea Linked to Heightened Mortality in Epilepsy
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM AES 2024
Noise and Artificial Light
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
If you’ve ever taken a red-eye flight you have probably received a little packet of items the airline hopes will make your night flight more comfortable. If you had shelled out for “extra leg room” or “more comfort” seating, your little kit may have included some one-size-never-fits-all socks, a toothbrush large enough to brush one tooth at a time, and a miniature tube of toothpaste the GEICO gecko would laugh at. I have no personal knowledge what the folks in first class are getting, but I suspect it comes in a calf skin Gucci pouch. But, regardless of where you are sitting, at a minimum your night comfort kit will come with an eye mask and ear plugs. Unfortunately, these freebies are wasted on me because I already use a sleep mask every night and simply turn off my hearing aids to mute the noise. But I appreciate their effort.
Light and sound are well-known sleep disruptors. Temperature gets less attention, but is nonetheless a potent contributor to a poor night’s sleep in my experience. Just by chance while I was recovering from my most recent jet lag, I encountered two papers from investigators who were curious about the association between healthy sleep and ambient light and noise.
The first paper looked at the relationship between artificial light at night (ALAN) and the incidence of insomnia. Looking at more than 300 Chinese cities, the investigators measured ALAN using satellite images and correlated the data with insomnia-related posts on social media. The researchers found when ALAN increased insomnia, related posts also increased. Not surprisingly, this relationship was greater in less populated cities during extreme temperatures and when air quality was poor.
The second paper came from University of Texas at Houston. Using Fitbit data from more than 3000 adolescents, the researchers looked for correlations between blood pressure, sleep health, and “median nighttime anthropogenic noise levels by ZIP code.” Turns out the Federal Highway Administration has a readily available map of these noise levels.
What the investigators found was that adequate sleep significantly reduces the risk of hypertension in adolescents. Not an unexpected finding to an ex-pediatrician like myself who is obsessed with the importance of sleep deprivation. However, the investigators and I were surprised that they had found no association between neighborhood noise alone or in combination with sleep health. I still suspect there is an association lurking there in the weeds of their data, but obviously it is not robust enough to float to the surface. It may be that in an acute situation noise can contribute to hypertension, but over time individuals adjust to the new sound level and their blood pressure settles down. Sleep is such a critical factor that it is not something our cardiovascular system can adapt to so easily. For various reasons most of us may already be functioning at the margins of sleep deprivation.
How then do we respond to observations by these two research teams? Do we take an approach similar to that the airlines have taken and prescribe, hand out, or sell ear plugs and sleep masks to every patient, or at least those with hypertension? This is what we could call the put-the-onus-on-the-patient approach, which seems to be the default when we lack the political will to take a bolder step.
The other path we could call the socio-environmental approach. The airlines have made a passing attempt at this by turning the cabin lights down on red-eye flights. I recently wrote about the “exposome,” which some investigators define as the total non-genetic exposures an individual endures during a lifetime and which in many situations has a negative effect on the individual’s health. These two papers clearly demonstrate that noise and nighttime artificial light are potent features of an uncountable number of individuals’ exposomes.
Unfortunately, it is going to require something far beyond these two relatively obscure studies to move the needle in the direction of a healthier population. It’s is not a stretch to put obesity and the attention deficit phenomenon under this same umbrella where our society needs to look at itself for the answers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
The Cause of All That Stress: Tonsillectomy?
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.
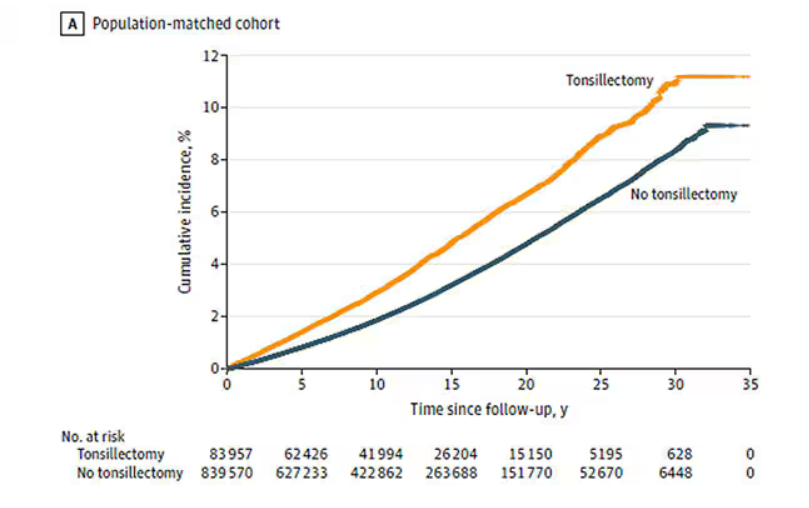
That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.
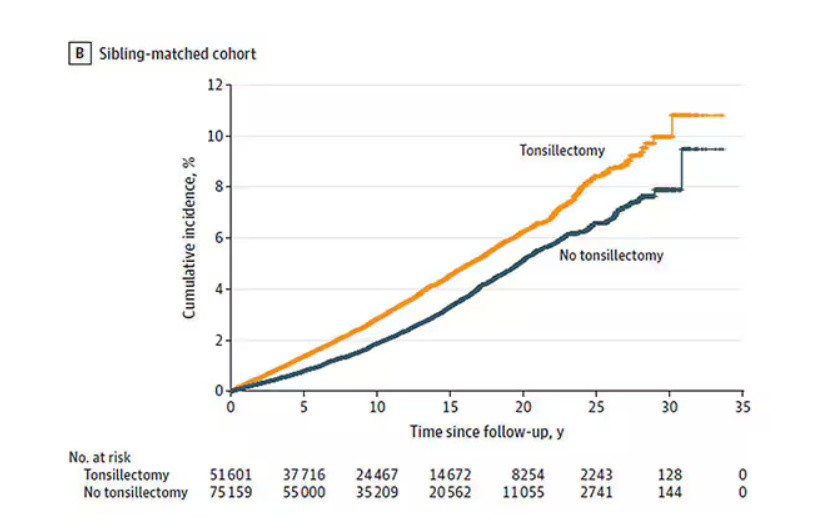
Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
More Biologics May Be Breaking Through for COPD
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.
No, Diet and Exercise Are Not Better Than Drugs for Obesity
They’re literally not better. Idealistically, sure, but literally not. And there’s really no debate. Meaning there’s never been a reproducible diet and exercise intervention that has led to anywhere near the average weight lost by those taking obesity medications. Furthermore, when it comes to the durability of weight lost, the gulf between outcomes with diet and exercise vs obesity medications is even more dramatic.
Looking to the literature, one of the most trotted out studies on lifestyle’s impact on weight over time is the Look AHEAD trial. Before useful obesity medications came on the scene, I trotted it out myself. Why? Because it was heartening when faced with the societal refrain that diet and exercise never worked to be able to show that yes, in fact they do. But how well?
Looking to Look AHEAD’s 4-year data (Obesity [Silver Spring]. 2011 Oct;19[10]:1987-1998), those randomized to the intensive lifestyle initiative arm averaged a 4.7% total body weight loss – an amount that remained the same at 8 years. But I chose 4 years because that’s a better comparison with the semaglutide SELECT trial that revealed at 4 years, the average sustained weight lost was more than double that of Look AHEAD’s, at 10.2%. Meanwhile the recently released SURMOUNT-4 study on tirzepatide reported that at 88 weeks, the average weight lost by participants was a near bariatric surgery level of 25.3% with no signs suggestive of pending regains.
Now maybe you want to cling to the notion that if you just try hard enough, your diet and exercise regime can beat our new meds. Well, it’s difficult to think of a more miserable, often actual vomit-inducing intervention, than the spectacle that used to air weekly on prime time called The Biggest Loser, where participants lived on a ranch and were berated and exercised all day long for the chance to lose the most and win a quarter of a million dollars. But even there, the meds prove to be superior. Although the short-term Biggest Loser data do look markedly better than meds (and than bariatric surgery), whereby the average participant lost 48.8% of their body weight during the grueling 7-month long, 24/7 competition, by postcompetition year 6, the average weight lost dropped to 12.7%.
Yet on November 26, when word came out that Medicare is likely to extend coverage to obesity medications for far more Americans, one of the most common refrains was something along the lines of yes, lifestyle modification is the best choice for dealing with obesity but it’s good that there will be medication options for those where that’s insufficient.
What?
The message is that people simply aren’t trying hard enough. This despite our comfort in knowing that medications have more of an impact than lifestyle on pretty much every other chronic disease. Nor can I recall any other circumstance when coverage of a remarkably effective drug was qualified by the suggestion that known-to-be-inferior interventions are still the best or favored choice.
At this point, obesity medications are plainly the first-line choice of treatment. They provide not only dramatically greater and more durable weight loss than lifestyle interventions, they have also been shown to very significantly reduce the risk for an ever-growing list of other medical concerns including heart attacks, strokes, type 2 diabetes, hypertension, sleep apnea, fatty liver disease, and more, while carrying minimal risk.
Let it also be said that improvements to diet and exercise are worth striving for at any weight, though one should not lose sight of the fact that perpetual, dramatic, intentional, behavior change in the name of health requires vast amounts of wide-ranging privilege to enact — amounts far beyond the average person’s abilities or physiologies (as demonstrated with obesity by decades of disappointing long-term lifestyle outcome data).
Let it also be said that some people will indeed find success solely through lifestyle and that not every person who meets the medical criteria for any medication’s prescription, including obesity medications, is required or encouraged to take it. The clinician’s job, however, at its most basic, is to inform patients who meet medical use criteria of their options, and if a medication is indicated, to inform them of that medication’s risks and benefits and expected outcomes, to help their patients come to their own treatment decisions.
It’s not a bad thing that we have medications that deliver better outcomes than lifestyle — in fact, it’s terrific, and thankfully that they do is true for pretty much every medical condition for which we have medication. That’s in fact why we have medications! And so this constant refrain of golly-gee wouldn’t it be better if we could just manage obesity with lifestyle changes needs to be put to rest — we literally know it wouldn’t be better, and it’s only weight bias that would lead this evidence-based statement to seem off-putting.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa, and Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He reported conflicts of interest with the Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article first appeared on Medscape.com.
They’re literally not better. Idealistically, sure, but literally not. And there’s really no debate. Meaning there’s never been a reproducible diet and exercise intervention that has led to anywhere near the average weight lost by those taking obesity medications. Furthermore, when it comes to the durability of weight lost, the gulf between outcomes with diet and exercise vs obesity medications is even more dramatic.
Looking to the literature, one of the most trotted out studies on lifestyle’s impact on weight over time is the Look AHEAD trial. Before useful obesity medications came on the scene, I trotted it out myself. Why? Because it was heartening when faced with the societal refrain that diet and exercise never worked to be able to show that yes, in fact they do. But how well?
Looking to Look AHEAD’s 4-year data (Obesity [Silver Spring]. 2011 Oct;19[10]:1987-1998), those randomized to the intensive lifestyle initiative arm averaged a 4.7% total body weight loss – an amount that remained the same at 8 years. But I chose 4 years because that’s a better comparison with the semaglutide SELECT trial that revealed at 4 years, the average sustained weight lost was more than double that of Look AHEAD’s, at 10.2%. Meanwhile the recently released SURMOUNT-4 study on tirzepatide reported that at 88 weeks, the average weight lost by participants was a near bariatric surgery level of 25.3% with no signs suggestive of pending regains.
Now maybe you want to cling to the notion that if you just try hard enough, your diet and exercise regime can beat our new meds. Well, it’s difficult to think of a more miserable, often actual vomit-inducing intervention, than the spectacle that used to air weekly on prime time called The Biggest Loser, where participants lived on a ranch and were berated and exercised all day long for the chance to lose the most and win a quarter of a million dollars. But even there, the meds prove to be superior. Although the short-term Biggest Loser data do look markedly better than meds (and than bariatric surgery), whereby the average participant lost 48.8% of their body weight during the grueling 7-month long, 24/7 competition, by postcompetition year 6, the average weight lost dropped to 12.7%.
Yet on November 26, when word came out that Medicare is likely to extend coverage to obesity medications for far more Americans, one of the most common refrains was something along the lines of yes, lifestyle modification is the best choice for dealing with obesity but it’s good that there will be medication options for those where that’s insufficient.
What?
The message is that people simply aren’t trying hard enough. This despite our comfort in knowing that medications have more of an impact than lifestyle on pretty much every other chronic disease. Nor can I recall any other circumstance when coverage of a remarkably effective drug was qualified by the suggestion that known-to-be-inferior interventions are still the best or favored choice.
At this point, obesity medications are plainly the first-line choice of treatment. They provide not only dramatically greater and more durable weight loss than lifestyle interventions, they have also been shown to very significantly reduce the risk for an ever-growing list of other medical concerns including heart attacks, strokes, type 2 diabetes, hypertension, sleep apnea, fatty liver disease, and more, while carrying minimal risk.
Let it also be said that improvements to diet and exercise are worth striving for at any weight, though one should not lose sight of the fact that perpetual, dramatic, intentional, behavior change in the name of health requires vast amounts of wide-ranging privilege to enact — amounts far beyond the average person’s abilities or physiologies (as demonstrated with obesity by decades of disappointing long-term lifestyle outcome data).
Let it also be said that some people will indeed find success solely through lifestyle and that not every person who meets the medical criteria for any medication’s prescription, including obesity medications, is required or encouraged to take it. The clinician’s job, however, at its most basic, is to inform patients who meet medical use criteria of their options, and if a medication is indicated, to inform them of that medication’s risks and benefits and expected outcomes, to help their patients come to their own treatment decisions.
It’s not a bad thing that we have medications that deliver better outcomes than lifestyle — in fact, it’s terrific, and thankfully that they do is true for pretty much every medical condition for which we have medication. That’s in fact why we have medications! And so this constant refrain of golly-gee wouldn’t it be better if we could just manage obesity with lifestyle changes needs to be put to rest — we literally know it wouldn’t be better, and it’s only weight bias that would lead this evidence-based statement to seem off-putting.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa, and Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He reported conflicts of interest with the Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article first appeared on Medscape.com.
They’re literally not better. Idealistically, sure, but literally not. And there’s really no debate. Meaning there’s never been a reproducible diet and exercise intervention that has led to anywhere near the average weight lost by those taking obesity medications. Furthermore, when it comes to the durability of weight lost, the gulf between outcomes with diet and exercise vs obesity medications is even more dramatic.
Looking to the literature, one of the most trotted out studies on lifestyle’s impact on weight over time is the Look AHEAD trial. Before useful obesity medications came on the scene, I trotted it out myself. Why? Because it was heartening when faced with the societal refrain that diet and exercise never worked to be able to show that yes, in fact they do. But how well?
Looking to Look AHEAD’s 4-year data (Obesity [Silver Spring]. 2011 Oct;19[10]:1987-1998), those randomized to the intensive lifestyle initiative arm averaged a 4.7% total body weight loss – an amount that remained the same at 8 years. But I chose 4 years because that’s a better comparison with the semaglutide SELECT trial that revealed at 4 years, the average sustained weight lost was more than double that of Look AHEAD’s, at 10.2%. Meanwhile the recently released SURMOUNT-4 study on tirzepatide reported that at 88 weeks, the average weight lost by participants was a near bariatric surgery level of 25.3% with no signs suggestive of pending regains.
Now maybe you want to cling to the notion that if you just try hard enough, your diet and exercise regime can beat our new meds. Well, it’s difficult to think of a more miserable, often actual vomit-inducing intervention, than the spectacle that used to air weekly on prime time called The Biggest Loser, where participants lived on a ranch and were berated and exercised all day long for the chance to lose the most and win a quarter of a million dollars. But even there, the meds prove to be superior. Although the short-term Biggest Loser data do look markedly better than meds (and than bariatric surgery), whereby the average participant lost 48.8% of their body weight during the grueling 7-month long, 24/7 competition, by postcompetition year 6, the average weight lost dropped to 12.7%.
Yet on November 26, when word came out that Medicare is likely to extend coverage to obesity medications for far more Americans, one of the most common refrains was something along the lines of yes, lifestyle modification is the best choice for dealing with obesity but it’s good that there will be medication options for those where that’s insufficient.
What?
The message is that people simply aren’t trying hard enough. This despite our comfort in knowing that medications have more of an impact than lifestyle on pretty much every other chronic disease. Nor can I recall any other circumstance when coverage of a remarkably effective drug was qualified by the suggestion that known-to-be-inferior interventions are still the best or favored choice.
At this point, obesity medications are plainly the first-line choice of treatment. They provide not only dramatically greater and more durable weight loss than lifestyle interventions, they have also been shown to very significantly reduce the risk for an ever-growing list of other medical concerns including heart attacks, strokes, type 2 diabetes, hypertension, sleep apnea, fatty liver disease, and more, while carrying minimal risk.
Let it also be said that improvements to diet and exercise are worth striving for at any weight, though one should not lose sight of the fact that perpetual, dramatic, intentional, behavior change in the name of health requires vast amounts of wide-ranging privilege to enact — amounts far beyond the average person’s abilities or physiologies (as demonstrated with obesity by decades of disappointing long-term lifestyle outcome data).
Let it also be said that some people will indeed find success solely through lifestyle and that not every person who meets the medical criteria for any medication’s prescription, including obesity medications, is required or encouraged to take it. The clinician’s job, however, at its most basic, is to inform patients who meet medical use criteria of their options, and if a medication is indicated, to inform them of that medication’s risks and benefits and expected outcomes, to help their patients come to their own treatment decisions.
It’s not a bad thing that we have medications that deliver better outcomes than lifestyle — in fact, it’s terrific, and thankfully that they do is true for pretty much every medical condition for which we have medication. That’s in fact why we have medications! And so this constant refrain of golly-gee wouldn’t it be better if we could just manage obesity with lifestyle changes needs to be put to rest — we literally know it wouldn’t be better, and it’s only weight bias that would lead this evidence-based statement to seem off-putting.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa, and Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He reported conflicts of interest with the Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article first appeared on Medscape.com.
New Data: The Most Promising Treatments for Long COVID
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
