User login
Evaluation of Internet Information About Rotator Cuff Repair
Patients are learning about health and disease more independently than before, but such self-education may pose a unique challenge for practicing physicians. Although educated patients can assist in the critical appraisal of treatment options,1 misinformed patients may have preconceived treatment biases and unrealistic expectations. More than 66 million Americans use the Internet daily, and recent surveys have shown 86% have used the Internet for health-related information.2,3 With Internet use increasing, the number of patients turning to the web for medical information is increasing as well.4 For many patients, this information can be useful in making decisions about their health and health care.5
Although accessing medical information from the Internet has grown exponentially, analysis of information quality has grown considerably slower.6 With no regulatory body monitoring content, there is easy circumvention of the peer review process, an essential feature of academic publishing.7 With no external regulation, the information retrieved may be incorrect, outdated, or misleading. Many orthopedic studies have analyzed Internet content about numerous diagnoses.3-6,8-18 Most of these studies have found this information highly variable and of poor quality.
We conducted a study to evaluate and analyze rotator cuff repair information available to the general public through the Internet; to assess changes in the quality of information over time; to determine if sites sponsored by academic institutions offered higher-quality information; and to assess whether the readability of the material varied according to DISCERN scores.
Rotator cuff repairs are among the most common surgeries performed by orthopedic surgeons. To our knowledge, this is the first study to assess the quality of web information about rotator cuff repairs. We hypothesized that the quality of information would positively correlate with the reading level of the material presented, that academic institutions would present the highest-quality information, and that the type of information presented would change over time.
Materials and Methods
We used the search phrase rotator cuff repair on the 3 most popular search engines: Google, Yahoo!, and Bing. Google is the dominant engine, taking 83.06% of total market share, followed by Yahoo! (6.86%) and Bing (4.27%).5 The first 50 websites identified by each search engine were selected for evaluation, excluding duplicates or overlapping websites. Similarly, advertisements and strictly video results lacking text were excluded. After each engine was queried, a master list of 150 websites was created for individual evaluation and assessment. To assess changes in results over time, we performed 2 searches, on November 16, 2011, and May 18, 2014.
The content of each website was analyzed for authorship, ability to contact the author, discussion of disorder, surgical treatment, complications, surgical eligibility, rehabilitation, other treatment options, and use of peer-reviewed sources. Authorship was placed in 1 of 6 categories:
1. Academic—university-affiliated physician or research group.
2. Private—physician or group without stated affiliation to an academic organization.
3. Industry—manufacturing or marketing company advertising a product or service for profit.
4. News source—bulletin or article without affiliation to a hospital or an academic institution.
5. Public education—individual or organization with noncommercial website providing third-party information (eg, Wikipedia, About.com).
6. Blog—website publishing an individual’s personal experiences in diary or journal form.
Websites were also assessed for accuracy and validity based on presence or absence of Health On the Net code (HONcode) certification and DISCERN score. Designed by the Health On the Net Foundation in 1996, HONcode provides a framework for disseminating high-quality medical information over the web.19 Website owners can request that their sites be evaluated for HONcode certification; a site that qualifies can display the HONcode seal.20 The DISCERN project, initially funded by the National Health Service in the United Kingdom, judges the quality of written information available on health-related websites.21 It determines the quality of a publication on the basis of 16 questions: The first 8 address the publication’s reliability, the next 7 involve specific details of treatment choices, and the last is an overall rating of the website.
Website readability was assessed with the Flesch-Kincaid test. This test, designed under contract with the US Navy in 1975, has been used in other orthopedic studies.19 Regression analysis was performed to check for correlation between website readability and DISCERN score. Analysis of variance was used to analyze differences between scores.
Results
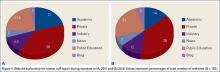
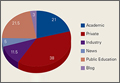
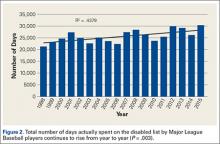
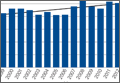
We performed a comprehensive analysis of the top 50 websites from each of the 3 search engines (N = 150 websites) (Figures 1–5, Table). Regarding authorship, our 2 searches demonstrated similar values (Figure 1). In 2011, 21% of websites were associated with an academic institution, 38% were authored by private physicians or hospital or physician groups not associated with an academic institution, 11.5% were industry-sponsored, 5% were news bulletins or media reports, 21.5% were public education websites, and 3% were personal blogs. Our 2014 search found a similar distribution of contributors. Between 2011 and 2014, the largest change was in academic authors, which decreased by 7%, from 21% to 14%. Percentage of websites authored by private physicians remained constant from the first to the second search: 38%.
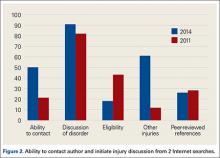
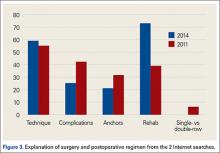
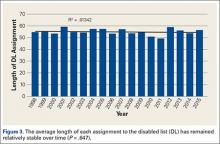
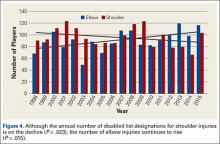
When the 2011 and 2014 website content was compared, several changes were noted. Percentage of websites providing an author contact method increased from 21% to 50% (Figure 2), percentage detailing rotator cuff repairs increased from 82% to 91%, and percentage introducing treatment options in addition to surgical management increased from 11.5% to 61%. Percentage discussing surgical eligibility, however, decreased from 43% to 18%. Percentage citing peer-reviewed sources remained relatively constant (28%, 26%), as did percentage discussing surgical technique for rotator cuff repair (55%, 59%) (Figure 3). A major decrease was found in percentage of websites discussing surgical complications, 42% in 2011 down to 25% in 2014, whereas a major increase was found in percentage discussing rehabilitation, from 39% in 2011 up to 73% in 2014. In 2014, no websites discussed double- versus single-row surgery—compared with 6% in 2011. False claims remained low between 2011 and 2014. In both searches, no website guaranteed a return to sport, and few made claims of painless or bloodless surgery.
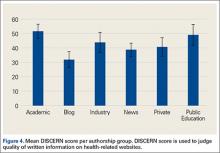
DISCERN scores for websites found during the 2014 search were averaged for each of the 6 authorship groups (Figure 4). The highest DISCERN scores were given to academic institution websites (51.6) and public education websites (49). For the academic websites, this difference was significant relative to news, blog, and private physician websites (Ps = .012, .001, .001) The lowest DISCERN scores were given to news organization websites and personal blogs. DISCERN scores were 43.8 for industry sources and 40.7 for private physician groups; the difference was not significant (P = .229). Overall mean DISCERN score for all websites was 44. Eleven percent of websites were HONcode-certified.
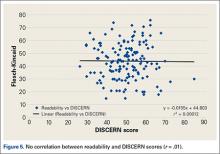
No correlation was found between website readability and DISCERN score; correlation coefficient r was .01 (Figure 5). For the websites in 2014, mean Flesch-Kincaid readability score was 50.17, and mean grade level was 10.98; coefficient of determination r2 was 0.00012.
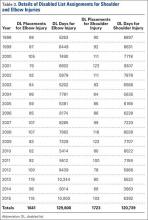
The Table compares our data with data from other orthopedic studies that have analyzed the quality of Internet information about various orthopedic injuries, diseases, and procedures.3-6,8,9,11-18 With its mean DISCERN score of 44, the present rotator cuff tear study was ranked third of 6 studies that have used this scoring system to analyze website content. Of these 6 studies, those reviewing osteosarcoma and juvenile idiopathic arthritis were ranked highest (mean scores, 49.8 and 48.9, respectively), and the study reviewing scoliosis surgery was ranked lowest (38.9). Bruce-Brand and colleagues9 recently found a mean DISCERN score of 41 for anterior cruciate ligament (ACL) reconstruction. When considering HONcode-certified websites, our Internet search for rotator cuff tears found the third lowest percentage, 10.5%, compared with the other studies (Table); the highest percentage, 30%, was found for websites discussing concussions in athletes. When considering authorship, our rotator cuff study found the third highest percentage, 76%, authored by academic centers, physicians, and public education websites; the highest percentage was found in websites discussing ACL reconstruction. Websites discussing ACL reconstruction also had the highest percentage of websites authored by industry.9
Discussion
To our knowledge, this is the first study specifically analyzing the quality of Internet information about rotator cuff repairs. A similar study, conducted by Starman and colleagues15 in 2010, addressed the quality of web information about 10 common sports medicine diagnoses, one of which was rotator cuff tears. In that study, only 16 of the websites included discussed rotator cuff tears. In addition, the authors used a customized, HONcode-based grading system to analyze each website, making their data difficult to compare across studies.
Ideally, a high-quality medical website should be written by a credible source and should cover a disorder, treatment options, eligibility, rehabilitation, and complications. As there is no standard grading system for analyzing web content about rotator cuff repairs, we analyzed the websites for specific information we thought should be included in a high-quality website (Figures 2, 3). When considering authorship, we found academic centers, private physicians, and educational sources comprised 76% of the sources; industry sources made up only 12%. Similar findings were noted by investigators analyzing Internet information about other orthopedic topics, including ACL reconstruction, lumbar arthroplasty, osteosarcoma, and cervical spine surgery.5,11,22 Studies analyzing websites for information on ACL tears and distal radius fractures found have a higher percentage of industry-sponsored websites.9,10
DISCERN showed that the highest-quality information came from websites with academic affiliations, consistent with previous studies,3,9,17 and its mean score (51.6) was significantly higher than the scores for private physician websites, news sites, and blogs (Ps = .001, .016, .001); the least reliable information was from personal blogs and news outlets. Of note, mean DISCERN score was higher for the industry websites we found than for private physician websites (43.8 vs 40.7), though the difference was not significant (P = .229). Previous investigators considered number of industry-sponsored websites as a marker of poor quality of information relating to a given topic; however, given the DISCERN scores in our study, this might not necessarily be true.6 Based on the present study’s data, websites affiliated with academic institutions would be recommended for patients searching for high-quality information about rotator cuff tears.
Given DISCERN scores across studies, information about rotator cuff tears ranked below information about osteosarcoma and juvenile idiopathic arthritis but above information about scoliosis, cervical spine surgery, and ACL reconstruction (Table). DISCERN scores must be compared across studies, as there are no definitions for good and poor DISCERN scores.
Of the 4 studies that analyzed percentage of websites citing peer-reviewed sources, only our study and the study of cervical spine surgery18 analyzed that percentage as well as DISCERN score. Percentage citing peer-reviewed sources was 26% for rotator cuff tears and 24% for cervical spine surgery; the respective DISCERN scores were 44 and 43.6. As only these 2 studies could be compared, no real correlation between percentage of websites citing peer-reviewed sources and the quality of the content on a given topic can be assessed. More research into this relationship is needed. One already delineated association is the correlation between HONcode-certified sites and high DISCERN scores.21 For high-quality medical information, physicians can direct their patients both to academic institution websites and to HONcode-certified websites.
When we compared the present study with previous investigations, we found a large difference between search results for a given topic. In 2013, Duncan and colleagues6 and Bruce-Brand and colleagues9 used similar study designs (eg, search terms, search engines) for their investigations of quality of web information. Their results, however, were widely different. For example, percentages of industry authorship were 4.5% (Duncan and colleagues6) and 64% (Bruce-Brand and colleagues9). This inconsistency between studies conducted during similar periods might be related to what appears at the top of the results queue for a search. Duncan and colleagues6 analyzed 200 websites, Bruce-Brand and colleagues9 only 45. Industries may have made financial arrangements and used search engine optimization techniques to have their websites listed first in search results.
In our study, we also analyzed how web information has changed over time. On the Internet, information changes daily, and we hypothesized that the content found during our 2 searches (2011, 2014) would yield different results. Surprisingly, the data were similar, particularly concerning authorship (Figures 1, 2). In both searches, the largest authorship source was private physician or physician groups (38% in 2011 and 2014). Other authorship sources showed little change in percentage between searches. As for content, we found both increases and decreases in specific web information. Ability to contact authors increased from 21% (2011) to 50% (2014). We think it is important that websites offer a communication channel to people who read the medical information the sites provide. Percentage of websites discussing nonoperative treatment options increased from 11.5% to 61%. Therefore, patients in 2014 were being introduced to more options (in addition to surgery) for managing shoulder pain—an improvement in quality of information between the searches. Percentage of websites discussing surgical eligibility, however, decreased from 43% to 18%—a negative development in information quality. Another decrease, from 42% to 25%, was found for websites discussing surgical complications. Given the data as a whole, and our finding both negative and positive changes, it appears the quality of web content has not improved significantly. Interestingly, no websites discussed double- versus single-row surgery in 2014, but 6% did so in 2011.
Lost in the discussion of quality and reliability of information is whether patients comprehend what they are reading.23 Yi and colleagues19 recentlyassessed the readability level of arthroscopy information in articles published online by the American Academy of Orthopaedic Surgeons (AAOS) and the Arthroscopy Association of North America (AANA). The investigators used the Flesch-Kincaid readability test to determine readability level in terms of grade level. They found that the majority of the patient education articles on the AAOS and AANA sites had a readability level far above the national average; only 4 articles were written at or below the eighth-grade level, the current average reading level in the United States.24 Information that is not comprehensible is of no use to patients, and information that physicians and researchers consider high-quality might not be what patients consider high-quality. As we pursue higher-quality web content, we need to consider that its audience includes nonmedical readers, our patients. In the present study, we found that the readability of a website had no correlation with the site’s DISCERN score (Figure 5). Therefore, for information about rotator cuff repairs, higher-quality websites are no harder than lower-quality sites for patients to comprehend. The Flesch-Kincaid readability test is flawed in that it considers only total number of syllables per word and words per sentence, not nontextual elements of patient education materials, such as illustrations on a website. The 10.98 mean grade level found in our study is higher than the levels found for most studies reviewed by Yi and colleagues.19
This study had several limitations. During an Internet search, the number of websites a user visits drops precipitously after the first page of results. Studies have shown the top 20 sites in a given search receive 97% of the views, and the top 3 receive 58.4%. Whether patients visit websites far down in the list of 150 we found in our given search is unknown. Last, the Flesch-Kincaid readability test is flawed in several ways but nevertheless is used extensively in research. Grading is based on number of words and syllables used in a given sentence; it does not take into account the complexity or common usage of a given word or definition. Therefore, websites may receive low Flesch-Kincaid scores—indicating ease of reading—despite their use of complex medical terminology and jargon that complicate patients’ comprehension of the material.
Conclusion
Numerous authors have evaluated orthopedic patients’ accessing of medical information from the Internet. Although the Internet makes access easier, unreliable content can lead patients to develop certain notions about the direction of their care and certain expectations regarding their clinical outcomes. With there being no regulatory body monitoring content, the peer review process, an essential feature of academic publishing, can be easily circumvented.25
In this study, the highest-quality websites had academic affiliations. Quality of information about rotator cuff repairs was similar to what was found for other orthopedic topics in comparable studies. Surprisingly, there was little change in authorship and content of web information between our 2 search periods (2011, 2014). Although there has been a rapid increase in the number of medical websites, quality of content seems not to have changed significantly. Patients look to physicians for guidance but increasingly are accessing the Internet for additional information. It is essential that physicians understand the quality of information available on the Internet when counseling patients regarding surgery.
1. Brunnekreef JJ, Schreurs BW. Total hip arthroplasty: what information do we offer patients on websites of hospitals? BMC Health Serv Res. 2011;11:83.
2. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
3. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the Internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
4. Groves ND, Humphreys HW, Williams AJ, Jones A. Effect of informational Internet web pages on patients’ decision making: randomised controlled trial regarding choice of spinal or general anaesthesia for orthopaedic surgery. Anaesthesia. 2010;65(3):277-282.
5. Purcell K, Brenner J, Rainie L. Search Engine Use 2012. Washington, DC: Pew Internet & American Life Project; 2012.
6. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the Internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
7. Lichtenfeld LJ. Can the beast be tamed? The woeful tale of accurate health information on the Internet. Ann Surg Oncol. 2012;19(3):701-702.
8. Ahmed OH, Sullivan SJ, Schneiders AG, McCrory PR. Concussion information online: evaluation of information quality, content and readability of concussion-related websites. Br J Sports Med. 2012;46(9):675-683.
9. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the Internet. Arthroscopy. 2013;29(6):1095-1100.
10. Dy JC, Taylor SA, Patel RM, Kitay A, Roberts TR, Daluiski A. The effect of search term on the quality and accuracy of online information regarding distal radius fractures. J Hand Surg Am. 2012;37(9):1881-1887.
11. Garcia RM, Messerschmitt PJ, Ahn NU. An evaluation of information on the Internet of a new device: the lumbar artificial disc replacement. J Spinal Disord Tech. 2009;22(1):52-57.
12. Gosselin MM, Mulcahey MK, Feller E, Hulstyn MJ. Examining Internet resources on gender differences in ACL injuries: what patients are reading. Knee. 2013;20(3):196-202.
13. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
14. Morr S, Shanti N, Carrer A, Kubeck J, Gerling MC. Quality of information concerning cervical disc herniation on the Internet. Spine J. 2010;10(4):350-354.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of Internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Stinson JN, Tucker L, Huber A, et al. Surfing for juvenile idiopathic arthritis: perspectives on quality and content of information on the Internet. J Rheumatol. 2009;36(8):1755-1762.
17. Sullivan TB, Anderson JS, Ahn UM, Ahn NU. Can Internet information on vertebroplasty be a reliable means of patient self-education? Clin Orthop Relat Res. 2014;472(5):1597-1604.
18. Weil AG, Bojanowski MW, Jamart J, Gustin T, Lévêque M. Evaluation of the quality of information on the Internet available to patients undergoing cervical spine surgery. World Neurosurg. 2014;82(1-2):e31-e39.
19. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
20. Boyer C, Selby M, Scherrer JR, Appel RD. The Health On the Net code of conduct for medical and health websites. Comput Biol Med. 1998;28(5):603-610.
21. Silberg WM, Lundberg GD, Musacchio RA. Assessing, controlling, and assuring the quality of medical information on the Internet: Caveant lector et viewor—Let the reader and viewer beware. JAMA. 1997;277(15):1244-1245.
22. Fabricant PD, Dy CJ, Patel RM, Blanco JS, Doyle SM. Internet search term affects the quality and accuracy of online information about developmental hip dysplasia. J Pediatr Orthop. 2013;33(4):361-365.
23. Aslam N, Bowyer D, Wainwright A, Theologis T, Benson M. Evaluation of Internet use by paediatric orthopaedic outpatients and the quality of information available. J Pediatr Orthop B. 2005;14(2):129-133.
24. Wetzler MJ. “I found it on the Internet”: how reliable and readable is patient information? Arthroscopy. 2013;29(6):967-968.
25. Qureshi SA, Koehler SM, Lin JD, Bird J, Garcia RM, Hecht AC. An evaluation of information on the Internet about a new device: the cervical artificial disc replacement. Spine. 2012;37(10):881-883.
Patients are learning about health and disease more independently than before, but such self-education may pose a unique challenge for practicing physicians. Although educated patients can assist in the critical appraisal of treatment options,1 misinformed patients may have preconceived treatment biases and unrealistic expectations. More than 66 million Americans use the Internet daily, and recent surveys have shown 86% have used the Internet for health-related information.2,3 With Internet use increasing, the number of patients turning to the web for medical information is increasing as well.4 For many patients, this information can be useful in making decisions about their health and health care.5
Although accessing medical information from the Internet has grown exponentially, analysis of information quality has grown considerably slower.6 With no regulatory body monitoring content, there is easy circumvention of the peer review process, an essential feature of academic publishing.7 With no external regulation, the information retrieved may be incorrect, outdated, or misleading. Many orthopedic studies have analyzed Internet content about numerous diagnoses.3-6,8-18 Most of these studies have found this information highly variable and of poor quality.
We conducted a study to evaluate and analyze rotator cuff repair information available to the general public through the Internet; to assess changes in the quality of information over time; to determine if sites sponsored by academic institutions offered higher-quality information; and to assess whether the readability of the material varied according to DISCERN scores.
Rotator cuff repairs are among the most common surgeries performed by orthopedic surgeons. To our knowledge, this is the first study to assess the quality of web information about rotator cuff repairs. We hypothesized that the quality of information would positively correlate with the reading level of the material presented, that academic institutions would present the highest-quality information, and that the type of information presented would change over time.
Materials and Methods
We used the search phrase rotator cuff repair on the 3 most popular search engines: Google, Yahoo!, and Bing. Google is the dominant engine, taking 83.06% of total market share, followed by Yahoo! (6.86%) and Bing (4.27%).5 The first 50 websites identified by each search engine were selected for evaluation, excluding duplicates or overlapping websites. Similarly, advertisements and strictly video results lacking text were excluded. After each engine was queried, a master list of 150 websites was created for individual evaluation and assessment. To assess changes in results over time, we performed 2 searches, on November 16, 2011, and May 18, 2014.
The content of each website was analyzed for authorship, ability to contact the author, discussion of disorder, surgical treatment, complications, surgical eligibility, rehabilitation, other treatment options, and use of peer-reviewed sources. Authorship was placed in 1 of 6 categories:
1. Academic—university-affiliated physician or research group.
2. Private—physician or group without stated affiliation to an academic organization.
3. Industry—manufacturing or marketing company advertising a product or service for profit.
4. News source—bulletin or article without affiliation to a hospital or an academic institution.
5. Public education—individual or organization with noncommercial website providing third-party information (eg, Wikipedia, About.com).
6. Blog—website publishing an individual’s personal experiences in diary or journal form.
Websites were also assessed for accuracy and validity based on presence or absence of Health On the Net code (HONcode) certification and DISCERN score. Designed by the Health On the Net Foundation in 1996, HONcode provides a framework for disseminating high-quality medical information over the web.19 Website owners can request that their sites be evaluated for HONcode certification; a site that qualifies can display the HONcode seal.20 The DISCERN project, initially funded by the National Health Service in the United Kingdom, judges the quality of written information available on health-related websites.21 It determines the quality of a publication on the basis of 16 questions: The first 8 address the publication’s reliability, the next 7 involve specific details of treatment choices, and the last is an overall rating of the website.
Website readability was assessed with the Flesch-Kincaid test. This test, designed under contract with the US Navy in 1975, has been used in other orthopedic studies.19 Regression analysis was performed to check for correlation between website readability and DISCERN score. Analysis of variance was used to analyze differences between scores.
Results
We performed a comprehensive analysis of the top 50 websites from each of the 3 search engines (N = 150 websites) (Figures 1–5, Table). Regarding authorship, our 2 searches demonstrated similar values (Figure 1). In 2011, 21% of websites were associated with an academic institution, 38% were authored by private physicians or hospital or physician groups not associated with an academic institution, 11.5% were industry-sponsored, 5% were news bulletins or media reports, 21.5% were public education websites, and 3% were personal blogs. Our 2014 search found a similar distribution of contributors. Between 2011 and 2014, the largest change was in academic authors, which decreased by 7%, from 21% to 14%. Percentage of websites authored by private physicians remained constant from the first to the second search: 38%.
When the 2011 and 2014 website content was compared, several changes were noted. Percentage of websites providing an author contact method increased from 21% to 50% (Figure 2), percentage detailing rotator cuff repairs increased from 82% to 91%, and percentage introducing treatment options in addition to surgical management increased from 11.5% to 61%. Percentage discussing surgical eligibility, however, decreased from 43% to 18%. Percentage citing peer-reviewed sources remained relatively constant (28%, 26%), as did percentage discussing surgical technique for rotator cuff repair (55%, 59%) (Figure 3). A major decrease was found in percentage of websites discussing surgical complications, 42% in 2011 down to 25% in 2014, whereas a major increase was found in percentage discussing rehabilitation, from 39% in 2011 up to 73% in 2014. In 2014, no websites discussed double- versus single-row surgery—compared with 6% in 2011. False claims remained low between 2011 and 2014. In both searches, no website guaranteed a return to sport, and few made claims of painless or bloodless surgery.
DISCERN scores for websites found during the 2014 search were averaged for each of the 6 authorship groups (Figure 4). The highest DISCERN scores were given to academic institution websites (51.6) and public education websites (49). For the academic websites, this difference was significant relative to news, blog, and private physician websites (Ps = .012, .001, .001) The lowest DISCERN scores were given to news organization websites and personal blogs. DISCERN scores were 43.8 for industry sources and 40.7 for private physician groups; the difference was not significant (P = .229). Overall mean DISCERN score for all websites was 44. Eleven percent of websites were HONcode-certified.
No correlation was found between website readability and DISCERN score; correlation coefficient r was .01 (Figure 5). For the websites in 2014, mean Flesch-Kincaid readability score was 50.17, and mean grade level was 10.98; coefficient of determination r2 was 0.00012.
The Table compares our data with data from other orthopedic studies that have analyzed the quality of Internet information about various orthopedic injuries, diseases, and procedures.3-6,8,9,11-18 With its mean DISCERN score of 44, the present rotator cuff tear study was ranked third of 6 studies that have used this scoring system to analyze website content. Of these 6 studies, those reviewing osteosarcoma and juvenile idiopathic arthritis were ranked highest (mean scores, 49.8 and 48.9, respectively), and the study reviewing scoliosis surgery was ranked lowest (38.9). Bruce-Brand and colleagues9 recently found a mean DISCERN score of 41 for anterior cruciate ligament (ACL) reconstruction. When considering HONcode-certified websites, our Internet search for rotator cuff tears found the third lowest percentage, 10.5%, compared with the other studies (Table); the highest percentage, 30%, was found for websites discussing concussions in athletes. When considering authorship, our rotator cuff study found the third highest percentage, 76%, authored by academic centers, physicians, and public education websites; the highest percentage was found in websites discussing ACL reconstruction. Websites discussing ACL reconstruction also had the highest percentage of websites authored by industry.9
Discussion
To our knowledge, this is the first study specifically analyzing the quality of Internet information about rotator cuff repairs. A similar study, conducted by Starman and colleagues15 in 2010, addressed the quality of web information about 10 common sports medicine diagnoses, one of which was rotator cuff tears. In that study, only 16 of the websites included discussed rotator cuff tears. In addition, the authors used a customized, HONcode-based grading system to analyze each website, making their data difficult to compare across studies.
Ideally, a high-quality medical website should be written by a credible source and should cover a disorder, treatment options, eligibility, rehabilitation, and complications. As there is no standard grading system for analyzing web content about rotator cuff repairs, we analyzed the websites for specific information we thought should be included in a high-quality website (Figures 2, 3). When considering authorship, we found academic centers, private physicians, and educational sources comprised 76% of the sources; industry sources made up only 12%. Similar findings were noted by investigators analyzing Internet information about other orthopedic topics, including ACL reconstruction, lumbar arthroplasty, osteosarcoma, and cervical spine surgery.5,11,22 Studies analyzing websites for information on ACL tears and distal radius fractures found have a higher percentage of industry-sponsored websites.9,10
DISCERN showed that the highest-quality information came from websites with academic affiliations, consistent with previous studies,3,9,17 and its mean score (51.6) was significantly higher than the scores for private physician websites, news sites, and blogs (Ps = .001, .016, .001); the least reliable information was from personal blogs and news outlets. Of note, mean DISCERN score was higher for the industry websites we found than for private physician websites (43.8 vs 40.7), though the difference was not significant (P = .229). Previous investigators considered number of industry-sponsored websites as a marker of poor quality of information relating to a given topic; however, given the DISCERN scores in our study, this might not necessarily be true.6 Based on the present study’s data, websites affiliated with academic institutions would be recommended for patients searching for high-quality information about rotator cuff tears.
Given DISCERN scores across studies, information about rotator cuff tears ranked below information about osteosarcoma and juvenile idiopathic arthritis but above information about scoliosis, cervical spine surgery, and ACL reconstruction (Table). DISCERN scores must be compared across studies, as there are no definitions for good and poor DISCERN scores.
Of the 4 studies that analyzed percentage of websites citing peer-reviewed sources, only our study and the study of cervical spine surgery18 analyzed that percentage as well as DISCERN score. Percentage citing peer-reviewed sources was 26% for rotator cuff tears and 24% for cervical spine surgery; the respective DISCERN scores were 44 and 43.6. As only these 2 studies could be compared, no real correlation between percentage of websites citing peer-reviewed sources and the quality of the content on a given topic can be assessed. More research into this relationship is needed. One already delineated association is the correlation between HONcode-certified sites and high DISCERN scores.21 For high-quality medical information, physicians can direct their patients both to academic institution websites and to HONcode-certified websites.
When we compared the present study with previous investigations, we found a large difference between search results for a given topic. In 2013, Duncan and colleagues6 and Bruce-Brand and colleagues9 used similar study designs (eg, search terms, search engines) for their investigations of quality of web information. Their results, however, were widely different. For example, percentages of industry authorship were 4.5% (Duncan and colleagues6) and 64% (Bruce-Brand and colleagues9). This inconsistency between studies conducted during similar periods might be related to what appears at the top of the results queue for a search. Duncan and colleagues6 analyzed 200 websites, Bruce-Brand and colleagues9 only 45. Industries may have made financial arrangements and used search engine optimization techniques to have their websites listed first in search results.
In our study, we also analyzed how web information has changed over time. On the Internet, information changes daily, and we hypothesized that the content found during our 2 searches (2011, 2014) would yield different results. Surprisingly, the data were similar, particularly concerning authorship (Figures 1, 2). In both searches, the largest authorship source was private physician or physician groups (38% in 2011 and 2014). Other authorship sources showed little change in percentage between searches. As for content, we found both increases and decreases in specific web information. Ability to contact authors increased from 21% (2011) to 50% (2014). We think it is important that websites offer a communication channel to people who read the medical information the sites provide. Percentage of websites discussing nonoperative treatment options increased from 11.5% to 61%. Therefore, patients in 2014 were being introduced to more options (in addition to surgery) for managing shoulder pain—an improvement in quality of information between the searches. Percentage of websites discussing surgical eligibility, however, decreased from 43% to 18%—a negative development in information quality. Another decrease, from 42% to 25%, was found for websites discussing surgical complications. Given the data as a whole, and our finding both negative and positive changes, it appears the quality of web content has not improved significantly. Interestingly, no websites discussed double- versus single-row surgery in 2014, but 6% did so in 2011.
Lost in the discussion of quality and reliability of information is whether patients comprehend what they are reading.23 Yi and colleagues19 recentlyassessed the readability level of arthroscopy information in articles published online by the American Academy of Orthopaedic Surgeons (AAOS) and the Arthroscopy Association of North America (AANA). The investigators used the Flesch-Kincaid readability test to determine readability level in terms of grade level. They found that the majority of the patient education articles on the AAOS and AANA sites had a readability level far above the national average; only 4 articles were written at or below the eighth-grade level, the current average reading level in the United States.24 Information that is not comprehensible is of no use to patients, and information that physicians and researchers consider high-quality might not be what patients consider high-quality. As we pursue higher-quality web content, we need to consider that its audience includes nonmedical readers, our patients. In the present study, we found that the readability of a website had no correlation with the site’s DISCERN score (Figure 5). Therefore, for information about rotator cuff repairs, higher-quality websites are no harder than lower-quality sites for patients to comprehend. The Flesch-Kincaid readability test is flawed in that it considers only total number of syllables per word and words per sentence, not nontextual elements of patient education materials, such as illustrations on a website. The 10.98 mean grade level found in our study is higher than the levels found for most studies reviewed by Yi and colleagues.19
This study had several limitations. During an Internet search, the number of websites a user visits drops precipitously after the first page of results. Studies have shown the top 20 sites in a given search receive 97% of the views, and the top 3 receive 58.4%. Whether patients visit websites far down in the list of 150 we found in our given search is unknown. Last, the Flesch-Kincaid readability test is flawed in several ways but nevertheless is used extensively in research. Grading is based on number of words and syllables used in a given sentence; it does not take into account the complexity or common usage of a given word or definition. Therefore, websites may receive low Flesch-Kincaid scores—indicating ease of reading—despite their use of complex medical terminology and jargon that complicate patients’ comprehension of the material.
Conclusion
Numerous authors have evaluated orthopedic patients’ accessing of medical information from the Internet. Although the Internet makes access easier, unreliable content can lead patients to develop certain notions about the direction of their care and certain expectations regarding their clinical outcomes. With there being no regulatory body monitoring content, the peer review process, an essential feature of academic publishing, can be easily circumvented.25
In this study, the highest-quality websites had academic affiliations. Quality of information about rotator cuff repairs was similar to what was found for other orthopedic topics in comparable studies. Surprisingly, there was little change in authorship and content of web information between our 2 search periods (2011, 2014). Although there has been a rapid increase in the number of medical websites, quality of content seems not to have changed significantly. Patients look to physicians for guidance but increasingly are accessing the Internet for additional information. It is essential that physicians understand the quality of information available on the Internet when counseling patients regarding surgery.
Patients are learning about health and disease more independently than before, but such self-education may pose a unique challenge for practicing physicians. Although educated patients can assist in the critical appraisal of treatment options,1 misinformed patients may have preconceived treatment biases and unrealistic expectations. More than 66 million Americans use the Internet daily, and recent surveys have shown 86% have used the Internet for health-related information.2,3 With Internet use increasing, the number of patients turning to the web for medical information is increasing as well.4 For many patients, this information can be useful in making decisions about their health and health care.5
Although accessing medical information from the Internet has grown exponentially, analysis of information quality has grown considerably slower.6 With no regulatory body monitoring content, there is easy circumvention of the peer review process, an essential feature of academic publishing.7 With no external regulation, the information retrieved may be incorrect, outdated, or misleading. Many orthopedic studies have analyzed Internet content about numerous diagnoses.3-6,8-18 Most of these studies have found this information highly variable and of poor quality.
We conducted a study to evaluate and analyze rotator cuff repair information available to the general public through the Internet; to assess changes in the quality of information over time; to determine if sites sponsored by academic institutions offered higher-quality information; and to assess whether the readability of the material varied according to DISCERN scores.
Rotator cuff repairs are among the most common surgeries performed by orthopedic surgeons. To our knowledge, this is the first study to assess the quality of web information about rotator cuff repairs. We hypothesized that the quality of information would positively correlate with the reading level of the material presented, that academic institutions would present the highest-quality information, and that the type of information presented would change over time.
Materials and Methods
We used the search phrase rotator cuff repair on the 3 most popular search engines: Google, Yahoo!, and Bing. Google is the dominant engine, taking 83.06% of total market share, followed by Yahoo! (6.86%) and Bing (4.27%).5 The first 50 websites identified by each search engine were selected for evaluation, excluding duplicates or overlapping websites. Similarly, advertisements and strictly video results lacking text were excluded. After each engine was queried, a master list of 150 websites was created for individual evaluation and assessment. To assess changes in results over time, we performed 2 searches, on November 16, 2011, and May 18, 2014.
The content of each website was analyzed for authorship, ability to contact the author, discussion of disorder, surgical treatment, complications, surgical eligibility, rehabilitation, other treatment options, and use of peer-reviewed sources. Authorship was placed in 1 of 6 categories:
1. Academic—university-affiliated physician or research group.
2. Private—physician or group without stated affiliation to an academic organization.
3. Industry—manufacturing or marketing company advertising a product or service for profit.
4. News source—bulletin or article without affiliation to a hospital or an academic institution.
5. Public education—individual or organization with noncommercial website providing third-party information (eg, Wikipedia, About.com).
6. Blog—website publishing an individual’s personal experiences in diary or journal form.
Websites were also assessed for accuracy and validity based on presence or absence of Health On the Net code (HONcode) certification and DISCERN score. Designed by the Health On the Net Foundation in 1996, HONcode provides a framework for disseminating high-quality medical information over the web.19 Website owners can request that their sites be evaluated for HONcode certification; a site that qualifies can display the HONcode seal.20 The DISCERN project, initially funded by the National Health Service in the United Kingdom, judges the quality of written information available on health-related websites.21 It determines the quality of a publication on the basis of 16 questions: The first 8 address the publication’s reliability, the next 7 involve specific details of treatment choices, and the last is an overall rating of the website.
Website readability was assessed with the Flesch-Kincaid test. This test, designed under contract with the US Navy in 1975, has been used in other orthopedic studies.19 Regression analysis was performed to check for correlation between website readability and DISCERN score. Analysis of variance was used to analyze differences between scores.
Results
We performed a comprehensive analysis of the top 50 websites from each of the 3 search engines (N = 150 websites) (Figures 1–5, Table). Regarding authorship, our 2 searches demonstrated similar values (Figure 1). In 2011, 21% of websites were associated with an academic institution, 38% were authored by private physicians or hospital or physician groups not associated with an academic institution, 11.5% were industry-sponsored, 5% were news bulletins or media reports, 21.5% were public education websites, and 3% were personal blogs. Our 2014 search found a similar distribution of contributors. Between 2011 and 2014, the largest change was in academic authors, which decreased by 7%, from 21% to 14%. Percentage of websites authored by private physicians remained constant from the first to the second search: 38%.
When the 2011 and 2014 website content was compared, several changes were noted. Percentage of websites providing an author contact method increased from 21% to 50% (Figure 2), percentage detailing rotator cuff repairs increased from 82% to 91%, and percentage introducing treatment options in addition to surgical management increased from 11.5% to 61%. Percentage discussing surgical eligibility, however, decreased from 43% to 18%. Percentage citing peer-reviewed sources remained relatively constant (28%, 26%), as did percentage discussing surgical technique for rotator cuff repair (55%, 59%) (Figure 3). A major decrease was found in percentage of websites discussing surgical complications, 42% in 2011 down to 25% in 2014, whereas a major increase was found in percentage discussing rehabilitation, from 39% in 2011 up to 73% in 2014. In 2014, no websites discussed double- versus single-row surgery—compared with 6% in 2011. False claims remained low between 2011 and 2014. In both searches, no website guaranteed a return to sport, and few made claims of painless or bloodless surgery.
DISCERN scores for websites found during the 2014 search were averaged for each of the 6 authorship groups (Figure 4). The highest DISCERN scores were given to academic institution websites (51.6) and public education websites (49). For the academic websites, this difference was significant relative to news, blog, and private physician websites (Ps = .012, .001, .001) The lowest DISCERN scores were given to news organization websites and personal blogs. DISCERN scores were 43.8 for industry sources and 40.7 for private physician groups; the difference was not significant (P = .229). Overall mean DISCERN score for all websites was 44. Eleven percent of websites were HONcode-certified.
No correlation was found between website readability and DISCERN score; correlation coefficient r was .01 (Figure 5). For the websites in 2014, mean Flesch-Kincaid readability score was 50.17, and mean grade level was 10.98; coefficient of determination r2 was 0.00012.
The Table compares our data with data from other orthopedic studies that have analyzed the quality of Internet information about various orthopedic injuries, diseases, and procedures.3-6,8,9,11-18 With its mean DISCERN score of 44, the present rotator cuff tear study was ranked third of 6 studies that have used this scoring system to analyze website content. Of these 6 studies, those reviewing osteosarcoma and juvenile idiopathic arthritis were ranked highest (mean scores, 49.8 and 48.9, respectively), and the study reviewing scoliosis surgery was ranked lowest (38.9). Bruce-Brand and colleagues9 recently found a mean DISCERN score of 41 for anterior cruciate ligament (ACL) reconstruction. When considering HONcode-certified websites, our Internet search for rotator cuff tears found the third lowest percentage, 10.5%, compared with the other studies (Table); the highest percentage, 30%, was found for websites discussing concussions in athletes. When considering authorship, our rotator cuff study found the third highest percentage, 76%, authored by academic centers, physicians, and public education websites; the highest percentage was found in websites discussing ACL reconstruction. Websites discussing ACL reconstruction also had the highest percentage of websites authored by industry.9
Discussion
To our knowledge, this is the first study specifically analyzing the quality of Internet information about rotator cuff repairs. A similar study, conducted by Starman and colleagues15 in 2010, addressed the quality of web information about 10 common sports medicine diagnoses, one of which was rotator cuff tears. In that study, only 16 of the websites included discussed rotator cuff tears. In addition, the authors used a customized, HONcode-based grading system to analyze each website, making their data difficult to compare across studies.
Ideally, a high-quality medical website should be written by a credible source and should cover a disorder, treatment options, eligibility, rehabilitation, and complications. As there is no standard grading system for analyzing web content about rotator cuff repairs, we analyzed the websites for specific information we thought should be included in a high-quality website (Figures 2, 3). When considering authorship, we found academic centers, private physicians, and educational sources comprised 76% of the sources; industry sources made up only 12%. Similar findings were noted by investigators analyzing Internet information about other orthopedic topics, including ACL reconstruction, lumbar arthroplasty, osteosarcoma, and cervical spine surgery.5,11,22 Studies analyzing websites for information on ACL tears and distal radius fractures found have a higher percentage of industry-sponsored websites.9,10
DISCERN showed that the highest-quality information came from websites with academic affiliations, consistent with previous studies,3,9,17 and its mean score (51.6) was significantly higher than the scores for private physician websites, news sites, and blogs (Ps = .001, .016, .001); the least reliable information was from personal blogs and news outlets. Of note, mean DISCERN score was higher for the industry websites we found than for private physician websites (43.8 vs 40.7), though the difference was not significant (P = .229). Previous investigators considered number of industry-sponsored websites as a marker of poor quality of information relating to a given topic; however, given the DISCERN scores in our study, this might not necessarily be true.6 Based on the present study’s data, websites affiliated with academic institutions would be recommended for patients searching for high-quality information about rotator cuff tears.
Given DISCERN scores across studies, information about rotator cuff tears ranked below information about osteosarcoma and juvenile idiopathic arthritis but above information about scoliosis, cervical spine surgery, and ACL reconstruction (Table). DISCERN scores must be compared across studies, as there are no definitions for good and poor DISCERN scores.
Of the 4 studies that analyzed percentage of websites citing peer-reviewed sources, only our study and the study of cervical spine surgery18 analyzed that percentage as well as DISCERN score. Percentage citing peer-reviewed sources was 26% for rotator cuff tears and 24% for cervical spine surgery; the respective DISCERN scores were 44 and 43.6. As only these 2 studies could be compared, no real correlation between percentage of websites citing peer-reviewed sources and the quality of the content on a given topic can be assessed. More research into this relationship is needed. One already delineated association is the correlation between HONcode-certified sites and high DISCERN scores.21 For high-quality medical information, physicians can direct their patients both to academic institution websites and to HONcode-certified websites.
When we compared the present study with previous investigations, we found a large difference between search results for a given topic. In 2013, Duncan and colleagues6 and Bruce-Brand and colleagues9 used similar study designs (eg, search terms, search engines) for their investigations of quality of web information. Their results, however, were widely different. For example, percentages of industry authorship were 4.5% (Duncan and colleagues6) and 64% (Bruce-Brand and colleagues9). This inconsistency between studies conducted during similar periods might be related to what appears at the top of the results queue for a search. Duncan and colleagues6 analyzed 200 websites, Bruce-Brand and colleagues9 only 45. Industries may have made financial arrangements and used search engine optimization techniques to have their websites listed first in search results.
In our study, we also analyzed how web information has changed over time. On the Internet, information changes daily, and we hypothesized that the content found during our 2 searches (2011, 2014) would yield different results. Surprisingly, the data were similar, particularly concerning authorship (Figures 1, 2). In both searches, the largest authorship source was private physician or physician groups (38% in 2011 and 2014). Other authorship sources showed little change in percentage between searches. As for content, we found both increases and decreases in specific web information. Ability to contact authors increased from 21% (2011) to 50% (2014). We think it is important that websites offer a communication channel to people who read the medical information the sites provide. Percentage of websites discussing nonoperative treatment options increased from 11.5% to 61%. Therefore, patients in 2014 were being introduced to more options (in addition to surgery) for managing shoulder pain—an improvement in quality of information between the searches. Percentage of websites discussing surgical eligibility, however, decreased from 43% to 18%—a negative development in information quality. Another decrease, from 42% to 25%, was found for websites discussing surgical complications. Given the data as a whole, and our finding both negative and positive changes, it appears the quality of web content has not improved significantly. Interestingly, no websites discussed double- versus single-row surgery in 2014, but 6% did so in 2011.
Lost in the discussion of quality and reliability of information is whether patients comprehend what they are reading.23 Yi and colleagues19 recentlyassessed the readability level of arthroscopy information in articles published online by the American Academy of Orthopaedic Surgeons (AAOS) and the Arthroscopy Association of North America (AANA). The investigators used the Flesch-Kincaid readability test to determine readability level in terms of grade level. They found that the majority of the patient education articles on the AAOS and AANA sites had a readability level far above the national average; only 4 articles were written at or below the eighth-grade level, the current average reading level in the United States.24 Information that is not comprehensible is of no use to patients, and information that physicians and researchers consider high-quality might not be what patients consider high-quality. As we pursue higher-quality web content, we need to consider that its audience includes nonmedical readers, our patients. In the present study, we found that the readability of a website had no correlation with the site’s DISCERN score (Figure 5). Therefore, for information about rotator cuff repairs, higher-quality websites are no harder than lower-quality sites for patients to comprehend. The Flesch-Kincaid readability test is flawed in that it considers only total number of syllables per word and words per sentence, not nontextual elements of patient education materials, such as illustrations on a website. The 10.98 mean grade level found in our study is higher than the levels found for most studies reviewed by Yi and colleagues.19
This study had several limitations. During an Internet search, the number of websites a user visits drops precipitously after the first page of results. Studies have shown the top 20 sites in a given search receive 97% of the views, and the top 3 receive 58.4%. Whether patients visit websites far down in the list of 150 we found in our given search is unknown. Last, the Flesch-Kincaid readability test is flawed in several ways but nevertheless is used extensively in research. Grading is based on number of words and syllables used in a given sentence; it does not take into account the complexity or common usage of a given word or definition. Therefore, websites may receive low Flesch-Kincaid scores—indicating ease of reading—despite their use of complex medical terminology and jargon that complicate patients’ comprehension of the material.
Conclusion
Numerous authors have evaluated orthopedic patients’ accessing of medical information from the Internet. Although the Internet makes access easier, unreliable content can lead patients to develop certain notions about the direction of their care and certain expectations regarding their clinical outcomes. With there being no regulatory body monitoring content, the peer review process, an essential feature of academic publishing, can be easily circumvented.25
In this study, the highest-quality websites had academic affiliations. Quality of information about rotator cuff repairs was similar to what was found for other orthopedic topics in comparable studies. Surprisingly, there was little change in authorship and content of web information between our 2 search periods (2011, 2014). Although there has been a rapid increase in the number of medical websites, quality of content seems not to have changed significantly. Patients look to physicians for guidance but increasingly are accessing the Internet for additional information. It is essential that physicians understand the quality of information available on the Internet when counseling patients regarding surgery.
1. Brunnekreef JJ, Schreurs BW. Total hip arthroplasty: what information do we offer patients on websites of hospitals? BMC Health Serv Res. 2011;11:83.
2. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
3. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the Internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
4. Groves ND, Humphreys HW, Williams AJ, Jones A. Effect of informational Internet web pages on patients’ decision making: randomised controlled trial regarding choice of spinal or general anaesthesia for orthopaedic surgery. Anaesthesia. 2010;65(3):277-282.
5. Purcell K, Brenner J, Rainie L. Search Engine Use 2012. Washington, DC: Pew Internet & American Life Project; 2012.
6. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the Internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
7. Lichtenfeld LJ. Can the beast be tamed? The woeful tale of accurate health information on the Internet. Ann Surg Oncol. 2012;19(3):701-702.
8. Ahmed OH, Sullivan SJ, Schneiders AG, McCrory PR. Concussion information online: evaluation of information quality, content and readability of concussion-related websites. Br J Sports Med. 2012;46(9):675-683.
9. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the Internet. Arthroscopy. 2013;29(6):1095-1100.
10. Dy JC, Taylor SA, Patel RM, Kitay A, Roberts TR, Daluiski A. The effect of search term on the quality and accuracy of online information regarding distal radius fractures. J Hand Surg Am. 2012;37(9):1881-1887.
11. Garcia RM, Messerschmitt PJ, Ahn NU. An evaluation of information on the Internet of a new device: the lumbar artificial disc replacement. J Spinal Disord Tech. 2009;22(1):52-57.
12. Gosselin MM, Mulcahey MK, Feller E, Hulstyn MJ. Examining Internet resources on gender differences in ACL injuries: what patients are reading. Knee. 2013;20(3):196-202.
13. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
14. Morr S, Shanti N, Carrer A, Kubeck J, Gerling MC. Quality of information concerning cervical disc herniation on the Internet. Spine J. 2010;10(4):350-354.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of Internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Stinson JN, Tucker L, Huber A, et al. Surfing for juvenile idiopathic arthritis: perspectives on quality and content of information on the Internet. J Rheumatol. 2009;36(8):1755-1762.
17. Sullivan TB, Anderson JS, Ahn UM, Ahn NU. Can Internet information on vertebroplasty be a reliable means of patient self-education? Clin Orthop Relat Res. 2014;472(5):1597-1604.
18. Weil AG, Bojanowski MW, Jamart J, Gustin T, Lévêque M. Evaluation of the quality of information on the Internet available to patients undergoing cervical spine surgery. World Neurosurg. 2014;82(1-2):e31-e39.
19. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
20. Boyer C, Selby M, Scherrer JR, Appel RD. The Health On the Net code of conduct for medical and health websites. Comput Biol Med. 1998;28(5):603-610.
21. Silberg WM, Lundberg GD, Musacchio RA. Assessing, controlling, and assuring the quality of medical information on the Internet: Caveant lector et viewor—Let the reader and viewer beware. JAMA. 1997;277(15):1244-1245.
22. Fabricant PD, Dy CJ, Patel RM, Blanco JS, Doyle SM. Internet search term affects the quality and accuracy of online information about developmental hip dysplasia. J Pediatr Orthop. 2013;33(4):361-365.
23. Aslam N, Bowyer D, Wainwright A, Theologis T, Benson M. Evaluation of Internet use by paediatric orthopaedic outpatients and the quality of information available. J Pediatr Orthop B. 2005;14(2):129-133.
24. Wetzler MJ. “I found it on the Internet”: how reliable and readable is patient information? Arthroscopy. 2013;29(6):967-968.
25. Qureshi SA, Koehler SM, Lin JD, Bird J, Garcia RM, Hecht AC. An evaluation of information on the Internet about a new device: the cervical artificial disc replacement. Spine. 2012;37(10):881-883.
1. Brunnekreef JJ, Schreurs BW. Total hip arthroplasty: what information do we offer patients on websites of hospitals? BMC Health Serv Res. 2011;11:83.
2. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
3. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the Internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
4. Groves ND, Humphreys HW, Williams AJ, Jones A. Effect of informational Internet web pages on patients’ decision making: randomised controlled trial regarding choice of spinal or general anaesthesia for orthopaedic surgery. Anaesthesia. 2010;65(3):277-282.
5. Purcell K, Brenner J, Rainie L. Search Engine Use 2012. Washington, DC: Pew Internet & American Life Project; 2012.
6. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the Internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
7. Lichtenfeld LJ. Can the beast be tamed? The woeful tale of accurate health information on the Internet. Ann Surg Oncol. 2012;19(3):701-702.
8. Ahmed OH, Sullivan SJ, Schneiders AG, McCrory PR. Concussion information online: evaluation of information quality, content and readability of concussion-related websites. Br J Sports Med. 2012;46(9):675-683.
9. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the Internet. Arthroscopy. 2013;29(6):1095-1100.
10. Dy JC, Taylor SA, Patel RM, Kitay A, Roberts TR, Daluiski A. The effect of search term on the quality and accuracy of online information regarding distal radius fractures. J Hand Surg Am. 2012;37(9):1881-1887.
11. Garcia RM, Messerschmitt PJ, Ahn NU. An evaluation of information on the Internet of a new device: the lumbar artificial disc replacement. J Spinal Disord Tech. 2009;22(1):52-57.
12. Gosselin MM, Mulcahey MK, Feller E, Hulstyn MJ. Examining Internet resources on gender differences in ACL injuries: what patients are reading. Knee. 2013;20(3):196-202.
13. Lam CG, Roter DL, Cohen KJ. Survey of quality, readability, and social reach of websites on osteosarcoma in adolescents. Patient Educ Couns. 2013;90(1):82-87.
14. Morr S, Shanti N, Carrer A, Kubeck J, Gerling MC. Quality of information concerning cervical disc herniation on the Internet. Spine J. 2010;10(4):350-354.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of Internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Stinson JN, Tucker L, Huber A, et al. Surfing for juvenile idiopathic arthritis: perspectives on quality and content of information on the Internet. J Rheumatol. 2009;36(8):1755-1762.
17. Sullivan TB, Anderson JS, Ahn UM, Ahn NU. Can Internet information on vertebroplasty be a reliable means of patient self-education? Clin Orthop Relat Res. 2014;472(5):1597-1604.
18. Weil AG, Bojanowski MW, Jamart J, Gustin T, Lévêque M. Evaluation of the quality of information on the Internet available to patients undergoing cervical spine surgery. World Neurosurg. 2014;82(1-2):e31-e39.
19. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
20. Boyer C, Selby M, Scherrer JR, Appel RD. The Health On the Net code of conduct for medical and health websites. Comput Biol Med. 1998;28(5):603-610.
21. Silberg WM, Lundberg GD, Musacchio RA. Assessing, controlling, and assuring the quality of medical information on the Internet: Caveant lector et viewor—Let the reader and viewer beware. JAMA. 1997;277(15):1244-1245.
22. Fabricant PD, Dy CJ, Patel RM, Blanco JS, Doyle SM. Internet search term affects the quality and accuracy of online information about developmental hip dysplasia. J Pediatr Orthop. 2013;33(4):361-365.
23. Aslam N, Bowyer D, Wainwright A, Theologis T, Benson M. Evaluation of Internet use by paediatric orthopaedic outpatients and the quality of information available. J Pediatr Orthop B. 2005;14(2):129-133.
24. Wetzler MJ. “I found it on the Internet”: how reliable and readable is patient information? Arthroscopy. 2013;29(6):967-968.
25. Qureshi SA, Koehler SM, Lin JD, Bird J, Garcia RM, Hecht AC. An evaluation of information on the Internet about a new device: the cervical artificial disc replacement. Spine. 2012;37(10):881-883.
Shoulder Instability Management: A Survey of the American Shoulder and Elbow Surgeons
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
In Vivo Measurement of Rotator Cuff Tear Tension: Medial Versus Lateral Footprint Position
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Neurocognitive Deficits and Cerebral Desaturation During Shoulder Arthroscopy With Patient in Beach-Chair Position: A Review of the Current Literature
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
Cryo-Compression Therapy
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Latissimus Dorsi and Teres Major Injuries in Major League Baseball Pitchers: A Systematic Review
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.