User login
Is it psychosis, or an autoimmune encephalitis?
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
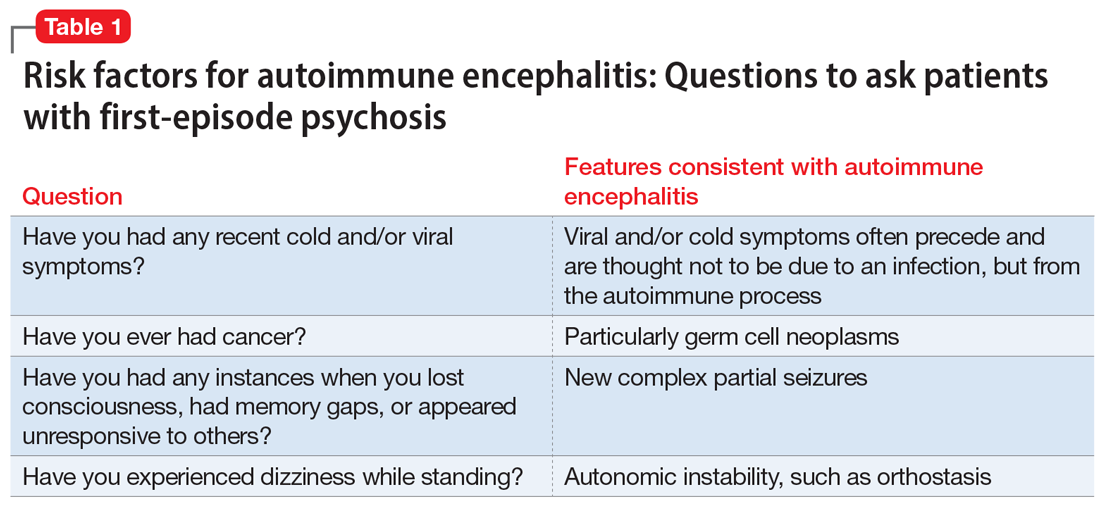
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.
CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
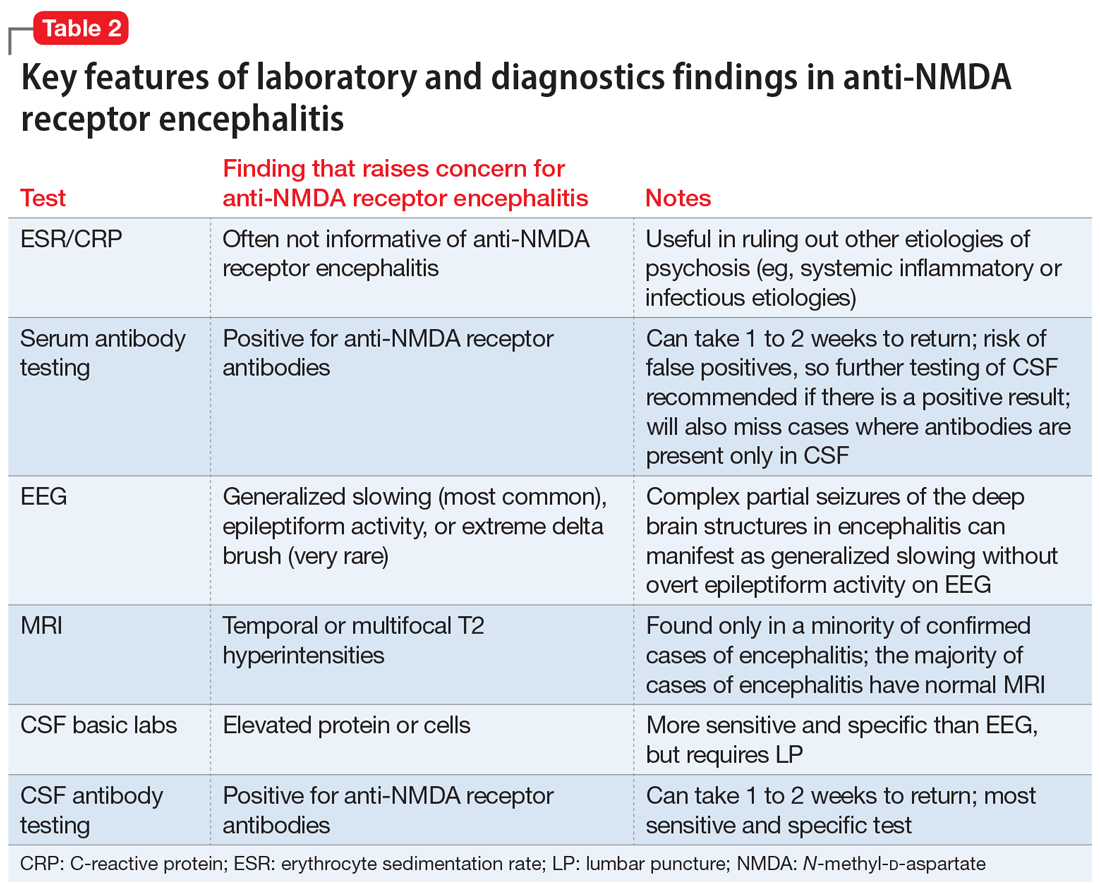
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.
When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.
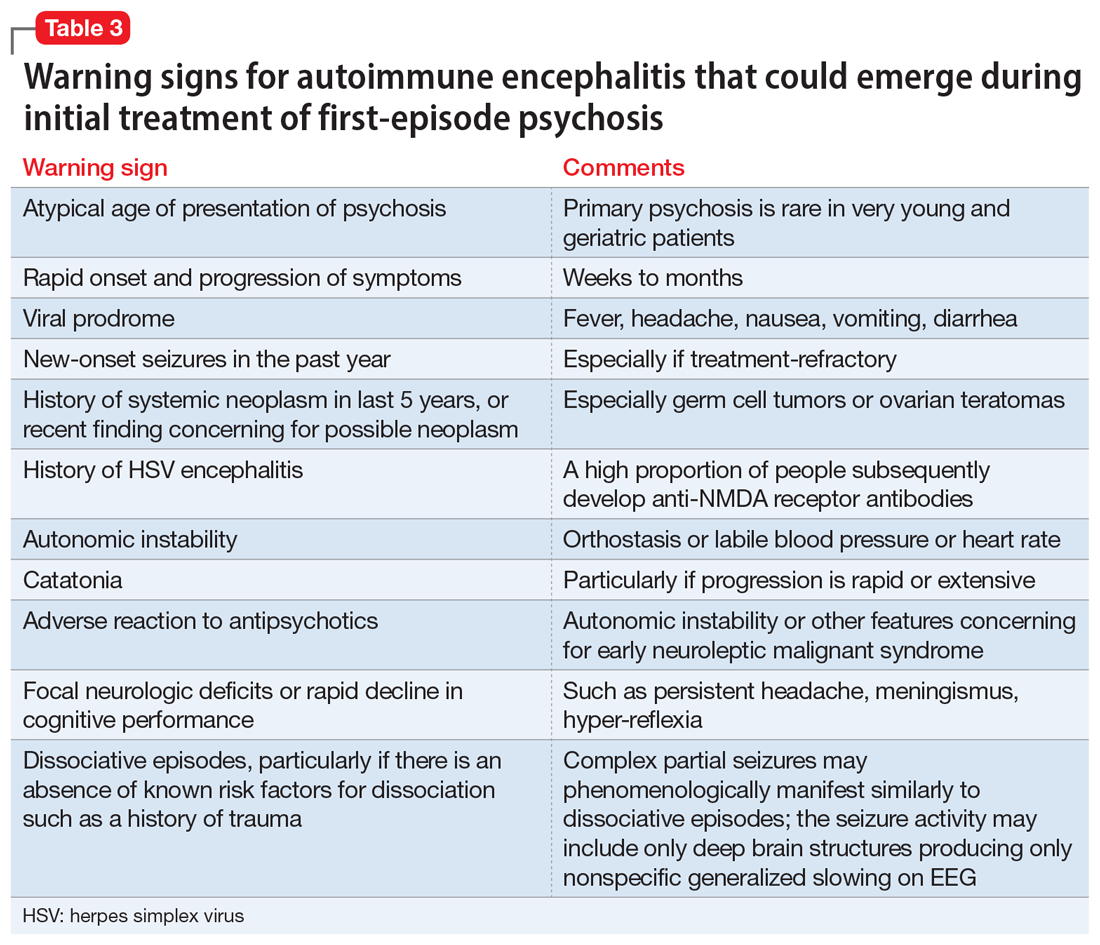
The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.

CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.

When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.

The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.

CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.

When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.

The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
Impaired cognition in a patient with schizophrenia and HIV
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.
During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.
The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
Does schizophrenia need a name change?
The term schizophrenia carries an incredible load with it.
It is not just a moniker for a serious mental condition but also a tool to support discrimination, shame, and condemnation, as multiple recent studies and surveys have shown.
The evidence suggests that many of the insensitivities of decades and centuries past, though certainly much improved, can still linger today. And when stigma is attached to a condition or status, it creates additional burdens on the people who are already enduring the challenges of their diagnosis.
There is a growing movement among patients and mental health experts to change the name of this complex condition because of both the added onus it places on patients and the fact that it’s simply clinically inaccurate. Opponents argue that the change will not create the sought-after results but instead, will just usher old negative attitudes into a new world.
Why the name change?
The term schizophrenia translates to “split mind,” which is misleading from the start. Mental health experts, people who live with the syndrome, and their advocates believe that changing the term to one that is more closely descriptive of the condition can lead to a more tolerant, understanding public.
In 2021, the Consumer Advisory Board at the Psychosis Research Program of the Massachusetts Mental Health Center Public Psychiatry Division of Beth Israel Deaconess Medical Center created a project to collect feedback from key stakeholders about the possibility of a name change. The survey was given to people with lived experience of mental illness and their family members, clinicians, researchers, government officials, and the general public. The results showed that nearly 75% of the people surveyed were ready to embrace a name change.
Matcheri S. Keshavan, MD, and Raquelle I. Mesholam-Gately, PhD, are two of the 13 authors of this study. In an interview, the researchers explained how the study was handled and what the results mean to them.
“About 5 years ago, we were all talking about this idea of renaming schizophrenia. I began thinking that first of all, it doesn’t accurately describe what the condition is, and there’s a lot of stigma associated with the word. We also discussed that the name ‘schizophrenia’ has been changed in several other Asian countries, and there have been some benefits associated with those changes, including people being more comfortable with seeking out care,” said Dr. Mesholam-Gately, psychologist and assistant professor of psychology in the department of psychiatry at Harvard University, Boston.
“We reviewed the literature that was out there already and then we put together a survey that we could give to a broad sample of stakeholders, including people with lived experiences, to get a sense of how stigmatizing they thought the word schizophrenia was and whether they feel that the name schizophrenia should be changed. Then we listed some alternate names for schizophrenia and asked how people felt about those alternate names,” continued Dr. Mesholam-Gately.
The alternative names that received the most support were “altered perception syndrome,” “psychosis spectrum syndrome,” and “neuro-emotional integration disorder.” Dr. Keshavan, a clinical psychiatrist and academic head of psychiatry at Beth Israel Deaconess, said diagnostic name changes have been adopted before in the field and have led to effective results.
“There are several examples in mental health that have gone through this change. For example, autism has been changed to autism spectrum disorder. Manic depressive [disorder] has been changed to bipolar disorder. Mental retardation has been changed to intellectual disability. And those kinds of changes have led to positive benefits and reducing stigma. People are willing to come in for care. For those reasons, we wanted to get the thinking started.”
The burden of stigma
The stigma associated with schizophrenia and mental illness in general is as palpable as it is detrimental. Having a mental illness is one thing, but the stigma of carrying such a label is an additional load that individuals must carry as well. Not only does a person with schizophrenia have to manage their symptoms and treatment, both medical and behavioral, but they also must dodge negative attitudes, misinformation, and discrimination that comes from an uneducated or judgmental public. This can lead to different forms of stigma – like self-stigma and label avoidance.
In a recent blog published by the National Alliance on Mental Illness, Casey Clabough, a person who lives with a diagnosis of schizophrenia, explained that people who have this serious mental illness can suffer from the backlash of the stigma. He explains that people with schizophrenia can misinterpret reality and behave in ways that the general public doesn’t understand or accept. As a result, they are labeled “crazy,” the public grows fearful of them, and they retreat to social isolation.
The stigma surrounding mental illness is perpetuated from several sources. Media and pop culture inaccurately portray schizophrenia as an out-of-control condition that makes someone prone to violence and more likely to commit crimes. In actuality, people living with schizophrenia are at increased risk of becoming victims of violence. One study found that people with schizophrenia are at least 14 times more likely to be victims of a violent crime than to be arrested for one.
A history of changes
The term “schizophrenia” is actually the result of a name change from over 100 years ago. The condition was first identified as a mental illness by Emil Kraepelin, MD, a German psychiatrist who studied the pathogenesis of neurologic and psychiatric disorders. In his studies of dementia in young adults, Dr. Kraepelin labeled the symptoms of what we now call schizophrenia as “dementia praecox,” or early dementia.
In 1908, a Swiss professor named Paul Eugen Bleuler, MD, challenged the accuracy of the term “dementia praecox” at a meeting of the German Psychiatric Association in Berlin. During this meeting, Dr. Bleuler argued that the term schizophrenia comes closer to describing the splitting of psychic functioning. Dr. Bleuler explained how schizophrenia has primary and secondary symptoms. The four primary symptoms (the four As) are:
- Abnormal associations
- Autistic behavior and thinking
- Abnormal affect
- Ambivalence
According to Dr. Bleuler, if an individual lacks adaptive capacity and support, these primary symptoms could lead to more pronounced secondary symptoms, such as social withdraw, hallucinations, and delusions.
In later years, more research has been done to gain a greater understanding of the illness. Kurt Schneider, a German psychiatrist, presented a group of select symptoms for diagnosing schizophrenia as First Rank Symptoms (FRS) in 1959. These symptoms may be experienced by people with psychosis.
The problem here is twofold. One, people who have bipolar disorder may also suffer from similar symptoms, which leads to problem number two: misdiagnosis. An examination of a collection of 21 studies on FRS used as a tool for schizophrenia diagnosis showed that FRS misdiagnosed almost 20% of individuals as having schizophrenia when, in fact, they didn’t have the illness.
A rose by any other name still smells sweet
There is apprehension about the name change from some mental health experts; not all respondents to the survey felt that a name change would help with stigma. Concerns range from potential confusion among medical professionals to changing the name prematurely before the newest revision of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, to having trouble applying for insurance coverages.
“There is a stigma, and people will have [negative] attitudes towards people with schizophrenia,” said William Carpenter, MD, professor of psychiatry and pharmacology at the University of Maryland School of Medicine, Baltimore. “That is going to occur no matter what kind of name you put to it. But the name itself sounds like you’ve been told you have the worst of all mental illnesses. Or you’re never going to get over this, which may be incorrect. So there’s self-stigma, and it’s based on these kinds of feelings.”
Both sides of the debate agree that one vital strategy for reducing stigma and discrimination is education. “Giving information about schizophrenia makes a difference in how people conceptualize and view schizophrenia,” he added.
“We don’t think that the name change alone is going to completely solve the problem,” Dr. Mesholam-Gately admitted. “There needs to be more public education and initiatives to help along with it. But we think that changing the name can be a part of reducing the stigma for people who experience the condition. That would be worth it.”
A version of this article first appeared on Medscape.com.
The term schizophrenia carries an incredible load with it.
It is not just a moniker for a serious mental condition but also a tool to support discrimination, shame, and condemnation, as multiple recent studies and surveys have shown.
The evidence suggests that many of the insensitivities of decades and centuries past, though certainly much improved, can still linger today. And when stigma is attached to a condition or status, it creates additional burdens on the people who are already enduring the challenges of their diagnosis.
There is a growing movement among patients and mental health experts to change the name of this complex condition because of both the added onus it places on patients and the fact that it’s simply clinically inaccurate. Opponents argue that the change will not create the sought-after results but instead, will just usher old negative attitudes into a new world.
Why the name change?
The term schizophrenia translates to “split mind,” which is misleading from the start. Mental health experts, people who live with the syndrome, and their advocates believe that changing the term to one that is more closely descriptive of the condition can lead to a more tolerant, understanding public.
In 2021, the Consumer Advisory Board at the Psychosis Research Program of the Massachusetts Mental Health Center Public Psychiatry Division of Beth Israel Deaconess Medical Center created a project to collect feedback from key stakeholders about the possibility of a name change. The survey was given to people with lived experience of mental illness and their family members, clinicians, researchers, government officials, and the general public. The results showed that nearly 75% of the people surveyed were ready to embrace a name change.
Matcheri S. Keshavan, MD, and Raquelle I. Mesholam-Gately, PhD, are two of the 13 authors of this study. In an interview, the researchers explained how the study was handled and what the results mean to them.
“About 5 years ago, we were all talking about this idea of renaming schizophrenia. I began thinking that first of all, it doesn’t accurately describe what the condition is, and there’s a lot of stigma associated with the word. We also discussed that the name ‘schizophrenia’ has been changed in several other Asian countries, and there have been some benefits associated with those changes, including people being more comfortable with seeking out care,” said Dr. Mesholam-Gately, psychologist and assistant professor of psychology in the department of psychiatry at Harvard University, Boston.
“We reviewed the literature that was out there already and then we put together a survey that we could give to a broad sample of stakeholders, including people with lived experiences, to get a sense of how stigmatizing they thought the word schizophrenia was and whether they feel that the name schizophrenia should be changed. Then we listed some alternate names for schizophrenia and asked how people felt about those alternate names,” continued Dr. Mesholam-Gately.
The alternative names that received the most support were “altered perception syndrome,” “psychosis spectrum syndrome,” and “neuro-emotional integration disorder.” Dr. Keshavan, a clinical psychiatrist and academic head of psychiatry at Beth Israel Deaconess, said diagnostic name changes have been adopted before in the field and have led to effective results.
“There are several examples in mental health that have gone through this change. For example, autism has been changed to autism spectrum disorder. Manic depressive [disorder] has been changed to bipolar disorder. Mental retardation has been changed to intellectual disability. And those kinds of changes have led to positive benefits and reducing stigma. People are willing to come in for care. For those reasons, we wanted to get the thinking started.”
The burden of stigma
The stigma associated with schizophrenia and mental illness in general is as palpable as it is detrimental. Having a mental illness is one thing, but the stigma of carrying such a label is an additional load that individuals must carry as well. Not only does a person with schizophrenia have to manage their symptoms and treatment, both medical and behavioral, but they also must dodge negative attitudes, misinformation, and discrimination that comes from an uneducated or judgmental public. This can lead to different forms of stigma – like self-stigma and label avoidance.
In a recent blog published by the National Alliance on Mental Illness, Casey Clabough, a person who lives with a diagnosis of schizophrenia, explained that people who have this serious mental illness can suffer from the backlash of the stigma. He explains that people with schizophrenia can misinterpret reality and behave in ways that the general public doesn’t understand or accept. As a result, they are labeled “crazy,” the public grows fearful of them, and they retreat to social isolation.
The stigma surrounding mental illness is perpetuated from several sources. Media and pop culture inaccurately portray schizophrenia as an out-of-control condition that makes someone prone to violence and more likely to commit crimes. In actuality, people living with schizophrenia are at increased risk of becoming victims of violence. One study found that people with schizophrenia are at least 14 times more likely to be victims of a violent crime than to be arrested for one.
A history of changes
The term “schizophrenia” is actually the result of a name change from over 100 years ago. The condition was first identified as a mental illness by Emil Kraepelin, MD, a German psychiatrist who studied the pathogenesis of neurologic and psychiatric disorders. In his studies of dementia in young adults, Dr. Kraepelin labeled the symptoms of what we now call schizophrenia as “dementia praecox,” or early dementia.
In 1908, a Swiss professor named Paul Eugen Bleuler, MD, challenged the accuracy of the term “dementia praecox” at a meeting of the German Psychiatric Association in Berlin. During this meeting, Dr. Bleuler argued that the term schizophrenia comes closer to describing the splitting of psychic functioning. Dr. Bleuler explained how schizophrenia has primary and secondary symptoms. The four primary symptoms (the four As) are:
- Abnormal associations
- Autistic behavior and thinking
- Abnormal affect
- Ambivalence
According to Dr. Bleuler, if an individual lacks adaptive capacity and support, these primary symptoms could lead to more pronounced secondary symptoms, such as social withdraw, hallucinations, and delusions.
In later years, more research has been done to gain a greater understanding of the illness. Kurt Schneider, a German psychiatrist, presented a group of select symptoms for diagnosing schizophrenia as First Rank Symptoms (FRS) in 1959. These symptoms may be experienced by people with psychosis.
The problem here is twofold. One, people who have bipolar disorder may also suffer from similar symptoms, which leads to problem number two: misdiagnosis. An examination of a collection of 21 studies on FRS used as a tool for schizophrenia diagnosis showed that FRS misdiagnosed almost 20% of individuals as having schizophrenia when, in fact, they didn’t have the illness.
A rose by any other name still smells sweet
There is apprehension about the name change from some mental health experts; not all respondents to the survey felt that a name change would help with stigma. Concerns range from potential confusion among medical professionals to changing the name prematurely before the newest revision of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, to having trouble applying for insurance coverages.
“There is a stigma, and people will have [negative] attitudes towards people with schizophrenia,” said William Carpenter, MD, professor of psychiatry and pharmacology at the University of Maryland School of Medicine, Baltimore. “That is going to occur no matter what kind of name you put to it. But the name itself sounds like you’ve been told you have the worst of all mental illnesses. Or you’re never going to get over this, which may be incorrect. So there’s self-stigma, and it’s based on these kinds of feelings.”
Both sides of the debate agree that one vital strategy for reducing stigma and discrimination is education. “Giving information about schizophrenia makes a difference in how people conceptualize and view schizophrenia,” he added.
“We don’t think that the name change alone is going to completely solve the problem,” Dr. Mesholam-Gately admitted. “There needs to be more public education and initiatives to help along with it. But we think that changing the name can be a part of reducing the stigma for people who experience the condition. That would be worth it.”
A version of this article first appeared on Medscape.com.
The term schizophrenia carries an incredible load with it.
It is not just a moniker for a serious mental condition but also a tool to support discrimination, shame, and condemnation, as multiple recent studies and surveys have shown.
The evidence suggests that many of the insensitivities of decades and centuries past, though certainly much improved, can still linger today. And when stigma is attached to a condition or status, it creates additional burdens on the people who are already enduring the challenges of their diagnosis.
There is a growing movement among patients and mental health experts to change the name of this complex condition because of both the added onus it places on patients and the fact that it’s simply clinically inaccurate. Opponents argue that the change will not create the sought-after results but instead, will just usher old negative attitudes into a new world.
Why the name change?
The term schizophrenia translates to “split mind,” which is misleading from the start. Mental health experts, people who live with the syndrome, and their advocates believe that changing the term to one that is more closely descriptive of the condition can lead to a more tolerant, understanding public.
In 2021, the Consumer Advisory Board at the Psychosis Research Program of the Massachusetts Mental Health Center Public Psychiatry Division of Beth Israel Deaconess Medical Center created a project to collect feedback from key stakeholders about the possibility of a name change. The survey was given to people with lived experience of mental illness and their family members, clinicians, researchers, government officials, and the general public. The results showed that nearly 75% of the people surveyed were ready to embrace a name change.
Matcheri S. Keshavan, MD, and Raquelle I. Mesholam-Gately, PhD, are two of the 13 authors of this study. In an interview, the researchers explained how the study was handled and what the results mean to them.
“About 5 years ago, we were all talking about this idea of renaming schizophrenia. I began thinking that first of all, it doesn’t accurately describe what the condition is, and there’s a lot of stigma associated with the word. We also discussed that the name ‘schizophrenia’ has been changed in several other Asian countries, and there have been some benefits associated with those changes, including people being more comfortable with seeking out care,” said Dr. Mesholam-Gately, psychologist and assistant professor of psychology in the department of psychiatry at Harvard University, Boston.
“We reviewed the literature that was out there already and then we put together a survey that we could give to a broad sample of stakeholders, including people with lived experiences, to get a sense of how stigmatizing they thought the word schizophrenia was and whether they feel that the name schizophrenia should be changed. Then we listed some alternate names for schizophrenia and asked how people felt about those alternate names,” continued Dr. Mesholam-Gately.
The alternative names that received the most support were “altered perception syndrome,” “psychosis spectrum syndrome,” and “neuro-emotional integration disorder.” Dr. Keshavan, a clinical psychiatrist and academic head of psychiatry at Beth Israel Deaconess, said diagnostic name changes have been adopted before in the field and have led to effective results.
“There are several examples in mental health that have gone through this change. For example, autism has been changed to autism spectrum disorder. Manic depressive [disorder] has been changed to bipolar disorder. Mental retardation has been changed to intellectual disability. And those kinds of changes have led to positive benefits and reducing stigma. People are willing to come in for care. For those reasons, we wanted to get the thinking started.”
The burden of stigma
The stigma associated with schizophrenia and mental illness in general is as palpable as it is detrimental. Having a mental illness is one thing, but the stigma of carrying such a label is an additional load that individuals must carry as well. Not only does a person with schizophrenia have to manage their symptoms and treatment, both medical and behavioral, but they also must dodge negative attitudes, misinformation, and discrimination that comes from an uneducated or judgmental public. This can lead to different forms of stigma – like self-stigma and label avoidance.
In a recent blog published by the National Alliance on Mental Illness, Casey Clabough, a person who lives with a diagnosis of schizophrenia, explained that people who have this serious mental illness can suffer from the backlash of the stigma. He explains that people with schizophrenia can misinterpret reality and behave in ways that the general public doesn’t understand or accept. As a result, they are labeled “crazy,” the public grows fearful of them, and they retreat to social isolation.
The stigma surrounding mental illness is perpetuated from several sources. Media and pop culture inaccurately portray schizophrenia as an out-of-control condition that makes someone prone to violence and more likely to commit crimes. In actuality, people living with schizophrenia are at increased risk of becoming victims of violence. One study found that people with schizophrenia are at least 14 times more likely to be victims of a violent crime than to be arrested for one.
A history of changes
The term “schizophrenia” is actually the result of a name change from over 100 years ago. The condition was first identified as a mental illness by Emil Kraepelin, MD, a German psychiatrist who studied the pathogenesis of neurologic and psychiatric disorders. In his studies of dementia in young adults, Dr. Kraepelin labeled the symptoms of what we now call schizophrenia as “dementia praecox,” or early dementia.
In 1908, a Swiss professor named Paul Eugen Bleuler, MD, challenged the accuracy of the term “dementia praecox” at a meeting of the German Psychiatric Association in Berlin. During this meeting, Dr. Bleuler argued that the term schizophrenia comes closer to describing the splitting of psychic functioning. Dr. Bleuler explained how schizophrenia has primary and secondary symptoms. The four primary symptoms (the four As) are:
- Abnormal associations
- Autistic behavior and thinking
- Abnormal affect
- Ambivalence
According to Dr. Bleuler, if an individual lacks adaptive capacity and support, these primary symptoms could lead to more pronounced secondary symptoms, such as social withdraw, hallucinations, and delusions.
In later years, more research has been done to gain a greater understanding of the illness. Kurt Schneider, a German psychiatrist, presented a group of select symptoms for diagnosing schizophrenia as First Rank Symptoms (FRS) in 1959. These symptoms may be experienced by people with psychosis.
The problem here is twofold. One, people who have bipolar disorder may also suffer from similar symptoms, which leads to problem number two: misdiagnosis. An examination of a collection of 21 studies on FRS used as a tool for schizophrenia diagnosis showed that FRS misdiagnosed almost 20% of individuals as having schizophrenia when, in fact, they didn’t have the illness.
A rose by any other name still smells sweet
There is apprehension about the name change from some mental health experts; not all respondents to the survey felt that a name change would help with stigma. Concerns range from potential confusion among medical professionals to changing the name prematurely before the newest revision of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, to having trouble applying for insurance coverages.
“There is a stigma, and people will have [negative] attitudes towards people with schizophrenia,” said William Carpenter, MD, professor of psychiatry and pharmacology at the University of Maryland School of Medicine, Baltimore. “That is going to occur no matter what kind of name you put to it. But the name itself sounds like you’ve been told you have the worst of all mental illnesses. Or you’re never going to get over this, which may be incorrect. So there’s self-stigma, and it’s based on these kinds of feelings.”
Both sides of the debate agree that one vital strategy for reducing stigma and discrimination is education. “Giving information about schizophrenia makes a difference in how people conceptualize and view schizophrenia,” he added.
“We don’t think that the name change alone is going to completely solve the problem,” Dr. Mesholam-Gately admitted. “There needs to be more public education and initiatives to help along with it. But we think that changing the name can be a part of reducing the stigma for people who experience the condition. That would be worth it.”
A version of this article first appeared on Medscape.com.
Telemental health linked with improvements in key outcomes
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.K. survey: Dermatologists want training in prescribing antipsychotics for delusional infestation
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT BAD 2022
3 steps to bend the curve of schizophrenia
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24
Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24
Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24
Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Adaptive changes to antipsychotics: How to avoid the consequences
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
Best strategy to prevent schizophrenia relapse yields unexpected results
A large meta-analysis sheds light on the best antipsychotic maintenance strategy to prevent relapse in clinically stable schizophrenia – with some unexpected results that have potential implications for changes to current guidelines.
Consistent with the researchers’ hypothesis, continuing antipsychotic treatment at the standard dose, switching to another antipsychotic, and reducing the dose were all significantly more effective than stopping antipsychotic treatment in preventing relapse.
However, contrary to the researchers’ hypothesis, which was based on current literature, switching to another antipsychotic was just as effective as continuing an antipsychotic at the standard dose.
Switching to another antipsychotic “does not increase the risk of relapse. This result was not expected, as previous literature suggested otherwise,” Giovanni Ostuzzi, MD, PhD, with University of Verona (Italy) said in an interview.
“On the other hand, reducing the dose below the standard range used in the acute phase carries a tangible risk of relapse, and should be limited to selected cases, for example those where the risk of withdrawing the treatment altogether is particularly high,” Dr. Ostuzzi said.
“These results should inform evidence-based guidelines, considering that clinical practices for relapse prevention are still heterogeneous and too often guided by clinical common sense only,” he added.
The study was published online in Lancet Psychiatry.
Guideline update warranted
The researchers evaluated the effect of different antipsychotic treatment strategies on risk for relapse in a network meta-analysis of 98 randomized controlled trials (RCTs) involving nearly 14,000 patients.
Compared to stopping the antipsychotic, all continuation strategies were effective in preventing relapse.
The risk for relapse was largely (and similarly) reduced when continuing the antipsychotic at the standard dose or switching to a different antipsychotic (relative risk, 0.37 and RR, 0.44, respectively), the researchers found.
Both strategies outperformed the strategy of reducing the antipsychotic dose below the standard (RR, 0.68), which was inferior to the other two strategies.
For every three patients continuing an antipsychotic at standard doses, one additional patient will avoid relapse, compared with patients stopping an antipsychotic, “which can be regarded as a large-effect magnitude according to commonly used thresholds and results from RCTs in acute schizophrenia,” the researchers write.
The number needed to treat (NNT) slightly increased to about 3.5 for patients who switched antipsychotic treatment – “still regarded as a large-effect magnitude,” they note.
“Currently, most psychiatrists are aware of the benefits of continuing antipsychotics in clinically stable individuals. However, they might face the necessity of changing the ongoing treatment strategy, generally because of burdening side effects, poor adherence, or both,” said Dr. Ostuzzi.
the investigators write.
More to the story
In an accompanying editorial, Marieke J.H. Begemann, PhD, University Medical Center Groningen (the Netherlands) and colleagues note the large number of patients included in the analysis provide “great credibility” to the findings, which are “trustworthy and important, yet only tell part of the story.”
They note that, while tapering information was often missing, antipsychotic discontinuation was probably abrupt for about two-thirds of the included studies.
“The issue of slow versus swift tapering is not yet settled, as there is a scarcity of RCTs that provide very gradual tapering over several months,” the editorialists write.
To fill this gap, several randomized trials are now in progress to specifically address the effects of gradual tapering or discontinuation vs. antipsychotic maintenance treatment in clinically stable schizophrenia.
“Time is pressing, as patients, their families, and clinicians need evidence-based data to weigh up the risks and benefits of maintaining, switching, or reducing medication with respect to a range of outcomes that are important to them, including social functioning, cognition, physical health, sexual health, and quality of life, thus going well beyond relapse prevention,” the editorialists note.
“Schizophrenia-spectrum disorders are heterogeneous with a largely unpredictable course, and we have known for a long time that a substantial proportion of patients who experienced a first psychosis can manage without antipsychotic medication. The challenge for future research is therefore to identify this subgroup on the basis of individual characteristics and guide them in tapering medication safely,” they add.
The study had no funding source. Dr. Ostuzzi reports no relevant financial relationships. A complete list of author disclosures is available with the original article. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large meta-analysis sheds light on the best antipsychotic maintenance strategy to prevent relapse in clinically stable schizophrenia – with some unexpected results that have potential implications for changes to current guidelines.
Consistent with the researchers’ hypothesis, continuing antipsychotic treatment at the standard dose, switching to another antipsychotic, and reducing the dose were all significantly more effective than stopping antipsychotic treatment in preventing relapse.
However, contrary to the researchers’ hypothesis, which was based on current literature, switching to another antipsychotic was just as effective as continuing an antipsychotic at the standard dose.
Switching to another antipsychotic “does not increase the risk of relapse. This result was not expected, as previous literature suggested otherwise,” Giovanni Ostuzzi, MD, PhD, with University of Verona (Italy) said in an interview.
“On the other hand, reducing the dose below the standard range used in the acute phase carries a tangible risk of relapse, and should be limited to selected cases, for example those where the risk of withdrawing the treatment altogether is particularly high,” Dr. Ostuzzi said.
“These results should inform evidence-based guidelines, considering that clinical practices for relapse prevention are still heterogeneous and too often guided by clinical common sense only,” he added.
The study was published online in Lancet Psychiatry.
Guideline update warranted
The researchers evaluated the effect of different antipsychotic treatment strategies on risk for relapse in a network meta-analysis of 98 randomized controlled trials (RCTs) involving nearly 14,000 patients.
Compared to stopping the antipsychotic, all continuation strategies were effective in preventing relapse.
The risk for relapse was largely (and similarly) reduced when continuing the antipsychotic at the standard dose or switching to a different antipsychotic (relative risk, 0.37 and RR, 0.44, respectively), the researchers found.
Both strategies outperformed the strategy of reducing the antipsychotic dose below the standard (RR, 0.68), which was inferior to the other two strategies.
For every three patients continuing an antipsychotic at standard doses, one additional patient will avoid relapse, compared with patients stopping an antipsychotic, “which can be regarded as a large-effect magnitude according to commonly used thresholds and results from RCTs in acute schizophrenia,” the researchers write.
The number needed to treat (NNT) slightly increased to about 3.5 for patients who switched antipsychotic treatment – “still regarded as a large-effect magnitude,” they note.
“Currently, most psychiatrists are aware of the benefits of continuing antipsychotics in clinically stable individuals. However, they might face the necessity of changing the ongoing treatment strategy, generally because of burdening side effects, poor adherence, or both,” said Dr. Ostuzzi.
the investigators write.
More to the story
In an accompanying editorial, Marieke J.H. Begemann, PhD, University Medical Center Groningen (the Netherlands) and colleagues note the large number of patients included in the analysis provide “great credibility” to the findings, which are “trustworthy and important, yet only tell part of the story.”
They note that, while tapering information was often missing, antipsychotic discontinuation was probably abrupt for about two-thirds of the included studies.
“The issue of slow versus swift tapering is not yet settled, as there is a scarcity of RCTs that provide very gradual tapering over several months,” the editorialists write.
To fill this gap, several randomized trials are now in progress to specifically address the effects of gradual tapering or discontinuation vs. antipsychotic maintenance treatment in clinically stable schizophrenia.
“Time is pressing, as patients, their families, and clinicians need evidence-based data to weigh up the risks and benefits of maintaining, switching, or reducing medication with respect to a range of outcomes that are important to them, including social functioning, cognition, physical health, sexual health, and quality of life, thus going well beyond relapse prevention,” the editorialists note.
“Schizophrenia-spectrum disorders are heterogeneous with a largely unpredictable course, and we have known for a long time that a substantial proportion of patients who experienced a first psychosis can manage without antipsychotic medication. The challenge for future research is therefore to identify this subgroup on the basis of individual characteristics and guide them in tapering medication safely,” they add.
The study had no funding source. Dr. Ostuzzi reports no relevant financial relationships. A complete list of author disclosures is available with the original article. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large meta-analysis sheds light on the best antipsychotic maintenance strategy to prevent relapse in clinically stable schizophrenia – with some unexpected results that have potential implications for changes to current guidelines.
Consistent with the researchers’ hypothesis, continuing antipsychotic treatment at the standard dose, switching to another antipsychotic, and reducing the dose were all significantly more effective than stopping antipsychotic treatment in preventing relapse.
However, contrary to the researchers’ hypothesis, which was based on current literature, switching to another antipsychotic was just as effective as continuing an antipsychotic at the standard dose.
Switching to another antipsychotic “does not increase the risk of relapse. This result was not expected, as previous literature suggested otherwise,” Giovanni Ostuzzi, MD, PhD, with University of Verona (Italy) said in an interview.
“On the other hand, reducing the dose below the standard range used in the acute phase carries a tangible risk of relapse, and should be limited to selected cases, for example those where the risk of withdrawing the treatment altogether is particularly high,” Dr. Ostuzzi said.
“These results should inform evidence-based guidelines, considering that clinical practices for relapse prevention are still heterogeneous and too often guided by clinical common sense only,” he added.
The study was published online in Lancet Psychiatry.
Guideline update warranted
The researchers evaluated the effect of different antipsychotic treatment strategies on risk for relapse in a network meta-analysis of 98 randomized controlled trials (RCTs) involving nearly 14,000 patients.
Compared to stopping the antipsychotic, all continuation strategies were effective in preventing relapse.
The risk for relapse was largely (and similarly) reduced when continuing the antipsychotic at the standard dose or switching to a different antipsychotic (relative risk, 0.37 and RR, 0.44, respectively), the researchers found.
Both strategies outperformed the strategy of reducing the antipsychotic dose below the standard (RR, 0.68), which was inferior to the other two strategies.
For every three patients continuing an antipsychotic at standard doses, one additional patient will avoid relapse, compared with patients stopping an antipsychotic, “which can be regarded as a large-effect magnitude according to commonly used thresholds and results from RCTs in acute schizophrenia,” the researchers write.
The number needed to treat (NNT) slightly increased to about 3.5 for patients who switched antipsychotic treatment – “still regarded as a large-effect magnitude,” they note.
“Currently, most psychiatrists are aware of the benefits of continuing antipsychotics in clinically stable individuals. However, they might face the necessity of changing the ongoing treatment strategy, generally because of burdening side effects, poor adherence, or both,” said Dr. Ostuzzi.
the investigators write.
More to the story
In an accompanying editorial, Marieke J.H. Begemann, PhD, University Medical Center Groningen (the Netherlands) and colleagues note the large number of patients included in the analysis provide “great credibility” to the findings, which are “trustworthy and important, yet only tell part of the story.”
They note that, while tapering information was often missing, antipsychotic discontinuation was probably abrupt for about two-thirds of the included studies.
“The issue of slow versus swift tapering is not yet settled, as there is a scarcity of RCTs that provide very gradual tapering over several months,” the editorialists write.
To fill this gap, several randomized trials are now in progress to specifically address the effects of gradual tapering or discontinuation vs. antipsychotic maintenance treatment in clinically stable schizophrenia.
“Time is pressing, as patients, their families, and clinicians need evidence-based data to weigh up the risks and benefits of maintaining, switching, or reducing medication with respect to a range of outcomes that are important to them, including social functioning, cognition, physical health, sexual health, and quality of life, thus going well beyond relapse prevention,” the editorialists note.
“Schizophrenia-spectrum disorders are heterogeneous with a largely unpredictable course, and we have known for a long time that a substantial proportion of patients who experienced a first psychosis can manage without antipsychotic medication. The challenge for future research is therefore to identify this subgroup on the basis of individual characteristics and guide them in tapering medication safely,” they add.
The study had no funding source. Dr. Ostuzzi reports no relevant financial relationships. A complete list of author disclosures is available with the original article. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Transdermal med may directly target hostility in schizophrenia
The results suggest that these effects are at least partially independent of general antipsychotic effects or effects on sedation or akathisia.
“It’s important not to assume that antipsychotics decrease hostility by having people feel more sedated and that the only way to treat someone hostile is by sedating them,” lead investigator Leslie Citrome, MD, MPH, clinical professor of psychiatry and behavioral sciences, New York Medical College, Valhalla, told this news organization.
“Our findings suggest that transdermal asenapine has a specific antihostility effect in patients with schizophrenia,” he added.
The study was published online in the Journal of Clinical Psychiatry.
A complex disorder
“Patients with schizophrenia are known to potentially exhibit aggressive, hostile behavior, especially during the acute phase of the illness, thus making effective management critical,” the authors write.
Dr. Citrome said schizophrenia is a complex condition that consists of five different symptom domains. These include positive (hallucinations, delusions), negative (amotivation, apathy), disorganization (cognitive symptoms), depression/anxiety, and excitability/hostility symptoms.
“These five domains are more or less independent of each other, in terms of treatment effects,” he noted.
Dr. Citrome has long been interested in the activity of antipsychotics and their impact on these various symptoms – particularly hostility – and recently published a review focusing on the impact of an array of antipsychotics on this symptom domain.
“What struck me here is that this is a transdermal system, a patch,” Dr. Citrome said. “None of the sedation that would ordinarily be associated with a sublingual asenapine would be present here.”
Dr. Citrome wanted to investigate whether the transdermal system would have an impact on hostility because, if so, “it would support the notion that hostility is an independent treatment target in schizophrenia.”
To investigate, Dr. Citrome and co-authors analyzed data from the pivotal HP-3070 phase 3 randomized, double-blind, placebo-controlled study of adults with schizophrenia who were randomly selected to receive either HP-3070 7.6 mg/24h, HP-3070 3.8 mg/24h, or placebo for 6 weeks.
The trial found that once-daily applications of HP-3070 demonstrated significant improvement in Positive and Negative Syndrome Scale (PANSS) total scores after 2-3 weeks of treatment, with the improvements sustained through week 6.
The current study was a post hoc analysis focusing specifically on 442 patients with hostility and agitation (defined as PANSS hostility item score > 1).
The outcome was the least-squares mean (LSM) changes in the PANSS hostility item. They also analyzed PANSS–Excited Component (PANSS-EC) from baseline to week 6 in all study participants. Findings were adjusted for the presence of somnolence or akathisia.
Demographic and baseline disease characteristics were “balanced” between the HP-3070–6.6-mg and the HP-3070–3.8-mg groups (n = 151 and n = 147, respectively) and the placebo group (n = 144) in the intent-to-treat analysis, with a mean (standard deviation) age of between 41.5 and 42.3 (11.6-11.9) years. Roughly three-quarters of participants were White, and most participants had a mean duration of between 15 and 16 years since diagnosis.
Independent effect
At week 6, the LSM mean change from baseline (CFB) in the PANSS hostility score was superior in both treatment groups at 6 weeks, compared with placebo (7.6 mg/24 hr: CFB, –0.4; 95% confidence interval, –0.6 to –0.2; P < .001; 3.8 mg/24 hr: CFB, –0.3; 95% CI, –0.6 to –0.1; P < .01).
The findings remained significant, even after the researchers adjusted for covariates.
For all patients, regardless of baseline PANSS hostility item score, PANSS-EC week 6 LSM CFB was greater in both treatment groups compared with placebo (7.6 mg/24 hr: CFB, –1.1; 95% CI, –1.9 to –0.4; P < .01; 3.8 mg/24 hr: CFB, –1.3; 95% CI, –2.0 to –0.6; P < .001).
Patients with a PANSS hostility score > 1 at baseline in both treatment groups also showed significant improvement in PANSS-EC score, compared with the placebo, beginning at week 2 and continuing through week 6.
“These effects of HP-3070 treatment on the PANSS hostility item score remained, even after adjustment for confounding variables, suggesting that the effect of HP-3070 on reducing symptoms of hostility may at be at least partially independent of general antipsychotic effects on hallucinations or delusions or the presence or absence of relevant medication-induced adverse effects, such as sedation or akathisia,” the authors comment.
They note that a limitation of the study is that it was conducted post hoc. In addition, the mean baseline PANSS hostility score was “low,” even among those with a score > 1, “translating to a severity level of minimal to mild,” which “limits the generalizability” of the analysis.
Important treatment target
Commenting on the study, Rifaat El-Mallakh, MD, MS, director of the Mood Disorders Research Program, department of psychiatry and behavioral sciences, University of Louisville (Ky.) School of Medicine, said, “aggression and hostility may exhibit themselves as symptoms of a psychotic illness or independent of psychosis and are an important treatment target.”
Dr. El-Mallakh, who was not involved with the study, said the “most effective anti-aggression medicine is clozapine.” He believes that it is this effect that “gives clozapine its stellar reputation,” rather than its antipsychotic effect.
“Of importance, the anti-aggression effect of clozapine is independent of its antipsychotic effect, which is an important point because if the behavior is rooted in psychosis, then successful treatment of psychosis with any agent should reduce aggression.”
The researchers “demonstrated that the effect was independent of psychosis” but because the study only recruited people with schizophrenia, the researchers “could not examine to see if the effect is independent of diagnosis,” Dr. El-Mallakh said.
“It is important to consider aggression/hostility as an independent behavior in pharmacologic studies, because it probably has its own biochemistry and neuroanatomy,” he added.
This study was funded by Hisamitsu Pharmaceutical. Dr. Citrome has received nonfinancial support from Hisamitsu Pharmaceutical and personal fees from Noven Pharmaceuticals during the conduct of the study; personal fees from numerous companies and organizations; and one-off ad hoc consulting for medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies outside the submitted work. He has stocks and received royalties from numerous companies and organizations. Dr. El-Mallakh is a speaker for Noven, as well as Indivior, Intracellular, Janssen, Lundbeck, Otsuka, Sunovion, and Teva.
A version of this article first appeared on Medscape.com.
The results suggest that these effects are at least partially independent of general antipsychotic effects or effects on sedation or akathisia.
“It’s important not to assume that antipsychotics decrease hostility by having people feel more sedated and that the only way to treat someone hostile is by sedating them,” lead investigator Leslie Citrome, MD, MPH, clinical professor of psychiatry and behavioral sciences, New York Medical College, Valhalla, told this news organization.
“Our findings suggest that transdermal asenapine has a specific antihostility effect in patients with schizophrenia,” he added.
The study was published online in the Journal of Clinical Psychiatry.
A complex disorder
“Patients with schizophrenia are known to potentially exhibit aggressive, hostile behavior, especially during the acute phase of the illness, thus making effective management critical,” the authors write.
Dr. Citrome said schizophrenia is a complex condition that consists of five different symptom domains. These include positive (hallucinations, delusions), negative (amotivation, apathy), disorganization (cognitive symptoms), depression/anxiety, and excitability/hostility symptoms.
“These five domains are more or less independent of each other, in terms of treatment effects,” he noted.
Dr. Citrome has long been interested in the activity of antipsychotics and their impact on these various symptoms – particularly hostility – and recently published a review focusing on the impact of an array of antipsychotics on this symptom domain.
“What struck me here is that this is a transdermal system, a patch,” Dr. Citrome said. “None of the sedation that would ordinarily be associated with a sublingual asenapine would be present here.”
Dr. Citrome wanted to investigate whether the transdermal system would have an impact on hostility because, if so, “it would support the notion that hostility is an independent treatment target in schizophrenia.”
To investigate, Dr. Citrome and co-authors analyzed data from the pivotal HP-3070 phase 3 randomized, double-blind, placebo-controlled study of adults with schizophrenia who were randomly selected to receive either HP-3070 7.6 mg/24h, HP-3070 3.8 mg/24h, or placebo for 6 weeks.
The trial found that once-daily applications of HP-3070 demonstrated significant improvement in Positive and Negative Syndrome Scale (PANSS) total scores after 2-3 weeks of treatment, with the improvements sustained through week 6.
The current study was a post hoc analysis focusing specifically on 442 patients with hostility and agitation (defined as PANSS hostility item score > 1).
The outcome was the least-squares mean (LSM) changes in the PANSS hostility item. They also analyzed PANSS–Excited Component (PANSS-EC) from baseline to week 6 in all study participants. Findings were adjusted for the presence of somnolence or akathisia.
Demographic and baseline disease characteristics were “balanced” between the HP-3070–6.6-mg and the HP-3070–3.8-mg groups (n = 151 and n = 147, respectively) and the placebo group (n = 144) in the intent-to-treat analysis, with a mean (standard deviation) age of between 41.5 and 42.3 (11.6-11.9) years. Roughly three-quarters of participants were White, and most participants had a mean duration of between 15 and 16 years since diagnosis.
Independent effect
At week 6, the LSM mean change from baseline (CFB) in the PANSS hostility score was superior in both treatment groups at 6 weeks, compared with placebo (7.6 mg/24 hr: CFB, –0.4; 95% confidence interval, –0.6 to –0.2; P < .001; 3.8 mg/24 hr: CFB, –0.3; 95% CI, –0.6 to –0.1; P < .01).
The findings remained significant, even after the researchers adjusted for covariates.
For all patients, regardless of baseline PANSS hostility item score, PANSS-EC week 6 LSM CFB was greater in both treatment groups compared with placebo (7.6 mg/24 hr: CFB, –1.1; 95% CI, –1.9 to –0.4; P < .01; 3.8 mg/24 hr: CFB, –1.3; 95% CI, –2.0 to –0.6; P < .001).
Patients with a PANSS hostility score > 1 at baseline in both treatment groups also showed significant improvement in PANSS-EC score, compared with the placebo, beginning at week 2 and continuing through week 6.
“These effects of HP-3070 treatment on the PANSS hostility item score remained, even after adjustment for confounding variables, suggesting that the effect of HP-3070 on reducing symptoms of hostility may at be at least partially independent of general antipsychotic effects on hallucinations or delusions or the presence or absence of relevant medication-induced adverse effects, such as sedation or akathisia,” the authors comment.
They note that a limitation of the study is that it was conducted post hoc. In addition, the mean baseline PANSS hostility score was “low,” even among those with a score > 1, “translating to a severity level of minimal to mild,” which “limits the generalizability” of the analysis.
Important treatment target
Commenting on the study, Rifaat El-Mallakh, MD, MS, director of the Mood Disorders Research Program, department of psychiatry and behavioral sciences, University of Louisville (Ky.) School of Medicine, said, “aggression and hostility may exhibit themselves as symptoms of a psychotic illness or independent of psychosis and are an important treatment target.”
Dr. El-Mallakh, who was not involved with the study, said the “most effective anti-aggression medicine is clozapine.” He believes that it is this effect that “gives clozapine its stellar reputation,” rather than its antipsychotic effect.
“Of importance, the anti-aggression effect of clozapine is independent of its antipsychotic effect, which is an important point because if the behavior is rooted in psychosis, then successful treatment of psychosis with any agent should reduce aggression.”
The researchers “demonstrated that the effect was independent of psychosis” but because the study only recruited people with schizophrenia, the researchers “could not examine to see if the effect is independent of diagnosis,” Dr. El-Mallakh said.
“It is important to consider aggression/hostility as an independent behavior in pharmacologic studies, because it probably has its own biochemistry and neuroanatomy,” he added.
This study was funded by Hisamitsu Pharmaceutical. Dr. Citrome has received nonfinancial support from Hisamitsu Pharmaceutical and personal fees from Noven Pharmaceuticals during the conduct of the study; personal fees from numerous companies and organizations; and one-off ad hoc consulting for medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies outside the submitted work. He has stocks and received royalties from numerous companies and organizations. Dr. El-Mallakh is a speaker for Noven, as well as Indivior, Intracellular, Janssen, Lundbeck, Otsuka, Sunovion, and Teva.
A version of this article first appeared on Medscape.com.
The results suggest that these effects are at least partially independent of general antipsychotic effects or effects on sedation or akathisia.
“It’s important not to assume that antipsychotics decrease hostility by having people feel more sedated and that the only way to treat someone hostile is by sedating them,” lead investigator Leslie Citrome, MD, MPH, clinical professor of psychiatry and behavioral sciences, New York Medical College, Valhalla, told this news organization.
“Our findings suggest that transdermal asenapine has a specific antihostility effect in patients with schizophrenia,” he added.
The study was published online in the Journal of Clinical Psychiatry.
A complex disorder
“Patients with schizophrenia are known to potentially exhibit aggressive, hostile behavior, especially during the acute phase of the illness, thus making effective management critical,” the authors write.
Dr. Citrome said schizophrenia is a complex condition that consists of five different symptom domains. These include positive (hallucinations, delusions), negative (amotivation, apathy), disorganization (cognitive symptoms), depression/anxiety, and excitability/hostility symptoms.
“These five domains are more or less independent of each other, in terms of treatment effects,” he noted.
Dr. Citrome has long been interested in the activity of antipsychotics and their impact on these various symptoms – particularly hostility – and recently published a review focusing on the impact of an array of antipsychotics on this symptom domain.
“What struck me here is that this is a transdermal system, a patch,” Dr. Citrome said. “None of the sedation that would ordinarily be associated with a sublingual asenapine would be present here.”
Dr. Citrome wanted to investigate whether the transdermal system would have an impact on hostility because, if so, “it would support the notion that hostility is an independent treatment target in schizophrenia.”
To investigate, Dr. Citrome and co-authors analyzed data from the pivotal HP-3070 phase 3 randomized, double-blind, placebo-controlled study of adults with schizophrenia who were randomly selected to receive either HP-3070 7.6 mg/24h, HP-3070 3.8 mg/24h, or placebo for 6 weeks.
The trial found that once-daily applications of HP-3070 demonstrated significant improvement in Positive and Negative Syndrome Scale (PANSS) total scores after 2-3 weeks of treatment, with the improvements sustained through week 6.
The current study was a post hoc analysis focusing specifically on 442 patients with hostility and agitation (defined as PANSS hostility item score > 1).
The outcome was the least-squares mean (LSM) changes in the PANSS hostility item. They also analyzed PANSS–Excited Component (PANSS-EC) from baseline to week 6 in all study participants. Findings were adjusted for the presence of somnolence or akathisia.
Demographic and baseline disease characteristics were “balanced” between the HP-3070–6.6-mg and the HP-3070–3.8-mg groups (n = 151 and n = 147, respectively) and the placebo group (n = 144) in the intent-to-treat analysis, with a mean (standard deviation) age of between 41.5 and 42.3 (11.6-11.9) years. Roughly three-quarters of participants were White, and most participants had a mean duration of between 15 and 16 years since diagnosis.
Independent effect
At week 6, the LSM mean change from baseline (CFB) in the PANSS hostility score was superior in both treatment groups at 6 weeks, compared with placebo (7.6 mg/24 hr: CFB, –0.4; 95% confidence interval, –0.6 to –0.2; P < .001; 3.8 mg/24 hr: CFB, –0.3; 95% CI, –0.6 to –0.1; P < .01).
The findings remained significant, even after the researchers adjusted for covariates.
For all patients, regardless of baseline PANSS hostility item score, PANSS-EC week 6 LSM CFB was greater in both treatment groups compared with placebo (7.6 mg/24 hr: CFB, –1.1; 95% CI, –1.9 to –0.4; P < .01; 3.8 mg/24 hr: CFB, –1.3; 95% CI, –2.0 to –0.6; P < .001).
Patients with a PANSS hostility score > 1 at baseline in both treatment groups also showed significant improvement in PANSS-EC score, compared with the placebo, beginning at week 2 and continuing through week 6.
“These effects of HP-3070 treatment on the PANSS hostility item score remained, even after adjustment for confounding variables, suggesting that the effect of HP-3070 on reducing symptoms of hostility may at be at least partially independent of general antipsychotic effects on hallucinations or delusions or the presence or absence of relevant medication-induced adverse effects, such as sedation or akathisia,” the authors comment.
They note that a limitation of the study is that it was conducted post hoc. In addition, the mean baseline PANSS hostility score was “low,” even among those with a score > 1, “translating to a severity level of minimal to mild,” which “limits the generalizability” of the analysis.
Important treatment target
Commenting on the study, Rifaat El-Mallakh, MD, MS, director of the Mood Disorders Research Program, department of psychiatry and behavioral sciences, University of Louisville (Ky.) School of Medicine, said, “aggression and hostility may exhibit themselves as symptoms of a psychotic illness or independent of psychosis and are an important treatment target.”
Dr. El-Mallakh, who was not involved with the study, said the “most effective anti-aggression medicine is clozapine.” He believes that it is this effect that “gives clozapine its stellar reputation,” rather than its antipsychotic effect.
“Of importance, the anti-aggression effect of clozapine is independent of its antipsychotic effect, which is an important point because if the behavior is rooted in psychosis, then successful treatment of psychosis with any agent should reduce aggression.”
The researchers “demonstrated that the effect was independent of psychosis” but because the study only recruited people with schizophrenia, the researchers “could not examine to see if the effect is independent of diagnosis,” Dr. El-Mallakh said.
“It is important to consider aggression/hostility as an independent behavior in pharmacologic studies, because it probably has its own biochemistry and neuroanatomy,” he added.
This study was funded by Hisamitsu Pharmaceutical. Dr. Citrome has received nonfinancial support from Hisamitsu Pharmaceutical and personal fees from Noven Pharmaceuticals during the conduct of the study; personal fees from numerous companies and organizations; and one-off ad hoc consulting for medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies outside the submitted work. He has stocks and received royalties from numerous companies and organizations. Dr. El-Mallakh is a speaker for Noven, as well as Indivior, Intracellular, Janssen, Lundbeck, Otsuka, Sunovion, and Teva.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Advance directives for psychiatric care reduce compulsory admissions
, new research shows.
Results of a randomized trial showed the peer worker PAD group had a 42% reduction in compulsory admission over the following 12 months. This study group also had lower symptom scores, greater rates of recovery, and increased empowerment, compared with patients assigned to usual care.
In addition to proving that PADs are effective in reducing compulsory admission, the results show that facilitation by peer workers is relevant, study investigator Aurélie Tinland, MD, PhD, Faculté de Médecine Timone, Aix-Marseille University, Marseille, France, told delegates attending the virtual European Psychiatric Association (EPA) 2022 Congress. The study was simultaneously published online in JAMA Psychiatry.
However, Dr. Tinland noted that more research that includes “harder to reach” populations is needed. In addition, greater use of PADs is also key to reducing compulsory admissions.
‘Most coercive’ country
The researchers note that respect for patient autonomy is a strong pillar of health care, such that “involuntary treatment should be unusual.” However, they point out that “compulsory psychiatric admissions are far too common in countries of all income levels.”
In France, said Dr. Tinland, 24% of psychiatric hospitalizations are compulsory. The country is ranked the sixth “most coercive” country in the world, and there are concerns about human rights in French psychiatric facilities.
She added that advance care statements are the most efficient tool for reducing coercion, with one study suggesting they could cut rates by 25%, compared with usual care.
However, she noted there is an “asymmetry” between medical professionals and patients and a risk of “undue influence” when clinicians facilitate the completion of care statements.
To examine the impact on clinical outcomes of peer-worker facilitated PADs, the researchers studied adults with a diagnosis of schizophrenia, bipolar I disorder, or schizoaffective disorder who were admitted to a psychiatric hospital within the previous 12 months. Peer workers are individuals who have lived experience with mental illness and help inform and guide current patients about care options in the event of a mental health crisis.
Study participants were randomly assigned 1:1 to an intervention group or a usual care control group. The intervention group received a PAD document and were assigned a peer worker while the usual care group received comprehensive information about the PAD concept at study entry and were free to complete it, but they were not connected with a peer worker.
The PAD document included information about future treatment and support preferences, early signs of relapse, and coping strategies. Participants could meet the peer worker in a place of their choice and be supported in drafting the document and in sharing it with health care professionals.
In all, 394 individuals completed the study. The majority (61%) of participants were male and 66% had completed post-secondary education. Schizophrenia was diagnosed in 45%, bipolar I disorder in 36%, and schizoaffective disorder in 19%.
Participants in the intervention group were significantly younger than those in the control group, with a mean of 37.4 years versus 41 years (P = .003) and were less likely to have one or more somatic comorbidities, at 61.2% versus 69.2%.
A PAD was completed by 54.6% of individuals in the intervention group versus 7.1% of controls (P < .001). The PAD was written with peer worker support by 41.3% of those in the intervention and by 2% of controls. Of those who completed a PAD, 75.7% met care facilitators, and 27.1% used it during a crisis over the following 12 months.
Results showed that the rate of compulsory admissions was significantly lower in the peer worker PAD group, at 27% versus 39.9% in control participants, at an odds ratio of 0.58 (P = .007).
Participants in the intervention group had lower symptoms on the modified Colorado Symptom Score than usual care patients with an effect size of -0.20 (P = .03) and higher scores on the Empowerment Scale (effect size 0.30, P = .003).
Scores on the Recovery Assessment Scale were also significantly higher in the peer worker PAD group versus controls with an effect size of 0.44 (P < .001). There were no significant differences, however, in overall admission rates, the quality of the therapeutic alliance, or quality of life.
Putting patients in the driver’s seat
Commenting on the findings, Robert Dabney Jr., MA, MDiv, peer apprentice program manager at the Depression and Bipolar Support Alliance, Chicago, said the study “tells us there are many benefits to completing a psychiatric advance directive, but perhaps the most powerful one is putting the person receiving mental health care in the driver’s seat of their own recovery.”
However, he noted that “many people living with mental health conditions don’t know the option exists to decide on their treatment plan in advance of a crisis.”
“This is where peer support specialists can come in. Having a peer who has been through similar experiences and can guide you through the process is as comforting as it is empowering. I have witnessed and experienced firsthand the power of peer support,” he said.
“It’s my personal hope and the goal of the Depression and Bipolar Support Alliance to empower more people to either become peer support specialists or seek out peer support services, because we know it improves and even saves lives,” Mr. Dabney added.
Virginia A. Brown, PhD, department of psychiatry & behavioral sciences, University of Texas at Austin Dell Medical School, noted there are huge differences between the health care systems in France and the United States.
She explained that two of the greatest barriers to PADs in the United States is that until 2016, filling one out was not billable and that “practitioners don’t know anything about advanced care plans.”
Dr. Brown said her own work shows that individuals who support patients during a crisis believe it would be “really helpful if we had some kind of document that we could share with the health care system that says: ‘Hey, look, I’m the designated person to speak for this patient, they’ve identified me through a document.’ So, people were actually describing a need for this document but didn’t know that it existed.”
Another problem is that in the United States, hospitals operate in a “closed system” and cannot talk to an unrelated hospital or to the police department “to get information to those first responders during an emergency about who to talk to about their wishes and preferences.”
“There are a lot of hurdles that we’ve got to get over to make a more robust system that protects the autonomy of people who live with serious mental illness,” Dr. Brown said, as “losing capacity during a crisis is time-limited, and it requires us to respond to it as a medical emergency.”
The study was supported by an institutional grant from the French 2017 National Program of Health Services Research. The Clinical Research Direction of Assistance Publique Hôpitaux de Marseille sponsored the trial. Dr. Tinland declares grants from the French Ministry of Health Directorate General of Health Care Services during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a randomized trial showed the peer worker PAD group had a 42% reduction in compulsory admission over the following 12 months. This study group also had lower symptom scores, greater rates of recovery, and increased empowerment, compared with patients assigned to usual care.
In addition to proving that PADs are effective in reducing compulsory admission, the results show that facilitation by peer workers is relevant, study investigator Aurélie Tinland, MD, PhD, Faculté de Médecine Timone, Aix-Marseille University, Marseille, France, told delegates attending the virtual European Psychiatric Association (EPA) 2022 Congress. The study was simultaneously published online in JAMA Psychiatry.
However, Dr. Tinland noted that more research that includes “harder to reach” populations is needed. In addition, greater use of PADs is also key to reducing compulsory admissions.
‘Most coercive’ country
The researchers note that respect for patient autonomy is a strong pillar of health care, such that “involuntary treatment should be unusual.” However, they point out that “compulsory psychiatric admissions are far too common in countries of all income levels.”
In France, said Dr. Tinland, 24% of psychiatric hospitalizations are compulsory. The country is ranked the sixth “most coercive” country in the world, and there are concerns about human rights in French psychiatric facilities.
She added that advance care statements are the most efficient tool for reducing coercion, with one study suggesting they could cut rates by 25%, compared with usual care.
However, she noted there is an “asymmetry” between medical professionals and patients and a risk of “undue influence” when clinicians facilitate the completion of care statements.
To examine the impact on clinical outcomes of peer-worker facilitated PADs, the researchers studied adults with a diagnosis of schizophrenia, bipolar I disorder, or schizoaffective disorder who were admitted to a psychiatric hospital within the previous 12 months. Peer workers are individuals who have lived experience with mental illness and help inform and guide current patients about care options in the event of a mental health crisis.
Study participants were randomly assigned 1:1 to an intervention group or a usual care control group. The intervention group received a PAD document and were assigned a peer worker while the usual care group received comprehensive information about the PAD concept at study entry and were free to complete it, but they were not connected with a peer worker.
The PAD document included information about future treatment and support preferences, early signs of relapse, and coping strategies. Participants could meet the peer worker in a place of their choice and be supported in drafting the document and in sharing it with health care professionals.
In all, 394 individuals completed the study. The majority (61%) of participants were male and 66% had completed post-secondary education. Schizophrenia was diagnosed in 45%, bipolar I disorder in 36%, and schizoaffective disorder in 19%.
Participants in the intervention group were significantly younger than those in the control group, with a mean of 37.4 years versus 41 years (P = .003) and were less likely to have one or more somatic comorbidities, at 61.2% versus 69.2%.
A PAD was completed by 54.6% of individuals in the intervention group versus 7.1% of controls (P < .001). The PAD was written with peer worker support by 41.3% of those in the intervention and by 2% of controls. Of those who completed a PAD, 75.7% met care facilitators, and 27.1% used it during a crisis over the following 12 months.
Results showed that the rate of compulsory admissions was significantly lower in the peer worker PAD group, at 27% versus 39.9% in control participants, at an odds ratio of 0.58 (P = .007).
Participants in the intervention group had lower symptoms on the modified Colorado Symptom Score than usual care patients with an effect size of -0.20 (P = .03) and higher scores on the Empowerment Scale (effect size 0.30, P = .003).
Scores on the Recovery Assessment Scale were also significantly higher in the peer worker PAD group versus controls with an effect size of 0.44 (P < .001). There were no significant differences, however, in overall admission rates, the quality of the therapeutic alliance, or quality of life.
Putting patients in the driver’s seat
Commenting on the findings, Robert Dabney Jr., MA, MDiv, peer apprentice program manager at the Depression and Bipolar Support Alliance, Chicago, said the study “tells us there are many benefits to completing a psychiatric advance directive, but perhaps the most powerful one is putting the person receiving mental health care in the driver’s seat of their own recovery.”
However, he noted that “many people living with mental health conditions don’t know the option exists to decide on their treatment plan in advance of a crisis.”
“This is where peer support specialists can come in. Having a peer who has been through similar experiences and can guide you through the process is as comforting as it is empowering. I have witnessed and experienced firsthand the power of peer support,” he said.
“It’s my personal hope and the goal of the Depression and Bipolar Support Alliance to empower more people to either become peer support specialists or seek out peer support services, because we know it improves and even saves lives,” Mr. Dabney added.
Virginia A. Brown, PhD, department of psychiatry & behavioral sciences, University of Texas at Austin Dell Medical School, noted there are huge differences between the health care systems in France and the United States.
She explained that two of the greatest barriers to PADs in the United States is that until 2016, filling one out was not billable and that “practitioners don’t know anything about advanced care plans.”
Dr. Brown said her own work shows that individuals who support patients during a crisis believe it would be “really helpful if we had some kind of document that we could share with the health care system that says: ‘Hey, look, I’m the designated person to speak for this patient, they’ve identified me through a document.’ So, people were actually describing a need for this document but didn’t know that it existed.”
Another problem is that in the United States, hospitals operate in a “closed system” and cannot talk to an unrelated hospital or to the police department “to get information to those first responders during an emergency about who to talk to about their wishes and preferences.”
“There are a lot of hurdles that we’ve got to get over to make a more robust system that protects the autonomy of people who live with serious mental illness,” Dr. Brown said, as “losing capacity during a crisis is time-limited, and it requires us to respond to it as a medical emergency.”
The study was supported by an institutional grant from the French 2017 National Program of Health Services Research. The Clinical Research Direction of Assistance Publique Hôpitaux de Marseille sponsored the trial. Dr. Tinland declares grants from the French Ministry of Health Directorate General of Health Care Services during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a randomized trial showed the peer worker PAD group had a 42% reduction in compulsory admission over the following 12 months. This study group also had lower symptom scores, greater rates of recovery, and increased empowerment, compared with patients assigned to usual care.
In addition to proving that PADs are effective in reducing compulsory admission, the results show that facilitation by peer workers is relevant, study investigator Aurélie Tinland, MD, PhD, Faculté de Médecine Timone, Aix-Marseille University, Marseille, France, told delegates attending the virtual European Psychiatric Association (EPA) 2022 Congress. The study was simultaneously published online in JAMA Psychiatry.
However, Dr. Tinland noted that more research that includes “harder to reach” populations is needed. In addition, greater use of PADs is also key to reducing compulsory admissions.
‘Most coercive’ country
The researchers note that respect for patient autonomy is a strong pillar of health care, such that “involuntary treatment should be unusual.” However, they point out that “compulsory psychiatric admissions are far too common in countries of all income levels.”
In France, said Dr. Tinland, 24% of psychiatric hospitalizations are compulsory. The country is ranked the sixth “most coercive” country in the world, and there are concerns about human rights in French psychiatric facilities.
She added that advance care statements are the most efficient tool for reducing coercion, with one study suggesting they could cut rates by 25%, compared with usual care.
However, she noted there is an “asymmetry” between medical professionals and patients and a risk of “undue influence” when clinicians facilitate the completion of care statements.
To examine the impact on clinical outcomes of peer-worker facilitated PADs, the researchers studied adults with a diagnosis of schizophrenia, bipolar I disorder, or schizoaffective disorder who were admitted to a psychiatric hospital within the previous 12 months. Peer workers are individuals who have lived experience with mental illness and help inform and guide current patients about care options in the event of a mental health crisis.
Study participants were randomly assigned 1:1 to an intervention group or a usual care control group. The intervention group received a PAD document and were assigned a peer worker while the usual care group received comprehensive information about the PAD concept at study entry and were free to complete it, but they were not connected with a peer worker.
The PAD document included information about future treatment and support preferences, early signs of relapse, and coping strategies. Participants could meet the peer worker in a place of their choice and be supported in drafting the document and in sharing it with health care professionals.
In all, 394 individuals completed the study. The majority (61%) of participants were male and 66% had completed post-secondary education. Schizophrenia was diagnosed in 45%, bipolar I disorder in 36%, and schizoaffective disorder in 19%.
Participants in the intervention group were significantly younger than those in the control group, with a mean of 37.4 years versus 41 years (P = .003) and were less likely to have one or more somatic comorbidities, at 61.2% versus 69.2%.
A PAD was completed by 54.6% of individuals in the intervention group versus 7.1% of controls (P < .001). The PAD was written with peer worker support by 41.3% of those in the intervention and by 2% of controls. Of those who completed a PAD, 75.7% met care facilitators, and 27.1% used it during a crisis over the following 12 months.
Results showed that the rate of compulsory admissions was significantly lower in the peer worker PAD group, at 27% versus 39.9% in control participants, at an odds ratio of 0.58 (P = .007).
Participants in the intervention group had lower symptoms on the modified Colorado Symptom Score than usual care patients with an effect size of -0.20 (P = .03) and higher scores on the Empowerment Scale (effect size 0.30, P = .003).
Scores on the Recovery Assessment Scale were also significantly higher in the peer worker PAD group versus controls with an effect size of 0.44 (P < .001). There were no significant differences, however, in overall admission rates, the quality of the therapeutic alliance, or quality of life.
Putting patients in the driver’s seat
Commenting on the findings, Robert Dabney Jr., MA, MDiv, peer apprentice program manager at the Depression and Bipolar Support Alliance, Chicago, said the study “tells us there are many benefits to completing a psychiatric advance directive, but perhaps the most powerful one is putting the person receiving mental health care in the driver’s seat of their own recovery.”
However, he noted that “many people living with mental health conditions don’t know the option exists to decide on their treatment plan in advance of a crisis.”
“This is where peer support specialists can come in. Having a peer who has been through similar experiences and can guide you through the process is as comforting as it is empowering. I have witnessed and experienced firsthand the power of peer support,” he said.
“It’s my personal hope and the goal of the Depression and Bipolar Support Alliance to empower more people to either become peer support specialists or seek out peer support services, because we know it improves and even saves lives,” Mr. Dabney added.
Virginia A. Brown, PhD, department of psychiatry & behavioral sciences, University of Texas at Austin Dell Medical School, noted there are huge differences between the health care systems in France and the United States.
She explained that two of the greatest barriers to PADs in the United States is that until 2016, filling one out was not billable and that “practitioners don’t know anything about advanced care plans.”
Dr. Brown said her own work shows that individuals who support patients during a crisis believe it would be “really helpful if we had some kind of document that we could share with the health care system that says: ‘Hey, look, I’m the designated person to speak for this patient, they’ve identified me through a document.’ So, people were actually describing a need for this document but didn’t know that it existed.”
Another problem is that in the United States, hospitals operate in a “closed system” and cannot talk to an unrelated hospital or to the police department “to get information to those first responders during an emergency about who to talk to about their wishes and preferences.”
“There are a lot of hurdles that we’ve got to get over to make a more robust system that protects the autonomy of people who live with serious mental illness,” Dr. Brown said, as “losing capacity during a crisis is time-limited, and it requires us to respond to it as a medical emergency.”
The study was supported by an institutional grant from the French 2017 National Program of Health Services Research. The Clinical Research Direction of Assistance Publique Hôpitaux de Marseille sponsored the trial. Dr. Tinland declares grants from the French Ministry of Health Directorate General of Health Care Services during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM EPA 2022