User login
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.
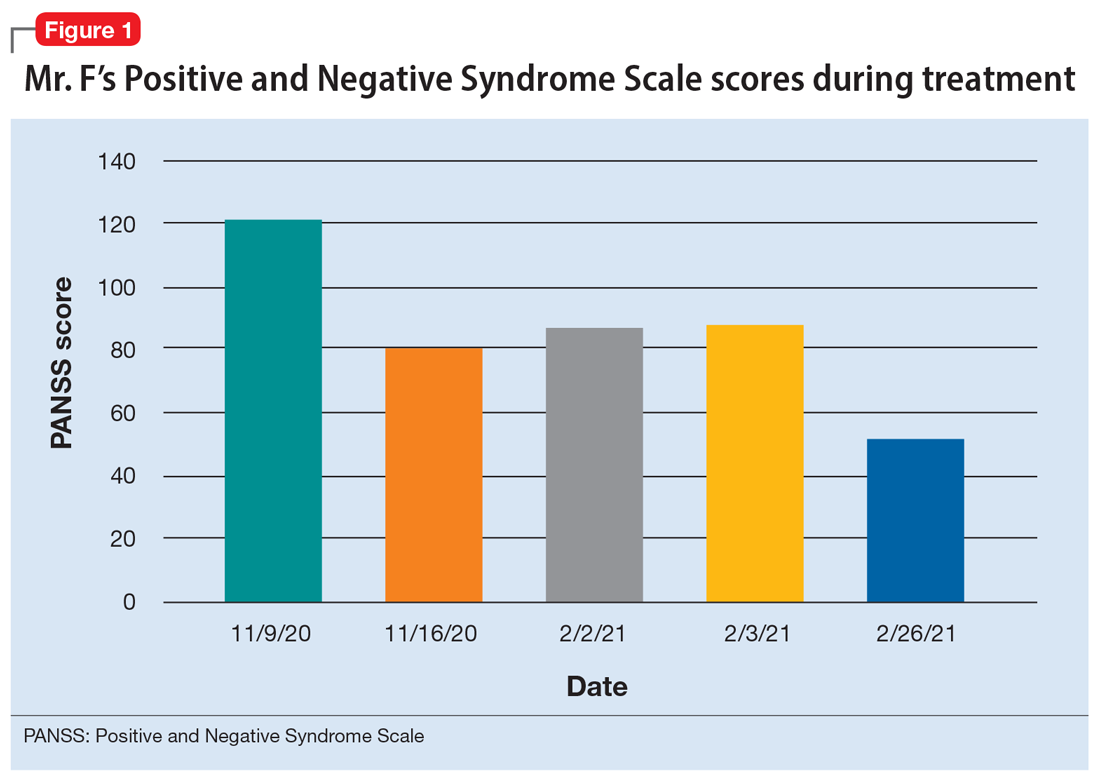
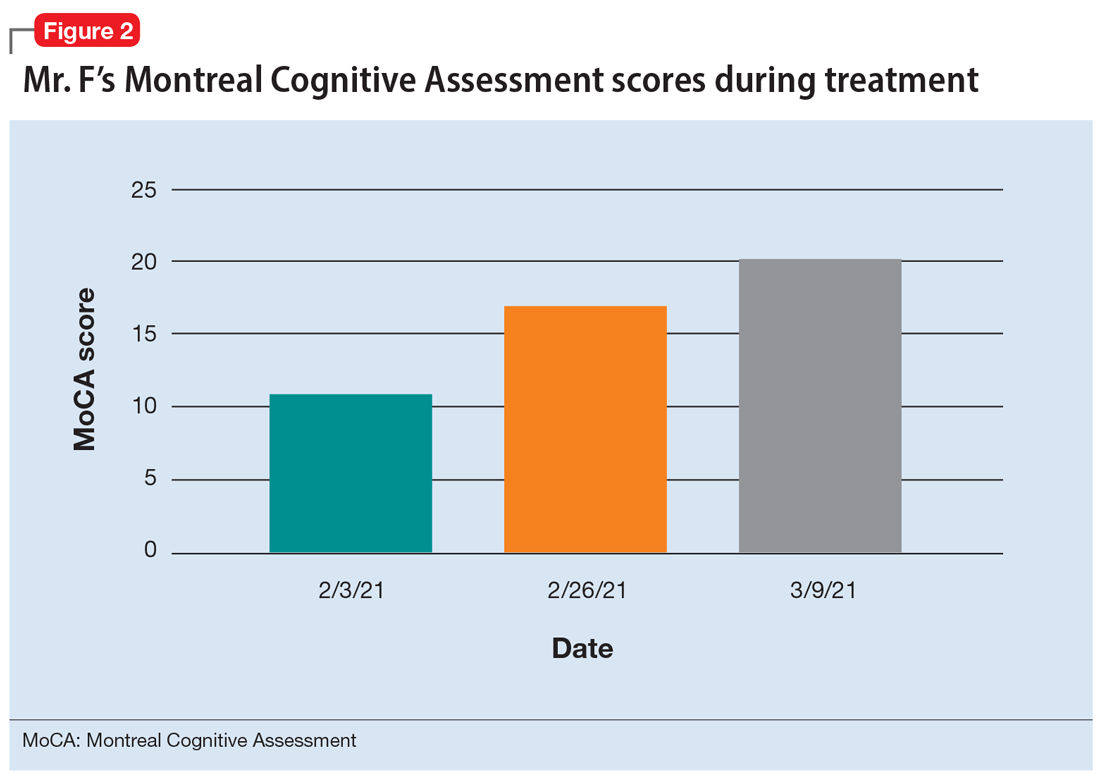
During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.
The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
