User login
RA Prevention: A Decade of Trials Provides Insights on What’s to Come
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)
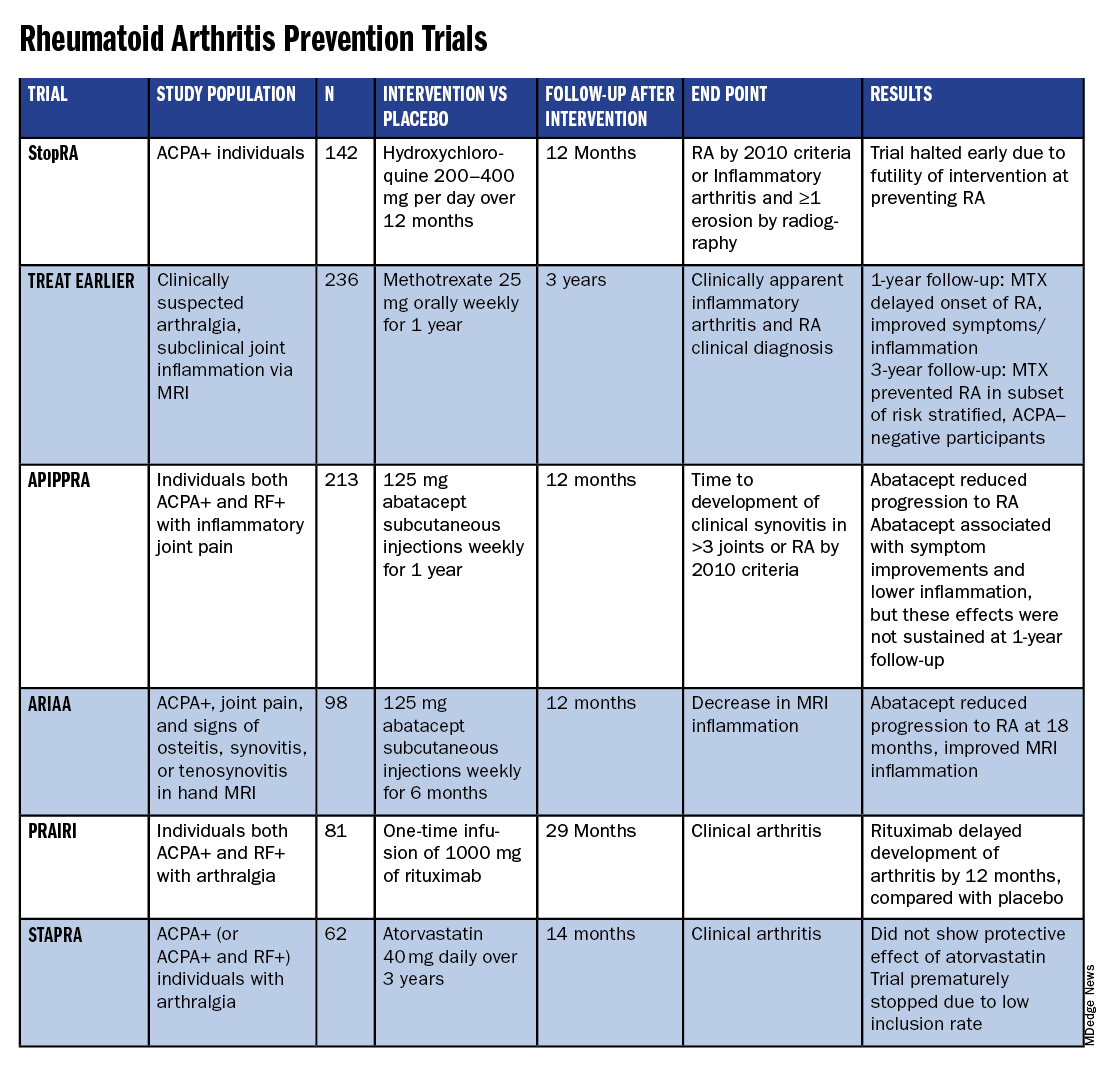
Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
What’s the Evidence Behind Popular Supplements in Rheumatology? Experts Weigh in
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Cannabis Often Used as a Substitute for Traditional Medications
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Therapeutic Drug Monitoring in Rheumatology: A Promising Outlook But Many Barriers to Overcome
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Few Differences Seen in RA Pain Outcomes for JAK Inhibitors, Biologics
TOPLINE:
Janus kinase (JAK) inhibitors had a marginally superior effect on pain relief when compared with tumor necrosis factor (TNF) inhibitors in patients with rheumatoid arthritis (RA), particularly when used as monotherapy and in those previously treated with at least two biologic disease-modifying antirheumatic drugs (DMARDs), but their pain reduction effects were similar to those of non–TNF inhibitor biologic DMARDs.
METHODOLOGY:
- Researchers aimed to compare the effect of JAK inhibitors and each class of biologic DMARDs such as TNF inhibitors, rituximab, abatacept, and interleukin (IL)-6 inhibitors on pain in patients with RA in clinical practice.
- They included 8430 patients with RA who were initiated on either a JAK inhibitor (n = 1827), TNF inhibitor (n = 6422), IL-6 inhibitor (n = 887), abatacept (n = 1102), or rituximab (n = 1149) in 2017-2019.
- Differences in the change in pain, assessed using a visual analog scale (VAS; 0-100 mm), from baseline to 3 months were compared between the treatment arms.
- The proportion of patients who continued their initial treatment with low pain levels (VAS pain, < 20 mm) at 12 months was also evaluated.
- The comparisons of treatment responses between JAK inhibitors and biologic DMARDs were analyzed using multivariate linear regression, adjusted for patient characteristics, comorbidities, current co-medication, and previous treatment.
TAKEAWAY:
- Pain scores improved from baseline to 3 months in all the treatment arms, with mean changes ranging from −20.1 mm (95% CI, −23.1 to −17.2) for IL-6 inhibitors to −16.6 mm (95% CI, −19.1 to −14.0) for rituximab.
- At 3 months, JAK inhibitors reduced pain scores by 4.0 mm (95% CI, 1.7-6.3) more than TNF inhibitors and by 3.9 mm (95% CI, 0.9-6.9) more than rituximab; however, the change in pain was not significantly different on comparing JAK inhibitors with abatacept or IL-6 inhibitors.
- The superior pain-reducing effects of JAK inhibitors over those of TNF inhibitors were more prominent in those who were previously treated with at least two biologic DMARDs and when the treatments were used as monotherapy.
- At 12 months, 19.5% of the patients receiving JAK inhibitors continued their treatment and achieved low pain levels, with the corresponding proportions ranging from 17% to 26% for biologic DMARDs; JAK inhibitors were more effective in reducing pain than TNF inhibitors, although the difference was not statistically significant.
IN PRACTICE:
“JAK inhibitors yield slightly better pain outcomes than TNF inhibitors. The magnitude of these effects is unlikely to be clinically meaningful in unselected groups of patients with RA,” experts from Feinberg School of Medicine, Northwestern University, Chicago, wrote in an accompanying editorial. “Specific subgroups, such as those who have tried at least two DMARDs, may experience greater effects,” they added.
SOURCE:
The study was led by Anna Eberhard, MD, Department of Clinical Sciences, Lund University, Malmö, Sweden. It was published online on September 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had a significant amount of missing data, particularly for follow-up evaluations, which may have introduced bias. The majority of patients were treated using baricitinib, potentially limiting the generalizability to other JAK inhibitors. Residual confounding could not be excluded despite adjustments for multiple relevant patient characteristics.
DISCLOSURES:
This study was supported by grants from The Swedish Research Council, The Swedish Rheumatism Association, and Lund University. Some authors declared receiving consulting fees, payments or honoraria, or grants or having other ties with pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Janus kinase (JAK) inhibitors had a marginally superior effect on pain relief when compared with tumor necrosis factor (TNF) inhibitors in patients with rheumatoid arthritis (RA), particularly when used as monotherapy and in those previously treated with at least two biologic disease-modifying antirheumatic drugs (DMARDs), but their pain reduction effects were similar to those of non–TNF inhibitor biologic DMARDs.
METHODOLOGY:
- Researchers aimed to compare the effect of JAK inhibitors and each class of biologic DMARDs such as TNF inhibitors, rituximab, abatacept, and interleukin (IL)-6 inhibitors on pain in patients with RA in clinical practice.
- They included 8430 patients with RA who were initiated on either a JAK inhibitor (n = 1827), TNF inhibitor (n = 6422), IL-6 inhibitor (n = 887), abatacept (n = 1102), or rituximab (n = 1149) in 2017-2019.
- Differences in the change in pain, assessed using a visual analog scale (VAS; 0-100 mm), from baseline to 3 months were compared between the treatment arms.
- The proportion of patients who continued their initial treatment with low pain levels (VAS pain, < 20 mm) at 12 months was also evaluated.
- The comparisons of treatment responses between JAK inhibitors and biologic DMARDs were analyzed using multivariate linear regression, adjusted for patient characteristics, comorbidities, current co-medication, and previous treatment.
TAKEAWAY:
- Pain scores improved from baseline to 3 months in all the treatment arms, with mean changes ranging from −20.1 mm (95% CI, −23.1 to −17.2) for IL-6 inhibitors to −16.6 mm (95% CI, −19.1 to −14.0) for rituximab.
- At 3 months, JAK inhibitors reduced pain scores by 4.0 mm (95% CI, 1.7-6.3) more than TNF inhibitors and by 3.9 mm (95% CI, 0.9-6.9) more than rituximab; however, the change in pain was not significantly different on comparing JAK inhibitors with abatacept or IL-6 inhibitors.
- The superior pain-reducing effects of JAK inhibitors over those of TNF inhibitors were more prominent in those who were previously treated with at least two biologic DMARDs and when the treatments were used as monotherapy.
- At 12 months, 19.5% of the patients receiving JAK inhibitors continued their treatment and achieved low pain levels, with the corresponding proportions ranging from 17% to 26% for biologic DMARDs; JAK inhibitors were more effective in reducing pain than TNF inhibitors, although the difference was not statistically significant.
IN PRACTICE:
“JAK inhibitors yield slightly better pain outcomes than TNF inhibitors. The magnitude of these effects is unlikely to be clinically meaningful in unselected groups of patients with RA,” experts from Feinberg School of Medicine, Northwestern University, Chicago, wrote in an accompanying editorial. “Specific subgroups, such as those who have tried at least two DMARDs, may experience greater effects,” they added.
SOURCE:
The study was led by Anna Eberhard, MD, Department of Clinical Sciences, Lund University, Malmö, Sweden. It was published online on September 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had a significant amount of missing data, particularly for follow-up evaluations, which may have introduced bias. The majority of patients were treated using baricitinib, potentially limiting the generalizability to other JAK inhibitors. Residual confounding could not be excluded despite adjustments for multiple relevant patient characteristics.
DISCLOSURES:
This study was supported by grants from The Swedish Research Council, The Swedish Rheumatism Association, and Lund University. Some authors declared receiving consulting fees, payments or honoraria, or grants or having other ties with pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Janus kinase (JAK) inhibitors had a marginally superior effect on pain relief when compared with tumor necrosis factor (TNF) inhibitors in patients with rheumatoid arthritis (RA), particularly when used as monotherapy and in those previously treated with at least two biologic disease-modifying antirheumatic drugs (DMARDs), but their pain reduction effects were similar to those of non–TNF inhibitor biologic DMARDs.
METHODOLOGY:
- Researchers aimed to compare the effect of JAK inhibitors and each class of biologic DMARDs such as TNF inhibitors, rituximab, abatacept, and interleukin (IL)-6 inhibitors on pain in patients with RA in clinical practice.
- They included 8430 patients with RA who were initiated on either a JAK inhibitor (n = 1827), TNF inhibitor (n = 6422), IL-6 inhibitor (n = 887), abatacept (n = 1102), or rituximab (n = 1149) in 2017-2019.
- Differences in the change in pain, assessed using a visual analog scale (VAS; 0-100 mm), from baseline to 3 months were compared between the treatment arms.
- The proportion of patients who continued their initial treatment with low pain levels (VAS pain, < 20 mm) at 12 months was also evaluated.
- The comparisons of treatment responses between JAK inhibitors and biologic DMARDs were analyzed using multivariate linear regression, adjusted for patient characteristics, comorbidities, current co-medication, and previous treatment.
TAKEAWAY:
- Pain scores improved from baseline to 3 months in all the treatment arms, with mean changes ranging from −20.1 mm (95% CI, −23.1 to −17.2) for IL-6 inhibitors to −16.6 mm (95% CI, −19.1 to −14.0) for rituximab.
- At 3 months, JAK inhibitors reduced pain scores by 4.0 mm (95% CI, 1.7-6.3) more than TNF inhibitors and by 3.9 mm (95% CI, 0.9-6.9) more than rituximab; however, the change in pain was not significantly different on comparing JAK inhibitors with abatacept or IL-6 inhibitors.
- The superior pain-reducing effects of JAK inhibitors over those of TNF inhibitors were more prominent in those who were previously treated with at least two biologic DMARDs and when the treatments were used as monotherapy.
- At 12 months, 19.5% of the patients receiving JAK inhibitors continued their treatment and achieved low pain levels, with the corresponding proportions ranging from 17% to 26% for biologic DMARDs; JAK inhibitors were more effective in reducing pain than TNF inhibitors, although the difference was not statistically significant.
IN PRACTICE:
“JAK inhibitors yield slightly better pain outcomes than TNF inhibitors. The magnitude of these effects is unlikely to be clinically meaningful in unselected groups of patients with RA,” experts from Feinberg School of Medicine, Northwestern University, Chicago, wrote in an accompanying editorial. “Specific subgroups, such as those who have tried at least two DMARDs, may experience greater effects,” they added.
SOURCE:
The study was led by Anna Eberhard, MD, Department of Clinical Sciences, Lund University, Malmö, Sweden. It was published online on September 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had a significant amount of missing data, particularly for follow-up evaluations, which may have introduced bias. The majority of patients were treated using baricitinib, potentially limiting the generalizability to other JAK inhibitors. Residual confounding could not be excluded despite adjustments for multiple relevant patient characteristics.
DISCLOSURES:
This study was supported by grants from The Swedish Research Council, The Swedish Rheumatism Association, and Lund University. Some authors declared receiving consulting fees, payments or honoraria, or grants or having other ties with pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Too Few Immunocompromised Veterans Are Getting Zoster Vaccinations
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New Scanner Creates Highly Detailed, 3D Images of Blood Vessels in Seconds
A new scanner can provide three-dimensional (3D) photoacoustic images of millimeter-scale veins and arteries in seconds.
The scanner, developed by researchers at University College London (UCL) in England, could help clinicians better visualize and track microvascular changes for a wide range of diseases, including cancer, rheumatoid arthritis (RA), and peripheral vascular disease (PVD).
The case studies “illustrate potential areas of application that warrant future, more comprehensive clinical studies,” the authors wrote. “Moreover, they demonstrate the feasibility of using the scanner on a real-world patient cohort where imaging is more challenging due to frailty, comorbidity, or pain that may limit their ability to tolerate prolonged scan times.”
The work was published online in Nature Biomedical Engineering.
Improving Photoacoustic Imaging
PAT works using the photoacoustic effect, a phenomenon where sound waves are generated when light is absorbed by a material. When pulsed light from a laser is directed at tissue, some of that light is absorbed and causes an increase in heat in the targeted area. This localized heat also increases pressure, which generates ultrasound waves that can be detected by specialized sensors.
While previous PAT scanners translated these sound waves to electric signals directly to generate imaging, UCL engineers developed a sensor in the early 2000s that can detect these ultrasound waves using light. The result was much clearer, 3D images.
“That was great, but the problem was it was very slow, and it would take 5 minutes to get an image,” explained Paul Beard, PhD, professor of biomedical photoacoustics at UCL and senior author of the study. “That’s fine if you’re imaging a dead mouse or an anesthetized mouse, but not so useful for human imaging,” he continued, where motion would blur the image.
In this new paper, Beard and colleagues outlined how they cut scanning times to an order of seconds (or fraction of a second) rather than minutes. While previous iterations could detect only acoustic waves from one point at a time, this new scanner can detect waves from multiple points simultaneously. The scanner can visualize veins and arteries up to 15 mm deep in human tissue and can also provide dynamic, 3D images of “time-varying tissue perfusion and other hemodynamic events,” the authors wrote.
With these types of scanners, there is always a trade-off between imaging quality and imaging speed, explained Srivalleesha Mallidi, PhD, an assistant professor of biomedical engineering at Tufts University in Medford, Massachusetts. She was not involved with the work.
“With the resolution that [the authors] are providing and the depth at which they are seeing the signals, it is one of the fastest systems,” she said.
Clinical Utility
Beard and colleagues also tested the scanner to visualize blood vessels in participants with RA, suspected PVD, and skin inflammation. The scanning images “illustrated how vascular abnormalities such as increased vessel tortuosity, which has previously been linked to PVD, and the neovascularization associated with inflammation can be visualized and quantified,” the authors wrote.
The next step, Beard noted, is testing whether these characteristics can be used as a marker for the progression of disease.
Nehal Mehta, MD, a cardiologist and professor of medicine at the George Washington University, Washington, DC, agreed that more longitudinal research is needed to understand how the abnormalities captured in these images can inform detection and diagnosis of various diseases.
“You don’t know whether these images look bad because of reverse causation — the disease is doing this — or true causation — that this is actually detecting the root cause of the disease,” he explained. “Until we have a bank of normal and abnormal scans, we don’t know what any of these things mean.”
Though still some time away from entering the clinic, Mehta likened the technology to the introduction of optical coherence tomography in the 1980s. Before being adapted for clinical use, researchers first needed to visualize differences between normal coronary vasculature and myocardial infarction.
“I think this is an amazingly strong first proof of concept,” Mehta said. “This technology is showing a true promise in the field imaging.”
The work was funded by grants from Cancer Research UK, the Engineering & Physical Sciences Research Council, Wellcome Trust, the European Research Council, and the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre. Beard and two coauthors are shareholders of DeepColor Imaging to which the intellectual property associated with the new scanner has been licensed, but the company was not involved in any of this research. Mallidi and Mehta had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new scanner can provide three-dimensional (3D) photoacoustic images of millimeter-scale veins and arteries in seconds.
The scanner, developed by researchers at University College London (UCL) in England, could help clinicians better visualize and track microvascular changes for a wide range of diseases, including cancer, rheumatoid arthritis (RA), and peripheral vascular disease (PVD).
The case studies “illustrate potential areas of application that warrant future, more comprehensive clinical studies,” the authors wrote. “Moreover, they demonstrate the feasibility of using the scanner on a real-world patient cohort where imaging is more challenging due to frailty, comorbidity, or pain that may limit their ability to tolerate prolonged scan times.”
The work was published online in Nature Biomedical Engineering.
Improving Photoacoustic Imaging
PAT works using the photoacoustic effect, a phenomenon where sound waves are generated when light is absorbed by a material. When pulsed light from a laser is directed at tissue, some of that light is absorbed and causes an increase in heat in the targeted area. This localized heat also increases pressure, which generates ultrasound waves that can be detected by specialized sensors.
While previous PAT scanners translated these sound waves to electric signals directly to generate imaging, UCL engineers developed a sensor in the early 2000s that can detect these ultrasound waves using light. The result was much clearer, 3D images.
“That was great, but the problem was it was very slow, and it would take 5 minutes to get an image,” explained Paul Beard, PhD, professor of biomedical photoacoustics at UCL and senior author of the study. “That’s fine if you’re imaging a dead mouse or an anesthetized mouse, but not so useful for human imaging,” he continued, where motion would blur the image.
In this new paper, Beard and colleagues outlined how they cut scanning times to an order of seconds (or fraction of a second) rather than minutes. While previous iterations could detect only acoustic waves from one point at a time, this new scanner can detect waves from multiple points simultaneously. The scanner can visualize veins and arteries up to 15 mm deep in human tissue and can also provide dynamic, 3D images of “time-varying tissue perfusion and other hemodynamic events,” the authors wrote.
With these types of scanners, there is always a trade-off between imaging quality and imaging speed, explained Srivalleesha Mallidi, PhD, an assistant professor of biomedical engineering at Tufts University in Medford, Massachusetts. She was not involved with the work.
“With the resolution that [the authors] are providing and the depth at which they are seeing the signals, it is one of the fastest systems,” she said.
Clinical Utility
Beard and colleagues also tested the scanner to visualize blood vessels in participants with RA, suspected PVD, and skin inflammation. The scanning images “illustrated how vascular abnormalities such as increased vessel tortuosity, which has previously been linked to PVD, and the neovascularization associated with inflammation can be visualized and quantified,” the authors wrote.
The next step, Beard noted, is testing whether these characteristics can be used as a marker for the progression of disease.
Nehal Mehta, MD, a cardiologist and professor of medicine at the George Washington University, Washington, DC, agreed that more longitudinal research is needed to understand how the abnormalities captured in these images can inform detection and diagnosis of various diseases.
“You don’t know whether these images look bad because of reverse causation — the disease is doing this — or true causation — that this is actually detecting the root cause of the disease,” he explained. “Until we have a bank of normal and abnormal scans, we don’t know what any of these things mean.”
Though still some time away from entering the clinic, Mehta likened the technology to the introduction of optical coherence tomography in the 1980s. Before being adapted for clinical use, researchers first needed to visualize differences between normal coronary vasculature and myocardial infarction.
“I think this is an amazingly strong first proof of concept,” Mehta said. “This technology is showing a true promise in the field imaging.”
The work was funded by grants from Cancer Research UK, the Engineering & Physical Sciences Research Council, Wellcome Trust, the European Research Council, and the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre. Beard and two coauthors are shareholders of DeepColor Imaging to which the intellectual property associated with the new scanner has been licensed, but the company was not involved in any of this research. Mallidi and Mehta had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new scanner can provide three-dimensional (3D) photoacoustic images of millimeter-scale veins and arteries in seconds.
The scanner, developed by researchers at University College London (UCL) in England, could help clinicians better visualize and track microvascular changes for a wide range of diseases, including cancer, rheumatoid arthritis (RA), and peripheral vascular disease (PVD).
The case studies “illustrate potential areas of application that warrant future, more comprehensive clinical studies,” the authors wrote. “Moreover, they demonstrate the feasibility of using the scanner on a real-world patient cohort where imaging is more challenging due to frailty, comorbidity, or pain that may limit their ability to tolerate prolonged scan times.”
The work was published online in Nature Biomedical Engineering.
Improving Photoacoustic Imaging
PAT works using the photoacoustic effect, a phenomenon where sound waves are generated when light is absorbed by a material. When pulsed light from a laser is directed at tissue, some of that light is absorbed and causes an increase in heat in the targeted area. This localized heat also increases pressure, which generates ultrasound waves that can be detected by specialized sensors.
While previous PAT scanners translated these sound waves to electric signals directly to generate imaging, UCL engineers developed a sensor in the early 2000s that can detect these ultrasound waves using light. The result was much clearer, 3D images.
“That was great, but the problem was it was very slow, and it would take 5 minutes to get an image,” explained Paul Beard, PhD, professor of biomedical photoacoustics at UCL and senior author of the study. “That’s fine if you’re imaging a dead mouse or an anesthetized mouse, but not so useful for human imaging,” he continued, where motion would blur the image.
In this new paper, Beard and colleagues outlined how they cut scanning times to an order of seconds (or fraction of a second) rather than minutes. While previous iterations could detect only acoustic waves from one point at a time, this new scanner can detect waves from multiple points simultaneously. The scanner can visualize veins and arteries up to 15 mm deep in human tissue and can also provide dynamic, 3D images of “time-varying tissue perfusion and other hemodynamic events,” the authors wrote.
With these types of scanners, there is always a trade-off between imaging quality and imaging speed, explained Srivalleesha Mallidi, PhD, an assistant professor of biomedical engineering at Tufts University in Medford, Massachusetts. She was not involved with the work.
“With the resolution that [the authors] are providing and the depth at which they are seeing the signals, it is one of the fastest systems,” she said.
Clinical Utility
Beard and colleagues also tested the scanner to visualize blood vessels in participants with RA, suspected PVD, and skin inflammation. The scanning images “illustrated how vascular abnormalities such as increased vessel tortuosity, which has previously been linked to PVD, and the neovascularization associated with inflammation can be visualized and quantified,” the authors wrote.
The next step, Beard noted, is testing whether these characteristics can be used as a marker for the progression of disease.
Nehal Mehta, MD, a cardiologist and professor of medicine at the George Washington University, Washington, DC, agreed that more longitudinal research is needed to understand how the abnormalities captured in these images can inform detection and diagnosis of various diseases.
“You don’t know whether these images look bad because of reverse causation — the disease is doing this — or true causation — that this is actually detecting the root cause of the disease,” he explained. “Until we have a bank of normal and abnormal scans, we don’t know what any of these things mean.”
Though still some time away from entering the clinic, Mehta likened the technology to the introduction of optical coherence tomography in the 1980s. Before being adapted for clinical use, researchers first needed to visualize differences between normal coronary vasculature and myocardial infarction.
“I think this is an amazingly strong first proof of concept,” Mehta said. “This technology is showing a true promise in the field imaging.”
The work was funded by grants from Cancer Research UK, the Engineering & Physical Sciences Research Council, Wellcome Trust, the European Research Council, and the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre. Beard and two coauthors are shareholders of DeepColor Imaging to which the intellectual property associated with the new scanner has been licensed, but the company was not involved in any of this research. Mallidi and Mehta had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE BIOMEDICAL ENGINEERING
Methotrexate in Preventing RA: Benefits in ACPA-Negative Patients, and Is It Cost Effective?
A 1-year course of methotrexate (MTX) in clinically suspected arthralgia may prevent the development of rheumatoid arthritis (RA) in at-risk individuals who test negative for anti-citrullinated protein antibody (ACPA), according to 4-year results from the TREAT EARLIER study.
While 2-year data did not show a preventive effect, researchers risk-stratified patients in this most recent data. The previous study also grouped all individuals together, while this new analysis separated patients by seropositivity.
“Heterogeneity in risk of rheumatoid arthritis development in ACPA–negative participants with clinically suspect arthralgia might have concealed a treatment effect due to dilution,” wrote senior author Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at Leiden University Medical Center, Leiden, the Netherlands, and colleagues. “Therefore, risk-stratified analyses are required to adequately assess the possibility of prevention of rheumatoid arthritis in people with clinically suspect arthralgia who are ACPA–negative.”
To qualify for the study, participants needed to have recent-onset joint pain that a treating rheumatologist suspected of progressing to RA. Second, participants had to have subclinical joint inflammation, detected via MRI.
These are “promising results” for a group where predicting risk for RA has been more difficult than for their ACPA–positive counterparts, said Kevin Deane, MD, PhD, a professor of medicine and rheumatologist at the University of Colorado School of Medicine, Aurora, who was not involved with the study. However, additional research is necessary to investigate these findings.
The clinical utility of this finding is also unclear, he noted, as it would be an “extensive process” for all ACPA–negative individuals with joint pain to undergo MRI screening, he continued.
“It’s hard to find people who would meet these criteria, and healthcare systems need to understand how ultimately this could be implemented in clinical care,” he said.
Adding Risk Stratification
The TREAT EARLIER trial included 236 participants; nearly two thirds were women, and 77% were ACPA–negative (specifically for anti-cyclic citrullinated peptide 2). Patients randomly assigned to active treatment received a single intramuscular glucocorticoid injection (methylprednisolone 120 mg) upon inclusion and then completed a 1-year course of MTX. The comparator group received a single placebo injection at the beginning of the trial and a 1-year course of placebo tablets. All trial screenings and visits were conducted at the Leiden University Medical Center.
At the 2-year mark, there was no difference in the development of RA between the treatment and placebo groups, although there was improvement in joint pain, physical functioning, and MRI-detected joint inflammation in all at-risk groups given MTX — ACPA–positive patients and those at high risk for clinical arthritis development.
MTX delayed the onset of RA, with a statistically significant difference between treatment and placebo at 6 and 12 months, but not at 24 months.
For this 4-year analysis, published in The Lancet Rheumatology, authors stratified patients at their time of enrollment according to their risk of developing RA based on a published model for predicting inflammatory arthritis. Predictors included ACPA positivity (2 points), rheumatoid factor positivity (1 point), more than two locations of subclinical inflammation on MRI (2 points), and presence of metacarpophalangeal extensor tenosynovitis on MRI (1 point).
Patients with at least 4 points were classified as “high-risk,” with a 70% or higher predicted risk of developing RA. Participants with 2-3 points were at “increased risk” — translating to a 25%-70% higher likelihood of developing the condition. Low-risk patients, with 0-1 points, had < 25% chance of developing RA.
Of the 182 ACPA–negative participants in the study, none were considered high risk, 66 (36%) were at increased risk, and 116 (64%) were at low risk.
Decreased Rates of RA Development
Of these ACPA–negative patients stratified as increased risk, 3 of 35 (9%) in the treatment group developed RA, compared with 9 of 31 (29%) in the placebo arm (hazard ratio [HR], 0.27; P = .034).
All 54 ACPA–positive patients enrolled in the study were classified as either increased risk or high risk, but the treatment showed no difference in the rate of RA development in this group, and more than half (56%) developed RA during 4 years of follow-up. However, Dr. van der Helm-van Mil noted that the 2-year data showed treatment improved the severity of subclinical inflammation and symptoms over time in these patients.
The 5-year data from the trial, including physical function and other measures of disease burden, will be analyzed in 2025, she said, and will reveal whether ACPA–negative patients treated with MTX had sustained improvements in these measures.
Additional studies are needed to validate these findings, Dr. van der Helm-van Mil said, but the results indicate that ACPA–positive and ACPA–negative patients “are different populations, and we should evaluate them separately.”
Future RA prevention studies should also risk stratify patients before enrollment so that patients at low risk of developing the disease are not included in the interventions. “You can’t expect a treatment effect if there is no risk for disease,” she added.
Is It Cost-Effective?
In a separate analysis, published in Annals of the Rheumatic Diseases, Dr. van der Helm-van Mil and colleagues sought to investigate the cost-effectiveness of the TREAT EARLIER intervention at 2 years of follow-up.
“There is an ongoing debate whether people with arthralgia at risk for RA should be treated with DMARDs [disease-modifying antirheumatic drugs]; however, the economic effects of an intervention in the arthralgia at risk phase are unknown.”
The analysis calculated healthcare productivity and work productivity costs from enrollment to 2 years of follow-up. To demonstrate effect, they also calculated change in quality-adjusted life years (QALYs).
Over the course of 2 years, estimated costs for the treatment arm were €4809 lower (−$5304) than the placebo arm per patient. Lower productivity costs accounted for 97% of this difference.
The treatment arm also resulted in a small improvement (+0.041) in QALYs, compared with placebo.
“These data provide the first evidence that first-line treatment aiming at secondary prevention in arthralgia at-risk for RA is cost-effective,” the authors wrote.
Dr. van der Helm-van Mil emphasized that this cost analysis used only 2-year follow-up data (rather than the newly published 4-year data) and did not differentiate ACPA–negative patients. Despite including a greater heterogeneity of patients, the intervention was still cost-effective. A future analysis that includes 5-year follow-up data and stratifies patients by ACPA status and excludes low-risk individuals could demonstrate more cost benefits to a temporary MTX regimen, she said.
Considering the costs of these preventive interventions is important, added Dr. Deane, who agreed that future analyses should examine cost-effectiveness in groups at high risk of developing RA. (Dr. Deane noted that he had reviewed this article for publication.) However, this analysis did not include the costs of screening patients before enrollment in the study.
“Additional factors that need to be considered are costs to find individuals who would meet the criteria for treatment,” he said, which would include getting an MRI to detect subclinical joint inflammation.
However, Dr. van der Helm-van Mil noted that both placebo and treatment groups received MRI scans, which would therefore not affect cost differences between the groups.
Future studies should also focus on longer-term outcomes, both Dr. Deane and Dr. van der Helm-van Mil agreed.
“Since RA is a chronic disease for which part of the patients require expensive biologicals, future cost-effectiveness analyses should also consider a lifetime horizon,” Dr. van der Helm-van Mil and colleagues wrote.
The TREAT EARLIER trial was funded by the Dutch Research Council and the Dutch Arthritis Society. The cost analysis study was funded by ZonMw and the Dutch Arthritis Society. Dr. Deane is a member of an American College of Rheumatology/European Alliance of Associations for Rheumatology task force for risk stratification in RA. He has received payments as a speaker for Werfen and Thermo Fisher Scientific and low-cost biomarker assays for research from Werfen. He has also received grant funding from Thermo Fisher Scientific, Gilead, and Boehringer Ingelheim. Dr. van der Helm-van Mil reported no disclosures.
A version of this article first appeared on Medscape.com.
A 1-year course of methotrexate (MTX) in clinically suspected arthralgia may prevent the development of rheumatoid arthritis (RA) in at-risk individuals who test negative for anti-citrullinated protein antibody (ACPA), according to 4-year results from the TREAT EARLIER study.
While 2-year data did not show a preventive effect, researchers risk-stratified patients in this most recent data. The previous study also grouped all individuals together, while this new analysis separated patients by seropositivity.
“Heterogeneity in risk of rheumatoid arthritis development in ACPA–negative participants with clinically suspect arthralgia might have concealed a treatment effect due to dilution,” wrote senior author Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at Leiden University Medical Center, Leiden, the Netherlands, and colleagues. “Therefore, risk-stratified analyses are required to adequately assess the possibility of prevention of rheumatoid arthritis in people with clinically suspect arthralgia who are ACPA–negative.”
To qualify for the study, participants needed to have recent-onset joint pain that a treating rheumatologist suspected of progressing to RA. Second, participants had to have subclinical joint inflammation, detected via MRI.
These are “promising results” for a group where predicting risk for RA has been more difficult than for their ACPA–positive counterparts, said Kevin Deane, MD, PhD, a professor of medicine and rheumatologist at the University of Colorado School of Medicine, Aurora, who was not involved with the study. However, additional research is necessary to investigate these findings.
The clinical utility of this finding is also unclear, he noted, as it would be an “extensive process” for all ACPA–negative individuals with joint pain to undergo MRI screening, he continued.
“It’s hard to find people who would meet these criteria, and healthcare systems need to understand how ultimately this could be implemented in clinical care,” he said.
Adding Risk Stratification
The TREAT EARLIER trial included 236 participants; nearly two thirds were women, and 77% were ACPA–negative (specifically for anti-cyclic citrullinated peptide 2). Patients randomly assigned to active treatment received a single intramuscular glucocorticoid injection (methylprednisolone 120 mg) upon inclusion and then completed a 1-year course of MTX. The comparator group received a single placebo injection at the beginning of the trial and a 1-year course of placebo tablets. All trial screenings and visits were conducted at the Leiden University Medical Center.
At the 2-year mark, there was no difference in the development of RA between the treatment and placebo groups, although there was improvement in joint pain, physical functioning, and MRI-detected joint inflammation in all at-risk groups given MTX — ACPA–positive patients and those at high risk for clinical arthritis development.
MTX delayed the onset of RA, with a statistically significant difference between treatment and placebo at 6 and 12 months, but not at 24 months.
For this 4-year analysis, published in The Lancet Rheumatology, authors stratified patients at their time of enrollment according to their risk of developing RA based on a published model for predicting inflammatory arthritis. Predictors included ACPA positivity (2 points), rheumatoid factor positivity (1 point), more than two locations of subclinical inflammation on MRI (2 points), and presence of metacarpophalangeal extensor tenosynovitis on MRI (1 point).
Patients with at least 4 points were classified as “high-risk,” with a 70% or higher predicted risk of developing RA. Participants with 2-3 points were at “increased risk” — translating to a 25%-70% higher likelihood of developing the condition. Low-risk patients, with 0-1 points, had < 25% chance of developing RA.
Of the 182 ACPA–negative participants in the study, none were considered high risk, 66 (36%) were at increased risk, and 116 (64%) were at low risk.
Decreased Rates of RA Development
Of these ACPA–negative patients stratified as increased risk, 3 of 35 (9%) in the treatment group developed RA, compared with 9 of 31 (29%) in the placebo arm (hazard ratio [HR], 0.27; P = .034).
All 54 ACPA–positive patients enrolled in the study were classified as either increased risk or high risk, but the treatment showed no difference in the rate of RA development in this group, and more than half (56%) developed RA during 4 years of follow-up. However, Dr. van der Helm-van Mil noted that the 2-year data showed treatment improved the severity of subclinical inflammation and symptoms over time in these patients.
The 5-year data from the trial, including physical function and other measures of disease burden, will be analyzed in 2025, she said, and will reveal whether ACPA–negative patients treated with MTX had sustained improvements in these measures.
Additional studies are needed to validate these findings, Dr. van der Helm-van Mil said, but the results indicate that ACPA–positive and ACPA–negative patients “are different populations, and we should evaluate them separately.”
Future RA prevention studies should also risk stratify patients before enrollment so that patients at low risk of developing the disease are not included in the interventions. “You can’t expect a treatment effect if there is no risk for disease,” she added.
Is It Cost-Effective?
In a separate analysis, published in Annals of the Rheumatic Diseases, Dr. van der Helm-van Mil and colleagues sought to investigate the cost-effectiveness of the TREAT EARLIER intervention at 2 years of follow-up.
“There is an ongoing debate whether people with arthralgia at risk for RA should be treated with DMARDs [disease-modifying antirheumatic drugs]; however, the economic effects of an intervention in the arthralgia at risk phase are unknown.”
The analysis calculated healthcare productivity and work productivity costs from enrollment to 2 years of follow-up. To demonstrate effect, they also calculated change in quality-adjusted life years (QALYs).
Over the course of 2 years, estimated costs for the treatment arm were €4809 lower (−$5304) than the placebo arm per patient. Lower productivity costs accounted for 97% of this difference.
The treatment arm also resulted in a small improvement (+0.041) in QALYs, compared with placebo.
“These data provide the first evidence that first-line treatment aiming at secondary prevention in arthralgia at-risk for RA is cost-effective,” the authors wrote.
Dr. van der Helm-van Mil emphasized that this cost analysis used only 2-year follow-up data (rather than the newly published 4-year data) and did not differentiate ACPA–negative patients. Despite including a greater heterogeneity of patients, the intervention was still cost-effective. A future analysis that includes 5-year follow-up data and stratifies patients by ACPA status and excludes low-risk individuals could demonstrate more cost benefits to a temporary MTX regimen, she said.
Considering the costs of these preventive interventions is important, added Dr. Deane, who agreed that future analyses should examine cost-effectiveness in groups at high risk of developing RA. (Dr. Deane noted that he had reviewed this article for publication.) However, this analysis did not include the costs of screening patients before enrollment in the study.
“Additional factors that need to be considered are costs to find individuals who would meet the criteria for treatment,” he said, which would include getting an MRI to detect subclinical joint inflammation.
However, Dr. van der Helm-van Mil noted that both placebo and treatment groups received MRI scans, which would therefore not affect cost differences between the groups.
Future studies should also focus on longer-term outcomes, both Dr. Deane and Dr. van der Helm-van Mil agreed.
“Since RA is a chronic disease for which part of the patients require expensive biologicals, future cost-effectiveness analyses should also consider a lifetime horizon,” Dr. van der Helm-van Mil and colleagues wrote.
The TREAT EARLIER trial was funded by the Dutch Research Council and the Dutch Arthritis Society. The cost analysis study was funded by ZonMw and the Dutch Arthritis Society. Dr. Deane is a member of an American College of Rheumatology/European Alliance of Associations for Rheumatology task force for risk stratification in RA. He has received payments as a speaker for Werfen and Thermo Fisher Scientific and low-cost biomarker assays for research from Werfen. He has also received grant funding from Thermo Fisher Scientific, Gilead, and Boehringer Ingelheim. Dr. van der Helm-van Mil reported no disclosures.
A version of this article first appeared on Medscape.com.
A 1-year course of methotrexate (MTX) in clinically suspected arthralgia may prevent the development of rheumatoid arthritis (RA) in at-risk individuals who test negative for anti-citrullinated protein antibody (ACPA), according to 4-year results from the TREAT EARLIER study.
While 2-year data did not show a preventive effect, researchers risk-stratified patients in this most recent data. The previous study also grouped all individuals together, while this new analysis separated patients by seropositivity.
“Heterogeneity in risk of rheumatoid arthritis development in ACPA–negative participants with clinically suspect arthralgia might have concealed a treatment effect due to dilution,” wrote senior author Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at Leiden University Medical Center, Leiden, the Netherlands, and colleagues. “Therefore, risk-stratified analyses are required to adequately assess the possibility of prevention of rheumatoid arthritis in people with clinically suspect arthralgia who are ACPA–negative.”
To qualify for the study, participants needed to have recent-onset joint pain that a treating rheumatologist suspected of progressing to RA. Second, participants had to have subclinical joint inflammation, detected via MRI.
These are “promising results” for a group where predicting risk for RA has been more difficult than for their ACPA–positive counterparts, said Kevin Deane, MD, PhD, a professor of medicine and rheumatologist at the University of Colorado School of Medicine, Aurora, who was not involved with the study. However, additional research is necessary to investigate these findings.
The clinical utility of this finding is also unclear, he noted, as it would be an “extensive process” for all ACPA–negative individuals with joint pain to undergo MRI screening, he continued.
“It’s hard to find people who would meet these criteria, and healthcare systems need to understand how ultimately this could be implemented in clinical care,” he said.
Adding Risk Stratification
The TREAT EARLIER trial included 236 participants; nearly two thirds were women, and 77% were ACPA–negative (specifically for anti-cyclic citrullinated peptide 2). Patients randomly assigned to active treatment received a single intramuscular glucocorticoid injection (methylprednisolone 120 mg) upon inclusion and then completed a 1-year course of MTX. The comparator group received a single placebo injection at the beginning of the trial and a 1-year course of placebo tablets. All trial screenings and visits were conducted at the Leiden University Medical Center.
At the 2-year mark, there was no difference in the development of RA between the treatment and placebo groups, although there was improvement in joint pain, physical functioning, and MRI-detected joint inflammation in all at-risk groups given MTX — ACPA–positive patients and those at high risk for clinical arthritis development.
MTX delayed the onset of RA, with a statistically significant difference between treatment and placebo at 6 and 12 months, but not at 24 months.
For this 4-year analysis, published in The Lancet Rheumatology, authors stratified patients at their time of enrollment according to their risk of developing RA based on a published model for predicting inflammatory arthritis. Predictors included ACPA positivity (2 points), rheumatoid factor positivity (1 point), more than two locations of subclinical inflammation on MRI (2 points), and presence of metacarpophalangeal extensor tenosynovitis on MRI (1 point).
Patients with at least 4 points were classified as “high-risk,” with a 70% or higher predicted risk of developing RA. Participants with 2-3 points were at “increased risk” — translating to a 25%-70% higher likelihood of developing the condition. Low-risk patients, with 0-1 points, had < 25% chance of developing RA.
Of the 182 ACPA–negative participants in the study, none were considered high risk, 66 (36%) were at increased risk, and 116 (64%) were at low risk.
Decreased Rates of RA Development
Of these ACPA–negative patients stratified as increased risk, 3 of 35 (9%) in the treatment group developed RA, compared with 9 of 31 (29%) in the placebo arm (hazard ratio [HR], 0.27; P = .034).
All 54 ACPA–positive patients enrolled in the study were classified as either increased risk or high risk, but the treatment showed no difference in the rate of RA development in this group, and more than half (56%) developed RA during 4 years of follow-up. However, Dr. van der Helm-van Mil noted that the 2-year data showed treatment improved the severity of subclinical inflammation and symptoms over time in these patients.
The 5-year data from the trial, including physical function and other measures of disease burden, will be analyzed in 2025, she said, and will reveal whether ACPA–negative patients treated with MTX had sustained improvements in these measures.
Additional studies are needed to validate these findings, Dr. van der Helm-van Mil said, but the results indicate that ACPA–positive and ACPA–negative patients “are different populations, and we should evaluate them separately.”
Future RA prevention studies should also risk stratify patients before enrollment so that patients at low risk of developing the disease are not included in the interventions. “You can’t expect a treatment effect if there is no risk for disease,” she added.
Is It Cost-Effective?
In a separate analysis, published in Annals of the Rheumatic Diseases, Dr. van der Helm-van Mil and colleagues sought to investigate the cost-effectiveness of the TREAT EARLIER intervention at 2 years of follow-up.
“There is an ongoing debate whether people with arthralgia at risk for RA should be treated with DMARDs [disease-modifying antirheumatic drugs]; however, the economic effects of an intervention in the arthralgia at risk phase are unknown.”
The analysis calculated healthcare productivity and work productivity costs from enrollment to 2 years of follow-up. To demonstrate effect, they also calculated change in quality-adjusted life years (QALYs).
Over the course of 2 years, estimated costs for the treatment arm were €4809 lower (−$5304) than the placebo arm per patient. Lower productivity costs accounted for 97% of this difference.
The treatment arm also resulted in a small improvement (+0.041) in QALYs, compared with placebo.
“These data provide the first evidence that first-line treatment aiming at secondary prevention in arthralgia at-risk for RA is cost-effective,” the authors wrote.
Dr. van der Helm-van Mil emphasized that this cost analysis used only 2-year follow-up data (rather than the newly published 4-year data) and did not differentiate ACPA–negative patients. Despite including a greater heterogeneity of patients, the intervention was still cost-effective. A future analysis that includes 5-year follow-up data and stratifies patients by ACPA status and excludes low-risk individuals could demonstrate more cost benefits to a temporary MTX regimen, she said.
Considering the costs of these preventive interventions is important, added Dr. Deane, who agreed that future analyses should examine cost-effectiveness in groups at high risk of developing RA. (Dr. Deane noted that he had reviewed this article for publication.) However, this analysis did not include the costs of screening patients before enrollment in the study.
“Additional factors that need to be considered are costs to find individuals who would meet the criteria for treatment,” he said, which would include getting an MRI to detect subclinical joint inflammation.
However, Dr. van der Helm-van Mil noted that both placebo and treatment groups received MRI scans, which would therefore not affect cost differences between the groups.
Future studies should also focus on longer-term outcomes, both Dr. Deane and Dr. van der Helm-van Mil agreed.
“Since RA is a chronic disease for which part of the patients require expensive biologicals, future cost-effectiveness analyses should also consider a lifetime horizon,” Dr. van der Helm-van Mil and colleagues wrote.
The TREAT EARLIER trial was funded by the Dutch Research Council and the Dutch Arthritis Society. The cost analysis study was funded by ZonMw and the Dutch Arthritis Society. Dr. Deane is a member of an American College of Rheumatology/European Alliance of Associations for Rheumatology task force for risk stratification in RA. He has received payments as a speaker for Werfen and Thermo Fisher Scientific and low-cost biomarker assays for research from Werfen. He has also received grant funding from Thermo Fisher Scientific, Gilead, and Boehringer Ingelheim. Dr. van der Helm-van Mil reported no disclosures.
A version of this article first appeared on Medscape.com.
ILD Subtypes in Rheumatoid Arthritis Carry Different Risk Factor Profiles
TOPLINE:
Older age, male sex, and seropositivity are linked to a higher risk for rheumatoid arthritis–interstitial lung disease (RA-ILD) with a usual interstitial pneumonia (UIP) pattern, while only seropositivity is associated with RA-ILD with a nonspecific interstitial pneumonia pattern (NSIP).
METHODOLOGY:
- Researchers conducted a case-control study using data from two cohorts in the Mass General Brigham Healthcare system to examine the risk factors associated with different subtypes of RA-ILD.
- They identified 208 patients with RA-ILD (mean age at RA diagnosis, 50.7 years; 67.3% women) and 547 control participants with RA but no ILD (mean age at RA diagnosis, 49.1 years; 78.1% women), who had high-resolution computed tomography (HRCT) imaging data available.
- RA-ILD subtypes such as RA-UIP, RA-NSIP, organizing pneumonia, and others were determined with HRCT scans.
- The associations between demographics, lifestyle, and serologic factors and RA-ILD subtypes were evaluated using multivariable logistic regression analysis.
TAKEAWAY:
- The RA-UIP subtype, the one with worst prognosis, was associated with older age during the time of RA diagnosis (odds ratio [OR], 1.03 per year; 95% CI, 1.01-1.05), male sex (OR, 2.15; 95% CI, 1.33-3.48), and seropositivity (OR, 2.08; 95% CI, 1.24-3.48).
- On the other hand, the RA-NSIP subtype was significantly associated only with seropositivity (OR, 3.21; 95% CI, 1.36-7.56).
- Nonfibrotic ILDs were significantly associated with positive smoking status (OR, 2.81; 95% CI, 1.52-5.21) and seropositivity (OR, 2.09; 95% CI, 1.19-3.67).
- The combination of male sex, seropositivity, and positive smoking status was associated with a nearly sevenfold increased risk for RA-UIP (OR, 6.89; 95% CI, 2.41-19.69), compared with having no RA-ILD risk factors.
IN PRACTICE:
“These findings suggest that RA-ILD subtypes may have distinct risk factor profiles and emphasize the importance of further efforts to understand RA-ILD disease heterogeneity to inform screening and prognostication strategies,” the authors wrote.
SOURCE:
The study was led by Gregory C. McDermott, MD, MPH, Brigham and Women’s Hospital, Boston, and was published online on September 11, 2024, in Arthritis Care & Research.
LIMITATIONS:
This study relied on HRCT imaging, which may have introduced selection bias within the control groups. RA disease activity measures were not available for the Mass General Brigham Biobank RA cohort, which limited the analysis of the influence of disease activity on the risk for RA-ILD. Both cohorts predominantly involved White patients, which may have limited the generalizability of the findings to more diverse populations.
DISCLOSURES:
Some authors were supported by the Rheumatology Research Foundation Scientist Development Award, a VERITY Pilot & Feasibility Research Award, the Société Française de Rhumatologie, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and other sources. The authors declared receiving grant support, consulting fees, and honoraria from various organizations and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Older age, male sex, and seropositivity are linked to a higher risk for rheumatoid arthritis–interstitial lung disease (RA-ILD) with a usual interstitial pneumonia (UIP) pattern, while only seropositivity is associated with RA-ILD with a nonspecific interstitial pneumonia pattern (NSIP).
METHODOLOGY:
- Researchers conducted a case-control study using data from two cohorts in the Mass General Brigham Healthcare system to examine the risk factors associated with different subtypes of RA-ILD.
- They identified 208 patients with RA-ILD (mean age at RA diagnosis, 50.7 years; 67.3% women) and 547 control participants with RA but no ILD (mean age at RA diagnosis, 49.1 years; 78.1% women), who had high-resolution computed tomography (HRCT) imaging data available.
- RA-ILD subtypes such as RA-UIP, RA-NSIP, organizing pneumonia, and others were determined with HRCT scans.
- The associations between demographics, lifestyle, and serologic factors and RA-ILD subtypes were evaluated using multivariable logistic regression analysis.
TAKEAWAY:
- The RA-UIP subtype, the one with worst prognosis, was associated with older age during the time of RA diagnosis (odds ratio [OR], 1.03 per year; 95% CI, 1.01-1.05), male sex (OR, 2.15; 95% CI, 1.33-3.48), and seropositivity (OR, 2.08; 95% CI, 1.24-3.48).
- On the other hand, the RA-NSIP subtype was significantly associated only with seropositivity (OR, 3.21; 95% CI, 1.36-7.56).
- Nonfibrotic ILDs were significantly associated with positive smoking status (OR, 2.81; 95% CI, 1.52-5.21) and seropositivity (OR, 2.09; 95% CI, 1.19-3.67).
- The combination of male sex, seropositivity, and positive smoking status was associated with a nearly sevenfold increased risk for RA-UIP (OR, 6.89; 95% CI, 2.41-19.69), compared with having no RA-ILD risk factors.
IN PRACTICE:
“These findings suggest that RA-ILD subtypes may have distinct risk factor profiles and emphasize the importance of further efforts to understand RA-ILD disease heterogeneity to inform screening and prognostication strategies,” the authors wrote.
SOURCE:
The study was led by Gregory C. McDermott, MD, MPH, Brigham and Women’s Hospital, Boston, and was published online on September 11, 2024, in Arthritis Care & Research.
LIMITATIONS:
This study relied on HRCT imaging, which may have introduced selection bias within the control groups. RA disease activity measures were not available for the Mass General Brigham Biobank RA cohort, which limited the analysis of the influence of disease activity on the risk for RA-ILD. Both cohorts predominantly involved White patients, which may have limited the generalizability of the findings to more diverse populations.
DISCLOSURES:
Some authors were supported by the Rheumatology Research Foundation Scientist Development Award, a VERITY Pilot & Feasibility Research Award, the Société Française de Rhumatologie, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and other sources. The authors declared receiving grant support, consulting fees, and honoraria from various organizations and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Older age, male sex, and seropositivity are linked to a higher risk for rheumatoid arthritis–interstitial lung disease (RA-ILD) with a usual interstitial pneumonia (UIP) pattern, while only seropositivity is associated with RA-ILD with a nonspecific interstitial pneumonia pattern (NSIP).
METHODOLOGY:
- Researchers conducted a case-control study using data from two cohorts in the Mass General Brigham Healthcare system to examine the risk factors associated with different subtypes of RA-ILD.
- They identified 208 patients with RA-ILD (mean age at RA diagnosis, 50.7 years; 67.3% women) and 547 control participants with RA but no ILD (mean age at RA diagnosis, 49.1 years; 78.1% women), who had high-resolution computed tomography (HRCT) imaging data available.
- RA-ILD subtypes such as RA-UIP, RA-NSIP, organizing pneumonia, and others were determined with HRCT scans.
- The associations between demographics, lifestyle, and serologic factors and RA-ILD subtypes were evaluated using multivariable logistic regression analysis.
TAKEAWAY:
- The RA-UIP subtype, the one with worst prognosis, was associated with older age during the time of RA diagnosis (odds ratio [OR], 1.03 per year; 95% CI, 1.01-1.05), male sex (OR, 2.15; 95% CI, 1.33-3.48), and seropositivity (OR, 2.08; 95% CI, 1.24-3.48).
- On the other hand, the RA-NSIP subtype was significantly associated only with seropositivity (OR, 3.21; 95% CI, 1.36-7.56).
- Nonfibrotic ILDs were significantly associated with positive smoking status (OR, 2.81; 95% CI, 1.52-5.21) and seropositivity (OR, 2.09; 95% CI, 1.19-3.67).
- The combination of male sex, seropositivity, and positive smoking status was associated with a nearly sevenfold increased risk for RA-UIP (OR, 6.89; 95% CI, 2.41-19.69), compared with having no RA-ILD risk factors.
IN PRACTICE:
“These findings suggest that RA-ILD subtypes may have distinct risk factor profiles and emphasize the importance of further efforts to understand RA-ILD disease heterogeneity to inform screening and prognostication strategies,” the authors wrote.
SOURCE:
The study was led by Gregory C. McDermott, MD, MPH, Brigham and Women’s Hospital, Boston, and was published online on September 11, 2024, in Arthritis Care & Research.
LIMITATIONS:
This study relied on HRCT imaging, which may have introduced selection bias within the control groups. RA disease activity measures were not available for the Mass General Brigham Biobank RA cohort, which limited the analysis of the influence of disease activity on the risk for RA-ILD. Both cohorts predominantly involved White patients, which may have limited the generalizability of the findings to more diverse populations.
DISCLOSURES:
Some authors were supported by the Rheumatology Research Foundation Scientist Development Award, a VERITY Pilot & Feasibility Research Award, the Société Française de Rhumatologie, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and other sources. The authors declared receiving grant support, consulting fees, and honoraria from various organizations and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Direct-to-Consumer Testing’s Expansion to Rheumatology Has Benefits but Potential Risks
When Jennifer Welsh, a 40-year-old from New Britain, Connecticut, visited her doctor about pain in her joints and neck, her doctor sent her to the emergency department (ED) to rule out meningitis. The ED did rule that out, as well as strep, so Ms. Welsh went to her follow-up appointment a few days later, hoping for answers or at least more tests to get those answers.
Instead, the doctor — a different one from the same practice as her primary care physician (PCP) — wouldn’t even talk to Ms. Welsh about her symptoms because she couldn’t see the ED’s results and refused to view the results that Ms. Welsh could pull up online.
“She just completely shut me down,” Ms. Welsh recalled. “It was a really awful appointment, and I left in tears. I was in physical pain, I had just been to the ER, nothing is really resolved, I’m stressed out about it, and this woman is completely dismissing me.”
She had been able to schedule an appointment with her regular PCP later that week, but after the harrowing experience with this doctor, she wondered if her PCP would order the rheumatoid arthritis (RA) test that Ms. Welsh suspected she needed. So, she took matters into her own hands.
“I was searching for what test to ask for from my doctor,” she said, and she found that she could order it on her own from a major lab company she was already familiar with. For around $100, “I could get it done and see what it says on my own,” she said.
But that’s not how it worked out. Her regular PCP apologized for the other doctor’s behavior and ordered the RA test as well as additional tests — and got results while Ms. Welsh still waited for the one she ordered to arrive over a week later.
At first, Ms. Welsh was grateful she could order the RA test without her doctor’s referral. “I felt it gave me a sense of control over the situation that I felt really not in control of, until the system failed me, and I didn’t get the results,” she said. But then, “not having someone I could call and get an answer about why my tests were delayed, why I wasn’t able to access them, why it was taking so long — it was definitely anxiety-inducing.”
A Growing Market
Ms. Welsh is one of a growing number of patients who are ordering direct-to-consumer (DTC) lab tests without the recommendation or guidance of a doctor. They’re offered online by labs ranging from well-established giants like Quest and Labcorp to smaller, potentially less vetted companies, although some smaller companies contract with larger companies like Quest. Combined, the DTC market is projected to be worth $2 billion by 2025.
Yet the burgeoning industry has also drawn critiques from both bioethicists and privacy experts. A research letter in JAMA in 2023, for example, found that less than half of the 21 companies identified in an online search declared Health Insurance Portability and Accountability Act compliance, while more than half “indicated the potential use of consumer data for research purposes either internally or through third-party sharing.” That study found the most commonly offered tests were related to diabetes, the thyroid, and vitamin levels, and hormone tests for men and women, such as testosterone or estradiol.
But a number of companies also offer tests related to rheumatologic conditions. A handful of tests offered by Labcorp, for example, could be used in rheumatology, such as tests for celiac antibodies or high-sensitivity C-reactive protein. Quest similarly offers a handful of autoimmune-related tests. But other companies offer a long slate of autoimmune or antibody tests.
The antinuclear antibody (ANA) test and RA panel offered by Quest are the same tests, run and analyzed in the same labs, as those ordered by physicians and hospitals, according to James Faix, MD, the medical director of immunology at Quest Diagnostics. Their RA panel includes rheumatoid factor and anti-cyclic citrullinated peptide as well as antibody to mutated citrullinated vimentin, “which may detect approximately 10%-15%” of patients who test negative to the first two.
Quest’s ANA test with reflex costs $112, and its RA panel costs $110, price points that are similar across other companies’ offerings. Labcorp declined to respond to questions about its DTC tests, and several smaller companies did not respond to queries about their offerings. It can therefore be hard to assess what’s included or what the quality is of many DTC tests, particularly from smaller, less established companies.
Oversight and Quality Control
Anthony Killeen, MD, PhD, president of the Association for Diagnostics & Laboratory Medicine (ADLM) and director of Clinical Laboratories at the University of Minnesota Medical Center in Minneapolis, said via email that the ADLM supports “expanding consumer access to direct-to-consumer laboratory testing services that have demonstrated analytical and clinical validity and clinical utility,” given the importance of individuals learning about their health status and becoming more involved in health decisions. But the ADLM also recommends “that only CLIA-certified laboratories perform direct-to-consumer testing,” he said.
“There are direct-to-consumer tests on the market that are not medical-grade laboratory tests and that may be performed in nonaccredited laboratories,” Dr. Killeen said. “We advise consumers to steer clear of such tests.” The ADLM also encourages consumers to “work with qualified healthcare providers when making decisions based off the results they receive from any direct-to-consumer tests” and recommends that DTC test companies “provide consumers with sufficient information and/or access to expert help to assist them in ordering tests and interpreting the results.”
Yet it’s unclear how much support, if any, consumers can receive in terms of understanding what their tests mean. Most of the companies in the 2023 study offered optional follow-up with a healthcare professional, but these professionals ranged from physicians to “health coaches,” and all the companies had disclaimers that “test results did not constitute medical advice.”
At Quest, the only company to respond to this news organization’s request for comment, consumer-initiated tests ordered online are first reviewed by a physician at PWNHealth, an independent, third-party physician network, to determine that it’s appropriate before the lab order is actually placed.
“Once results are available, individuals have the option to discuss their results with an independent physician at no extra cost,” Dr. Faix said. ANA or RA results outside the normal ranges may trigger a “call from a PWNHealth healthcare coordinator, who can help provide information, suggestions on next steps, and set up time for the individual to speak with an independent physician to discuss questions or concerns regarding the results,” he said.
“Our goal is not to replace the role of a healthcare provider,” Dr. Faix said. “We are providing an alternate way for people to engage with the healthcare system that offers convenience, gives people more control over their own healthcare journeys, and meets them where they are, supporting both consumers and their care teams.” The company has expanded its offerings from an initial 30 tests made available in 2018 to over 130 today, deciding which to offer “based on consumer research and expertise of clinical experts.” The company has also “seen steady interest in our two consumer rheumatology offerings,” Dr. Faix said.
The DTC Landscape in Rheumatology
Within rheumatology, among the most popular tests is for ANA, based on the experience of Alfred Kim, MD, PhD, associate professor of medicine at Washington University School of Medicine in St Louis, Missouri.
“For a lot of people, losing control over their health is maybe the most frightening experience they can have, so I think a lot of patients use this as a way to kind of have ownership over their health,” Dr. Kim said. “Let’s say they’ve been to four doctors. No one can explain what’s going on. They’re getting frustrated, and so they just turn to solutions where they feel like they have ownership over the situation.”
Though the market is undoubtedly growing, the growth appears uneven across geography and institution types. Kim has seen a “fair number of referrals,” with patients coming in with results from a DTC test. Michael Putman, MD, MSci, assistant professor of medicine at the Medical College of Wisconsin in Milwaukee, hasn’t seen it much. “I know that patients can get testing done themselves independently, but I don’t have people routinely coming in with tests they’ve ordered in advance of our appointment,” Dr. Putman said, but, like Dr. Kim, he recognizes why patients might seek them out.
“I’m a big fan of patient empowerment, and I do think that medicine serves a gatekeeper role that sometimes can be a little too far,” Dr. Putman said. “I think there is value to patients being able to get more information and try to understand what is happening in their bodies. I have a lot of compassion for someone who would try to find testing outside of the normal channels.”
Indeed, bringing these test results to a visit could be informative in some scenarios. A negative ANA test, for example, pretty much excludes lupus 100%, Dr. Kim said. But a positive ANA doesn’t tell him much, and if his clinical suspicion for a condition is high, he likely would order that test anyway, even if the patient came in with their own results. Dr. Putman also pointed out that the vast majority of tests used in rheumatology have a high rate of false positives.
“I think that will be the major area where this causes quite a lot of grief to patients and some frustration to some providers,” he said. A rheumatoid factor test like the one Ms. Welsh ordered, for example, might test positive in 10 out of 100 people randomly gathered in a room, but the majority of those individuals would not have RA, he said.
That test is another popular rheumatology one, according to Timothy Niewold, MD, vice chair for research in the Hospital for Special Surgery Department of Medicine in New York City. Among the possible reasons people might order these tests are the delay in diagnosis that can often occur with a lot of rheumatologic conditions and that “it can take a while to see a rheumatologist, depending on what part of the country you’re in and what the availability is,” he said. He’s not surprised to see tests for Sjögren disease among the offerings, for example, because it’s a condition that’s difficult to diagnose but reasonably common within autoimmune diseases.
Risks vs Benefits
DTC testing is not an answer to the national shortage of rheumatologists, however, especially given the risks that Dr. Niewold, Dr. Putman, and Dr. Kim worry outweigh potential benefits. On the one hand, getting online test results may help expedite a referral to a specialist, Dr. Niewold said. But a long wait for that appointment could then easily become a bigger source of anxiety than comfort, Dr. Putman said.
“It’s a trade-off where you are accepting a lot more people getting false-positive diagnoses and spending months thinking they have some disease where they might not, in exchange for a couple people who would have had a delayed diagnosis,” Dr. Putman said. “There’s an enormous amount of existential suffering,” that’s familiar to rheumatologists because some patients may dread the diagnosis of a rheumatic disease the way they might fear a cancer diagnosis, especially if they have lost a family member to a condition that they suspect they share, he said. “To put yourself into an existential catastrophe — that’s not a small harm.”
Dr. Niewold agreed, pointing out that patients with a positive ANA test may “get unnecessarily worried and stay up all night reading about lupus, getting scared for weeks on end before seeing a specialist.” And there are financial harms as well for patients who may order the same test multiple times, or a whole slate of tests, that they don’t need for hundreds or thousands of dollars. There’s also the lost time and effort of researching a condition or even seeking out support groups that patients may pursue, Dr. Niewold said.
The likely biggest risk to individuals, however, is the potential for overdiagnosis or misdiagnosis.
“If someone comes in and they’ve read the textbook on lupus and they have a positive ANA, it’s really hard as a rheumatologist to walk that back,” Dr. Putman said. “The human mind is a powerful thing,” he added, and people who get a positive test will likely start to notice things like joint pain or a rash on their cheeks and begin attributing it to a diagnosis they risk convincing themselves they have. “When people come into your clinic not knowing what a disease would look like and they just tell you how they’re feeling, it’s a much cleaner and more honest way to approach diagnosis.”
Most patients likely don’t realize, for example, that none of the tests rheumatologists usually order are diagnostic in and of themselves, Dr. Niewold said. “They’re all kind of like stars in the constellation of a diagnosis,” he said. “They’re helpful, but none of them is sufficient by itself.”
Dr. Killeen agreed, noting that “consumers might not understand the nuances of these tests well enough to know whether it is appropriate to order them or how to interpret the results correctly.” Given the long-term implications of a diagnosis for a rheumatologic disease, “I would have concerns about consumers ordering and interpreting rheumatologic tests without working closely with their physicians,” Dr. Killeen said. “The main concern that lab experts have about direct-to-consumer tests is the potential for people to get misleading results and/or to misinterpret their results, which in turn could lead to people not getting the treatment they need or getting treatment when they don’t need any at all.”
It’s one thing for patients to come in asking for a particular treatment they may not need but which a doctor may be able to dissuade them from seeking. But Dr. Kim also pointed out the risk that patients may decide to treat themselves with therapies that haven’t undergone rigorous testing or haven’t been recommended by a physician.
“We tend to have people who come in with a pretty clear idea of what they want done, but the problem is, we don’t know if their reasoning is correct from a clinical perspective,” Dr. Kim said. Companies offer these tests with the belief that they’re “providing patients a choice, an option to take ownership,” he said, “but the potential harm can be realized very quickly because there are going to be people who are misdiagnosing themselves and, worse yet, may then pursue their own treatment plan that’s going in the opposite direction of where we think it needs to go.”
Or, on the flip side, if a patient erroneously believes they have the answer to what ails them, it may delay diagnosis of a more serious condition that’s rarer or harder to detect. Kim pointed to, for example, intravascular lymphoma, which is notoriously as difficult to identify as it is rare and aggressive. If a patient’s confirmation bias has led them to believe they have an autoimmune condition, they may not receive the more serious diagnosis until it’s advanced too far to treat.
Patient-Provider Relationship Friction
Another concern is how these tests may lead to confusion and frustration that can erode the patient-provider relationship, particularly because most patients don’t know how to interpret the results or understand the bigger context in which the results have to be interpreted. Many patients may think a test can come back with a binary answer, a positive or negative, and that means they do or don’t have a condition. That’s generally true for pregnancy tests, COVID tests, and sexually transmitted infection tests — the kinds of tests that have long been available to consumers and which have fairly straightforward answers.
But physicians know that’s not the case for many conditions, particularly those in rheumatology.
“In rheumatic diseases, because the tests have such marginal value in terms of diagnosis, almost always we develop a suspicion before we even think about ordering the tests, and then that dictates whether or not we cross that threshold,” Dr. Kim said. “A negative test doesn’t exclude the fact that you may have disease X, but a positive test also doesn’t mean you have disease X. All they provide is an idea of the risk.”
But some patients who come in with DTC test results have “already made the decision in their mind that they have a certain condition,” Dr. Kim said. “This is obviously dangerous because the majority of these patients do not have the condition they think they have, and it leaves a very uncomfortable feeling after the visit because they feel like they’ve been either betrayed by me or by the test, and they leave more confused.”
Patients may also come in with tests that a doctor isn’t familiar with or isn’t sure how to interpret on its own, at least for that particular patient.
“For ANA testing, we have a pretty good idea of its positive and negative predictive value because it’s ordered so much, but for many of these tests being offered, there are specific autoantibodies, and we tend to only get them in people where there’s a clinical suspicion,” Dr. Kim said. “Within that very specific context, we kind of understand what that value means, but if you give it to the general public, then those numbers aren’t as applicable and most likely overestimate the risk of disease.”
Even if providers consider the results of a DTC test in their differential, they may want to be sure it’s from a trustworthy source. “If a provider is uncertain about whether a direct-to-consumer testing company is reputable or about whether a direct-to-consumer test result is reliable, I would encourage them to consult with their laboratory medicine colleagues,” Dr. Killeen said.
Responding to Patients
Like any other patient coming to a clinical visit, the most common reason patients are likely ordering these tests is that they’re seeking answers. Kim doesn’t typically see patients doing their own monitoring for diagnosed conditions between visits — the expense would add up too quickly — or testing for genetic markers, which likely wouldn’t be very helpful either.
“Even though most of our diseases probably have a genetic underpinning, how much it contributes is always unclear,” Dr. Kim said. Even conditions with clear genetic variants, such as familial Mediterranean fever, spondyloarthritis, and Behçet disease, can only support a diagnosis, not diagnose it on its own, Dr. Killeen said. And these are not among the tests currently available on most DTC company sites.
While there are also tests that can offer information about genetic risks for certain medications, such as a thiopurine methyltransferase test to find out if a patient lacks the enzyme needed to break down the immunosuppressant drug azathioprine, Kim hasn’t seen patients seeking these out either.
“The more global and more compassionate way to think about this is that we have a lot of people who are struggling to understand what’s going on with their bodies, and most physicians really don’t know what the next steps are for these people,” Dr. Kim said. “They’re desperate, and their quality of life is so poor that they’re going to take extreme steps to try to manage their own frustration with this condition.”
That means clinicians’ most powerful tools when patients come in with DTC test results are their listening skills.
“Empathy is the most important thing, just being able to share the patient’s frustration to the point where they had to take matters into their own hands,” Dr. Kim said. “I think a lot of rheumatologists are actually pretty comfortable being in this position.”
Additionally, doctors should know that some patients may be engaging in attempts to self-diagnose, self-treat, or otherwise self-manage their symptoms or perceived condition. “They just need to be aware and try to make sure there’s no harm being done,” Dr. Kim said.
Ms. Welsh didn’t seek treatment or diagnosis on her own, but getting her test also did not give her the control she was seeking. “Looking back, it was kind of a waste of money, but it felt good in the moment,” Ms. Welsh said. “I was so upset, and I wanted that control, and in the end, it didn’t get me results any sooner, and it didn’t give me peace of mind.”
It was Ms. Welsh’s primary care doctor listening to her concerns, ordering the same test she had ordered with several others, and working with her to seek answers that reassured her that her provider cared about her well-being.
“A lot of what I do in my business is reassure people that you know what they have is treatable or not going to end their life as they know it,” Dr. Putman said. “And you certainly can’t reassure them if they’re not in your clinic yet.”
Dr. Putman has participated in clinical trials with AbbVie, consulting with Novartis and GSK, and clinical trials and consulting with Amgen and AstraZeneca. Dr. Niewold reported receiving research grants from EMD Serono and Zenas BioPharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, AstraZeneca, S3 Connected Health, Flagship Pioneering, and Guidepoint. Dr. Kim reported sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, Novartis, and CRISPR Therapeutics; royalties from Kypha; and consulting/speaking for Amgen, ANI Pharmaceuticals, Atara Biotherapeutics, Aurinia Pharmaceuticals, CARGO Therapeutics, Exagen Diagnostics, GSK, Hinge Bio, Kypha, Progentec Diagnostics, Synthekine, and UpToDate. Dr. Killeen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
When Jennifer Welsh, a 40-year-old from New Britain, Connecticut, visited her doctor about pain in her joints and neck, her doctor sent her to the emergency department (ED) to rule out meningitis. The ED did rule that out, as well as strep, so Ms. Welsh went to her follow-up appointment a few days later, hoping for answers or at least more tests to get those answers.
Instead, the doctor — a different one from the same practice as her primary care physician (PCP) — wouldn’t even talk to Ms. Welsh about her symptoms because she couldn’t see the ED’s results and refused to view the results that Ms. Welsh could pull up online.
“She just completely shut me down,” Ms. Welsh recalled. “It was a really awful appointment, and I left in tears. I was in physical pain, I had just been to the ER, nothing is really resolved, I’m stressed out about it, and this woman is completely dismissing me.”
She had been able to schedule an appointment with her regular PCP later that week, but after the harrowing experience with this doctor, she wondered if her PCP would order the rheumatoid arthritis (RA) test that Ms. Welsh suspected she needed. So, she took matters into her own hands.
“I was searching for what test to ask for from my doctor,” she said, and she found that she could order it on her own from a major lab company she was already familiar with. For around $100, “I could get it done and see what it says on my own,” she said.
But that’s not how it worked out. Her regular PCP apologized for the other doctor’s behavior and ordered the RA test as well as additional tests — and got results while Ms. Welsh still waited for the one she ordered to arrive over a week later.
At first, Ms. Welsh was grateful she could order the RA test without her doctor’s referral. “I felt it gave me a sense of control over the situation that I felt really not in control of, until the system failed me, and I didn’t get the results,” she said. But then, “not having someone I could call and get an answer about why my tests were delayed, why I wasn’t able to access them, why it was taking so long — it was definitely anxiety-inducing.”
A Growing Market
Ms. Welsh is one of a growing number of patients who are ordering direct-to-consumer (DTC) lab tests without the recommendation or guidance of a doctor. They’re offered online by labs ranging from well-established giants like Quest and Labcorp to smaller, potentially less vetted companies, although some smaller companies contract with larger companies like Quest. Combined, the DTC market is projected to be worth $2 billion by 2025.
Yet the burgeoning industry has also drawn critiques from both bioethicists and privacy experts. A research letter in JAMA in 2023, for example, found that less than half of the 21 companies identified in an online search declared Health Insurance Portability and Accountability Act compliance, while more than half “indicated the potential use of consumer data for research purposes either internally or through third-party sharing.” That study found the most commonly offered tests were related to diabetes, the thyroid, and vitamin levels, and hormone tests for men and women, such as testosterone or estradiol.
But a number of companies also offer tests related to rheumatologic conditions. A handful of tests offered by Labcorp, for example, could be used in rheumatology, such as tests for celiac antibodies or high-sensitivity C-reactive protein. Quest similarly offers a handful of autoimmune-related tests. But other companies offer a long slate of autoimmune or antibody tests.
The antinuclear antibody (ANA) test and RA panel offered by Quest are the same tests, run and analyzed in the same labs, as those ordered by physicians and hospitals, according to James Faix, MD, the medical director of immunology at Quest Diagnostics. Their RA panel includes rheumatoid factor and anti-cyclic citrullinated peptide as well as antibody to mutated citrullinated vimentin, “which may detect approximately 10%-15%” of patients who test negative to the first two.
Quest’s ANA test with reflex costs $112, and its RA panel costs $110, price points that are similar across other companies’ offerings. Labcorp declined to respond to questions about its DTC tests, and several smaller companies did not respond to queries about their offerings. It can therefore be hard to assess what’s included or what the quality is of many DTC tests, particularly from smaller, less established companies.
Oversight and Quality Control
Anthony Killeen, MD, PhD, president of the Association for Diagnostics & Laboratory Medicine (ADLM) and director of Clinical Laboratories at the University of Minnesota Medical Center in Minneapolis, said via email that the ADLM supports “expanding consumer access to direct-to-consumer laboratory testing services that have demonstrated analytical and clinical validity and clinical utility,” given the importance of individuals learning about their health status and becoming more involved in health decisions. But the ADLM also recommends “that only CLIA-certified laboratories perform direct-to-consumer testing,” he said.
“There are direct-to-consumer tests on the market that are not medical-grade laboratory tests and that may be performed in nonaccredited laboratories,” Dr. Killeen said. “We advise consumers to steer clear of such tests.” The ADLM also encourages consumers to “work with qualified healthcare providers when making decisions based off the results they receive from any direct-to-consumer tests” and recommends that DTC test companies “provide consumers with sufficient information and/or access to expert help to assist them in ordering tests and interpreting the results.”
Yet it’s unclear how much support, if any, consumers can receive in terms of understanding what their tests mean. Most of the companies in the 2023 study offered optional follow-up with a healthcare professional, but these professionals ranged from physicians to “health coaches,” and all the companies had disclaimers that “test results did not constitute medical advice.”
At Quest, the only company to respond to this news organization’s request for comment, consumer-initiated tests ordered online are first reviewed by a physician at PWNHealth, an independent, third-party physician network, to determine that it’s appropriate before the lab order is actually placed.
“Once results are available, individuals have the option to discuss their results with an independent physician at no extra cost,” Dr. Faix said. ANA or RA results outside the normal ranges may trigger a “call from a PWNHealth healthcare coordinator, who can help provide information, suggestions on next steps, and set up time for the individual to speak with an independent physician to discuss questions or concerns regarding the results,” he said.
“Our goal is not to replace the role of a healthcare provider,” Dr. Faix said. “We are providing an alternate way for people to engage with the healthcare system that offers convenience, gives people more control over their own healthcare journeys, and meets them where they are, supporting both consumers and their care teams.” The company has expanded its offerings from an initial 30 tests made available in 2018 to over 130 today, deciding which to offer “based on consumer research and expertise of clinical experts.” The company has also “seen steady interest in our two consumer rheumatology offerings,” Dr. Faix said.
The DTC Landscape in Rheumatology
Within rheumatology, among the most popular tests is for ANA, based on the experience of Alfred Kim, MD, PhD, associate professor of medicine at Washington University School of Medicine in St Louis, Missouri.
“For a lot of people, losing control over their health is maybe the most frightening experience they can have, so I think a lot of patients use this as a way to kind of have ownership over their health,” Dr. Kim said. “Let’s say they’ve been to four doctors. No one can explain what’s going on. They’re getting frustrated, and so they just turn to solutions where they feel like they have ownership over the situation.”
Though the market is undoubtedly growing, the growth appears uneven across geography and institution types. Kim has seen a “fair number of referrals,” with patients coming in with results from a DTC test. Michael Putman, MD, MSci, assistant professor of medicine at the Medical College of Wisconsin in Milwaukee, hasn’t seen it much. “I know that patients can get testing done themselves independently, but I don’t have people routinely coming in with tests they’ve ordered in advance of our appointment,” Dr. Putman said, but, like Dr. Kim, he recognizes why patients might seek them out.
“I’m a big fan of patient empowerment, and I do think that medicine serves a gatekeeper role that sometimes can be a little too far,” Dr. Putman said. “I think there is value to patients being able to get more information and try to understand what is happening in their bodies. I have a lot of compassion for someone who would try to find testing outside of the normal channels.”
Indeed, bringing these test results to a visit could be informative in some scenarios. A negative ANA test, for example, pretty much excludes lupus 100%, Dr. Kim said. But a positive ANA doesn’t tell him much, and if his clinical suspicion for a condition is high, he likely would order that test anyway, even if the patient came in with their own results. Dr. Putman also pointed out that the vast majority of tests used in rheumatology have a high rate of false positives.
“I think that will be the major area where this causes quite a lot of grief to patients and some frustration to some providers,” he said. A rheumatoid factor test like the one Ms. Welsh ordered, for example, might test positive in 10 out of 100 people randomly gathered in a room, but the majority of those individuals would not have RA, he said.
That test is another popular rheumatology one, according to Timothy Niewold, MD, vice chair for research in the Hospital for Special Surgery Department of Medicine in New York City. Among the possible reasons people might order these tests are the delay in diagnosis that can often occur with a lot of rheumatologic conditions and that “it can take a while to see a rheumatologist, depending on what part of the country you’re in and what the availability is,” he said. He’s not surprised to see tests for Sjögren disease among the offerings, for example, because it’s a condition that’s difficult to diagnose but reasonably common within autoimmune diseases.
Risks vs Benefits
DTC testing is not an answer to the national shortage of rheumatologists, however, especially given the risks that Dr. Niewold, Dr. Putman, and Dr. Kim worry outweigh potential benefits. On the one hand, getting online test results may help expedite a referral to a specialist, Dr. Niewold said. But a long wait for that appointment could then easily become a bigger source of anxiety than comfort, Dr. Putman said.
“It’s a trade-off where you are accepting a lot more people getting false-positive diagnoses and spending months thinking they have some disease where they might not, in exchange for a couple people who would have had a delayed diagnosis,” Dr. Putman said. “There’s an enormous amount of existential suffering,” that’s familiar to rheumatologists because some patients may dread the diagnosis of a rheumatic disease the way they might fear a cancer diagnosis, especially if they have lost a family member to a condition that they suspect they share, he said. “To put yourself into an existential catastrophe — that’s not a small harm.”
Dr. Niewold agreed, pointing out that patients with a positive ANA test may “get unnecessarily worried and stay up all night reading about lupus, getting scared for weeks on end before seeing a specialist.” And there are financial harms as well for patients who may order the same test multiple times, or a whole slate of tests, that they don’t need for hundreds or thousands of dollars. There’s also the lost time and effort of researching a condition or even seeking out support groups that patients may pursue, Dr. Niewold said.
The likely biggest risk to individuals, however, is the potential for overdiagnosis or misdiagnosis.
“If someone comes in and they’ve read the textbook on lupus and they have a positive ANA, it’s really hard as a rheumatologist to walk that back,” Dr. Putman said. “The human mind is a powerful thing,” he added, and people who get a positive test will likely start to notice things like joint pain or a rash on their cheeks and begin attributing it to a diagnosis they risk convincing themselves they have. “When people come into your clinic not knowing what a disease would look like and they just tell you how they’re feeling, it’s a much cleaner and more honest way to approach diagnosis.”
Most patients likely don’t realize, for example, that none of the tests rheumatologists usually order are diagnostic in and of themselves, Dr. Niewold said. “They’re all kind of like stars in the constellation of a diagnosis,” he said. “They’re helpful, but none of them is sufficient by itself.”
Dr. Killeen agreed, noting that “consumers might not understand the nuances of these tests well enough to know whether it is appropriate to order them or how to interpret the results correctly.” Given the long-term implications of a diagnosis for a rheumatologic disease, “I would have concerns about consumers ordering and interpreting rheumatologic tests without working closely with their physicians,” Dr. Killeen said. “The main concern that lab experts have about direct-to-consumer tests is the potential for people to get misleading results and/or to misinterpret their results, which in turn could lead to people not getting the treatment they need or getting treatment when they don’t need any at all.”
It’s one thing for patients to come in asking for a particular treatment they may not need but which a doctor may be able to dissuade them from seeking. But Dr. Kim also pointed out the risk that patients may decide to treat themselves with therapies that haven’t undergone rigorous testing or haven’t been recommended by a physician.
“We tend to have people who come in with a pretty clear idea of what they want done, but the problem is, we don’t know if their reasoning is correct from a clinical perspective,” Dr. Kim said. Companies offer these tests with the belief that they’re “providing patients a choice, an option to take ownership,” he said, “but the potential harm can be realized very quickly because there are going to be people who are misdiagnosing themselves and, worse yet, may then pursue their own treatment plan that’s going in the opposite direction of where we think it needs to go.”
Or, on the flip side, if a patient erroneously believes they have the answer to what ails them, it may delay diagnosis of a more serious condition that’s rarer or harder to detect. Kim pointed to, for example, intravascular lymphoma, which is notoriously as difficult to identify as it is rare and aggressive. If a patient’s confirmation bias has led them to believe they have an autoimmune condition, they may not receive the more serious diagnosis until it’s advanced too far to treat.
Patient-Provider Relationship Friction
Another concern is how these tests may lead to confusion and frustration that can erode the patient-provider relationship, particularly because most patients don’t know how to interpret the results or understand the bigger context in which the results have to be interpreted. Many patients may think a test can come back with a binary answer, a positive or negative, and that means they do or don’t have a condition. That’s generally true for pregnancy tests, COVID tests, and sexually transmitted infection tests — the kinds of tests that have long been available to consumers and which have fairly straightforward answers.
But physicians know that’s not the case for many conditions, particularly those in rheumatology.
“In rheumatic diseases, because the tests have such marginal value in terms of diagnosis, almost always we develop a suspicion before we even think about ordering the tests, and then that dictates whether or not we cross that threshold,” Dr. Kim said. “A negative test doesn’t exclude the fact that you may have disease X, but a positive test also doesn’t mean you have disease X. All they provide is an idea of the risk.”
But some patients who come in with DTC test results have “already made the decision in their mind that they have a certain condition,” Dr. Kim said. “This is obviously dangerous because the majority of these patients do not have the condition they think they have, and it leaves a very uncomfortable feeling after the visit because they feel like they’ve been either betrayed by me or by the test, and they leave more confused.”
Patients may also come in with tests that a doctor isn’t familiar with or isn’t sure how to interpret on its own, at least for that particular patient.
“For ANA testing, we have a pretty good idea of its positive and negative predictive value because it’s ordered so much, but for many of these tests being offered, there are specific autoantibodies, and we tend to only get them in people where there’s a clinical suspicion,” Dr. Kim said. “Within that very specific context, we kind of understand what that value means, but if you give it to the general public, then those numbers aren’t as applicable and most likely overestimate the risk of disease.”
Even if providers consider the results of a DTC test in their differential, they may want to be sure it’s from a trustworthy source. “If a provider is uncertain about whether a direct-to-consumer testing company is reputable or about whether a direct-to-consumer test result is reliable, I would encourage them to consult with their laboratory medicine colleagues,” Dr. Killeen said.
Responding to Patients
Like any other patient coming to a clinical visit, the most common reason patients are likely ordering these tests is that they’re seeking answers. Kim doesn’t typically see patients doing their own monitoring for diagnosed conditions between visits — the expense would add up too quickly — or testing for genetic markers, which likely wouldn’t be very helpful either.
“Even though most of our diseases probably have a genetic underpinning, how much it contributes is always unclear,” Dr. Kim said. Even conditions with clear genetic variants, such as familial Mediterranean fever, spondyloarthritis, and Behçet disease, can only support a diagnosis, not diagnose it on its own, Dr. Killeen said. And these are not among the tests currently available on most DTC company sites.
While there are also tests that can offer information about genetic risks for certain medications, such as a thiopurine methyltransferase test to find out if a patient lacks the enzyme needed to break down the immunosuppressant drug azathioprine, Kim hasn’t seen patients seeking these out either.
“The more global and more compassionate way to think about this is that we have a lot of people who are struggling to understand what’s going on with their bodies, and most physicians really don’t know what the next steps are for these people,” Dr. Kim said. “They’re desperate, and their quality of life is so poor that they’re going to take extreme steps to try to manage their own frustration with this condition.”
That means clinicians’ most powerful tools when patients come in with DTC test results are their listening skills.
“Empathy is the most important thing, just being able to share the patient’s frustration to the point where they had to take matters into their own hands,” Dr. Kim said. “I think a lot of rheumatologists are actually pretty comfortable being in this position.”
Additionally, doctors should know that some patients may be engaging in attempts to self-diagnose, self-treat, or otherwise self-manage their symptoms or perceived condition. “They just need to be aware and try to make sure there’s no harm being done,” Dr. Kim said.
Ms. Welsh didn’t seek treatment or diagnosis on her own, but getting her test also did not give her the control she was seeking. “Looking back, it was kind of a waste of money, but it felt good in the moment,” Ms. Welsh said. “I was so upset, and I wanted that control, and in the end, it didn’t get me results any sooner, and it didn’t give me peace of mind.”
It was Ms. Welsh’s primary care doctor listening to her concerns, ordering the same test she had ordered with several others, and working with her to seek answers that reassured her that her provider cared about her well-being.
“A lot of what I do in my business is reassure people that you know what they have is treatable or not going to end their life as they know it,” Dr. Putman said. “And you certainly can’t reassure them if they’re not in your clinic yet.”
Dr. Putman has participated in clinical trials with AbbVie, consulting with Novartis and GSK, and clinical trials and consulting with Amgen and AstraZeneca. Dr. Niewold reported receiving research grants from EMD Serono and Zenas BioPharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, AstraZeneca, S3 Connected Health, Flagship Pioneering, and Guidepoint. Dr. Kim reported sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, Novartis, and CRISPR Therapeutics; royalties from Kypha; and consulting/speaking for Amgen, ANI Pharmaceuticals, Atara Biotherapeutics, Aurinia Pharmaceuticals, CARGO Therapeutics, Exagen Diagnostics, GSK, Hinge Bio, Kypha, Progentec Diagnostics, Synthekine, and UpToDate. Dr. Killeen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
When Jennifer Welsh, a 40-year-old from New Britain, Connecticut, visited her doctor about pain in her joints and neck, her doctor sent her to the emergency department (ED) to rule out meningitis. The ED did rule that out, as well as strep, so Ms. Welsh went to her follow-up appointment a few days later, hoping for answers or at least more tests to get those answers.
Instead, the doctor — a different one from the same practice as her primary care physician (PCP) — wouldn’t even talk to Ms. Welsh about her symptoms because she couldn’t see the ED’s results and refused to view the results that Ms. Welsh could pull up online.
“She just completely shut me down,” Ms. Welsh recalled. “It was a really awful appointment, and I left in tears. I was in physical pain, I had just been to the ER, nothing is really resolved, I’m stressed out about it, and this woman is completely dismissing me.”
She had been able to schedule an appointment with her regular PCP later that week, but after the harrowing experience with this doctor, she wondered if her PCP would order the rheumatoid arthritis (RA) test that Ms. Welsh suspected she needed. So, she took matters into her own hands.
“I was searching for what test to ask for from my doctor,” she said, and she found that she could order it on her own from a major lab company she was already familiar with. For around $100, “I could get it done and see what it says on my own,” she said.
But that’s not how it worked out. Her regular PCP apologized for the other doctor’s behavior and ordered the RA test as well as additional tests — and got results while Ms. Welsh still waited for the one she ordered to arrive over a week later.
At first, Ms. Welsh was grateful she could order the RA test without her doctor’s referral. “I felt it gave me a sense of control over the situation that I felt really not in control of, until the system failed me, and I didn’t get the results,” she said. But then, “not having someone I could call and get an answer about why my tests were delayed, why I wasn’t able to access them, why it was taking so long — it was definitely anxiety-inducing.”
A Growing Market
Ms. Welsh is one of a growing number of patients who are ordering direct-to-consumer (DTC) lab tests without the recommendation or guidance of a doctor. They’re offered online by labs ranging from well-established giants like Quest and Labcorp to smaller, potentially less vetted companies, although some smaller companies contract with larger companies like Quest. Combined, the DTC market is projected to be worth $2 billion by 2025.
Yet the burgeoning industry has also drawn critiques from both bioethicists and privacy experts. A research letter in JAMA in 2023, for example, found that less than half of the 21 companies identified in an online search declared Health Insurance Portability and Accountability Act compliance, while more than half “indicated the potential use of consumer data for research purposes either internally or through third-party sharing.” That study found the most commonly offered tests were related to diabetes, the thyroid, and vitamin levels, and hormone tests for men and women, such as testosterone or estradiol.
But a number of companies also offer tests related to rheumatologic conditions. A handful of tests offered by Labcorp, for example, could be used in rheumatology, such as tests for celiac antibodies or high-sensitivity C-reactive protein. Quest similarly offers a handful of autoimmune-related tests. But other companies offer a long slate of autoimmune or antibody tests.
The antinuclear antibody (ANA) test and RA panel offered by Quest are the same tests, run and analyzed in the same labs, as those ordered by physicians and hospitals, according to James Faix, MD, the medical director of immunology at Quest Diagnostics. Their RA panel includes rheumatoid factor and anti-cyclic citrullinated peptide as well as antibody to mutated citrullinated vimentin, “which may detect approximately 10%-15%” of patients who test negative to the first two.
Quest’s ANA test with reflex costs $112, and its RA panel costs $110, price points that are similar across other companies’ offerings. Labcorp declined to respond to questions about its DTC tests, and several smaller companies did not respond to queries about their offerings. It can therefore be hard to assess what’s included or what the quality is of many DTC tests, particularly from smaller, less established companies.
Oversight and Quality Control
Anthony Killeen, MD, PhD, president of the Association for Diagnostics & Laboratory Medicine (ADLM) and director of Clinical Laboratories at the University of Minnesota Medical Center in Minneapolis, said via email that the ADLM supports “expanding consumer access to direct-to-consumer laboratory testing services that have demonstrated analytical and clinical validity and clinical utility,” given the importance of individuals learning about their health status and becoming more involved in health decisions. But the ADLM also recommends “that only CLIA-certified laboratories perform direct-to-consumer testing,” he said.
“There are direct-to-consumer tests on the market that are not medical-grade laboratory tests and that may be performed in nonaccredited laboratories,” Dr. Killeen said. “We advise consumers to steer clear of such tests.” The ADLM also encourages consumers to “work with qualified healthcare providers when making decisions based off the results they receive from any direct-to-consumer tests” and recommends that DTC test companies “provide consumers with sufficient information and/or access to expert help to assist them in ordering tests and interpreting the results.”
Yet it’s unclear how much support, if any, consumers can receive in terms of understanding what their tests mean. Most of the companies in the 2023 study offered optional follow-up with a healthcare professional, but these professionals ranged from physicians to “health coaches,” and all the companies had disclaimers that “test results did not constitute medical advice.”
At Quest, the only company to respond to this news organization’s request for comment, consumer-initiated tests ordered online are first reviewed by a physician at PWNHealth, an independent, third-party physician network, to determine that it’s appropriate before the lab order is actually placed.
“Once results are available, individuals have the option to discuss their results with an independent physician at no extra cost,” Dr. Faix said. ANA or RA results outside the normal ranges may trigger a “call from a PWNHealth healthcare coordinator, who can help provide information, suggestions on next steps, and set up time for the individual to speak with an independent physician to discuss questions or concerns regarding the results,” he said.
“Our goal is not to replace the role of a healthcare provider,” Dr. Faix said. “We are providing an alternate way for people to engage with the healthcare system that offers convenience, gives people more control over their own healthcare journeys, and meets them where they are, supporting both consumers and their care teams.” The company has expanded its offerings from an initial 30 tests made available in 2018 to over 130 today, deciding which to offer “based on consumer research and expertise of clinical experts.” The company has also “seen steady interest in our two consumer rheumatology offerings,” Dr. Faix said.
The DTC Landscape in Rheumatology
Within rheumatology, among the most popular tests is for ANA, based on the experience of Alfred Kim, MD, PhD, associate professor of medicine at Washington University School of Medicine in St Louis, Missouri.
“For a lot of people, losing control over their health is maybe the most frightening experience they can have, so I think a lot of patients use this as a way to kind of have ownership over their health,” Dr. Kim said. “Let’s say they’ve been to four doctors. No one can explain what’s going on. They’re getting frustrated, and so they just turn to solutions where they feel like they have ownership over the situation.”
Though the market is undoubtedly growing, the growth appears uneven across geography and institution types. Kim has seen a “fair number of referrals,” with patients coming in with results from a DTC test. Michael Putman, MD, MSci, assistant professor of medicine at the Medical College of Wisconsin in Milwaukee, hasn’t seen it much. “I know that patients can get testing done themselves independently, but I don’t have people routinely coming in with tests they’ve ordered in advance of our appointment,” Dr. Putman said, but, like Dr. Kim, he recognizes why patients might seek them out.
“I’m a big fan of patient empowerment, and I do think that medicine serves a gatekeeper role that sometimes can be a little too far,” Dr. Putman said. “I think there is value to patients being able to get more information and try to understand what is happening in their bodies. I have a lot of compassion for someone who would try to find testing outside of the normal channels.”
Indeed, bringing these test results to a visit could be informative in some scenarios. A negative ANA test, for example, pretty much excludes lupus 100%, Dr. Kim said. But a positive ANA doesn’t tell him much, and if his clinical suspicion for a condition is high, he likely would order that test anyway, even if the patient came in with their own results. Dr. Putman also pointed out that the vast majority of tests used in rheumatology have a high rate of false positives.
“I think that will be the major area where this causes quite a lot of grief to patients and some frustration to some providers,” he said. A rheumatoid factor test like the one Ms. Welsh ordered, for example, might test positive in 10 out of 100 people randomly gathered in a room, but the majority of those individuals would not have RA, he said.
That test is another popular rheumatology one, according to Timothy Niewold, MD, vice chair for research in the Hospital for Special Surgery Department of Medicine in New York City. Among the possible reasons people might order these tests are the delay in diagnosis that can often occur with a lot of rheumatologic conditions and that “it can take a while to see a rheumatologist, depending on what part of the country you’re in and what the availability is,” he said. He’s not surprised to see tests for Sjögren disease among the offerings, for example, because it’s a condition that’s difficult to diagnose but reasonably common within autoimmune diseases.
Risks vs Benefits
DTC testing is not an answer to the national shortage of rheumatologists, however, especially given the risks that Dr. Niewold, Dr. Putman, and Dr. Kim worry outweigh potential benefits. On the one hand, getting online test results may help expedite a referral to a specialist, Dr. Niewold said. But a long wait for that appointment could then easily become a bigger source of anxiety than comfort, Dr. Putman said.
“It’s a trade-off where you are accepting a lot more people getting false-positive diagnoses and spending months thinking they have some disease where they might not, in exchange for a couple people who would have had a delayed diagnosis,” Dr. Putman said. “There’s an enormous amount of existential suffering,” that’s familiar to rheumatologists because some patients may dread the diagnosis of a rheumatic disease the way they might fear a cancer diagnosis, especially if they have lost a family member to a condition that they suspect they share, he said. “To put yourself into an existential catastrophe — that’s not a small harm.”
Dr. Niewold agreed, pointing out that patients with a positive ANA test may “get unnecessarily worried and stay up all night reading about lupus, getting scared for weeks on end before seeing a specialist.” And there are financial harms as well for patients who may order the same test multiple times, or a whole slate of tests, that they don’t need for hundreds or thousands of dollars. There’s also the lost time and effort of researching a condition or even seeking out support groups that patients may pursue, Dr. Niewold said.
The likely biggest risk to individuals, however, is the potential for overdiagnosis or misdiagnosis.
“If someone comes in and they’ve read the textbook on lupus and they have a positive ANA, it’s really hard as a rheumatologist to walk that back,” Dr. Putman said. “The human mind is a powerful thing,” he added, and people who get a positive test will likely start to notice things like joint pain or a rash on their cheeks and begin attributing it to a diagnosis they risk convincing themselves they have. “When people come into your clinic not knowing what a disease would look like and they just tell you how they’re feeling, it’s a much cleaner and more honest way to approach diagnosis.”
Most patients likely don’t realize, for example, that none of the tests rheumatologists usually order are diagnostic in and of themselves, Dr. Niewold said. “They’re all kind of like stars in the constellation of a diagnosis,” he said. “They’re helpful, but none of them is sufficient by itself.”
Dr. Killeen agreed, noting that “consumers might not understand the nuances of these tests well enough to know whether it is appropriate to order them or how to interpret the results correctly.” Given the long-term implications of a diagnosis for a rheumatologic disease, “I would have concerns about consumers ordering and interpreting rheumatologic tests without working closely with their physicians,” Dr. Killeen said. “The main concern that lab experts have about direct-to-consumer tests is the potential for people to get misleading results and/or to misinterpret their results, which in turn could lead to people not getting the treatment they need or getting treatment when they don’t need any at all.”
It’s one thing for patients to come in asking for a particular treatment they may not need but which a doctor may be able to dissuade them from seeking. But Dr. Kim also pointed out the risk that patients may decide to treat themselves with therapies that haven’t undergone rigorous testing or haven’t been recommended by a physician.
“We tend to have people who come in with a pretty clear idea of what they want done, but the problem is, we don’t know if their reasoning is correct from a clinical perspective,” Dr. Kim said. Companies offer these tests with the belief that they’re “providing patients a choice, an option to take ownership,” he said, “but the potential harm can be realized very quickly because there are going to be people who are misdiagnosing themselves and, worse yet, may then pursue their own treatment plan that’s going in the opposite direction of where we think it needs to go.”
Or, on the flip side, if a patient erroneously believes they have the answer to what ails them, it may delay diagnosis of a more serious condition that’s rarer or harder to detect. Kim pointed to, for example, intravascular lymphoma, which is notoriously as difficult to identify as it is rare and aggressive. If a patient’s confirmation bias has led them to believe they have an autoimmune condition, they may not receive the more serious diagnosis until it’s advanced too far to treat.
Patient-Provider Relationship Friction
Another concern is how these tests may lead to confusion and frustration that can erode the patient-provider relationship, particularly because most patients don’t know how to interpret the results or understand the bigger context in which the results have to be interpreted. Many patients may think a test can come back with a binary answer, a positive or negative, and that means they do or don’t have a condition. That’s generally true for pregnancy tests, COVID tests, and sexually transmitted infection tests — the kinds of tests that have long been available to consumers and which have fairly straightforward answers.
But physicians know that’s not the case for many conditions, particularly those in rheumatology.
“In rheumatic diseases, because the tests have such marginal value in terms of diagnosis, almost always we develop a suspicion before we even think about ordering the tests, and then that dictates whether or not we cross that threshold,” Dr. Kim said. “A negative test doesn’t exclude the fact that you may have disease X, but a positive test also doesn’t mean you have disease X. All they provide is an idea of the risk.”
But some patients who come in with DTC test results have “already made the decision in their mind that they have a certain condition,” Dr. Kim said. “This is obviously dangerous because the majority of these patients do not have the condition they think they have, and it leaves a very uncomfortable feeling after the visit because they feel like they’ve been either betrayed by me or by the test, and they leave more confused.”
Patients may also come in with tests that a doctor isn’t familiar with or isn’t sure how to interpret on its own, at least for that particular patient.
“For ANA testing, we have a pretty good idea of its positive and negative predictive value because it’s ordered so much, but for many of these tests being offered, there are specific autoantibodies, and we tend to only get them in people where there’s a clinical suspicion,” Dr. Kim said. “Within that very specific context, we kind of understand what that value means, but if you give it to the general public, then those numbers aren’t as applicable and most likely overestimate the risk of disease.”
Even if providers consider the results of a DTC test in their differential, they may want to be sure it’s from a trustworthy source. “If a provider is uncertain about whether a direct-to-consumer testing company is reputable or about whether a direct-to-consumer test result is reliable, I would encourage them to consult with their laboratory medicine colleagues,” Dr. Killeen said.
Responding to Patients
Like any other patient coming to a clinical visit, the most common reason patients are likely ordering these tests is that they’re seeking answers. Kim doesn’t typically see patients doing their own monitoring for diagnosed conditions between visits — the expense would add up too quickly — or testing for genetic markers, which likely wouldn’t be very helpful either.
“Even though most of our diseases probably have a genetic underpinning, how much it contributes is always unclear,” Dr. Kim said. Even conditions with clear genetic variants, such as familial Mediterranean fever, spondyloarthritis, and Behçet disease, can only support a diagnosis, not diagnose it on its own, Dr. Killeen said. And these are not among the tests currently available on most DTC company sites.
While there are also tests that can offer information about genetic risks for certain medications, such as a thiopurine methyltransferase test to find out if a patient lacks the enzyme needed to break down the immunosuppressant drug azathioprine, Kim hasn’t seen patients seeking these out either.
“The more global and more compassionate way to think about this is that we have a lot of people who are struggling to understand what’s going on with their bodies, and most physicians really don’t know what the next steps are for these people,” Dr. Kim said. “They’re desperate, and their quality of life is so poor that they’re going to take extreme steps to try to manage their own frustration with this condition.”
That means clinicians’ most powerful tools when patients come in with DTC test results are their listening skills.
“Empathy is the most important thing, just being able to share the patient’s frustration to the point where they had to take matters into their own hands,” Dr. Kim said. “I think a lot of rheumatologists are actually pretty comfortable being in this position.”
Additionally, doctors should know that some patients may be engaging in attempts to self-diagnose, self-treat, or otherwise self-manage their symptoms or perceived condition. “They just need to be aware and try to make sure there’s no harm being done,” Dr. Kim said.
Ms. Welsh didn’t seek treatment or diagnosis on her own, but getting her test also did not give her the control she was seeking. “Looking back, it was kind of a waste of money, but it felt good in the moment,” Ms. Welsh said. “I was so upset, and I wanted that control, and in the end, it didn’t get me results any sooner, and it didn’t give me peace of mind.”
It was Ms. Welsh’s primary care doctor listening to her concerns, ordering the same test she had ordered with several others, and working with her to seek answers that reassured her that her provider cared about her well-being.
“A lot of what I do in my business is reassure people that you know what they have is treatable or not going to end their life as they know it,” Dr. Putman said. “And you certainly can’t reassure them if they’re not in your clinic yet.”
Dr. Putman has participated in clinical trials with AbbVie, consulting with Novartis and GSK, and clinical trials and consulting with Amgen and AstraZeneca. Dr. Niewold reported receiving research grants from EMD Serono and Zenas BioPharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, AstraZeneca, S3 Connected Health, Flagship Pioneering, and Guidepoint. Dr. Kim reported sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, Novartis, and CRISPR Therapeutics; royalties from Kypha; and consulting/speaking for Amgen, ANI Pharmaceuticals, Atara Biotherapeutics, Aurinia Pharmaceuticals, CARGO Therapeutics, Exagen Diagnostics, GSK, Hinge Bio, Kypha, Progentec Diagnostics, Synthekine, and UpToDate. Dr. Killeen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.



















