User login
For MD-IQ only
Cancer Data Trends 2024: Genitourinary Cancers
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
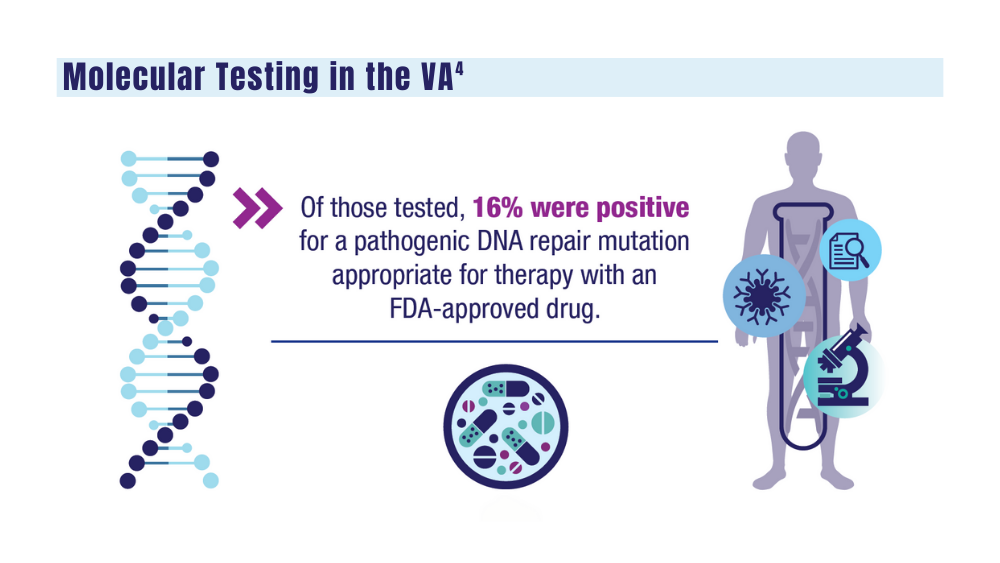
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
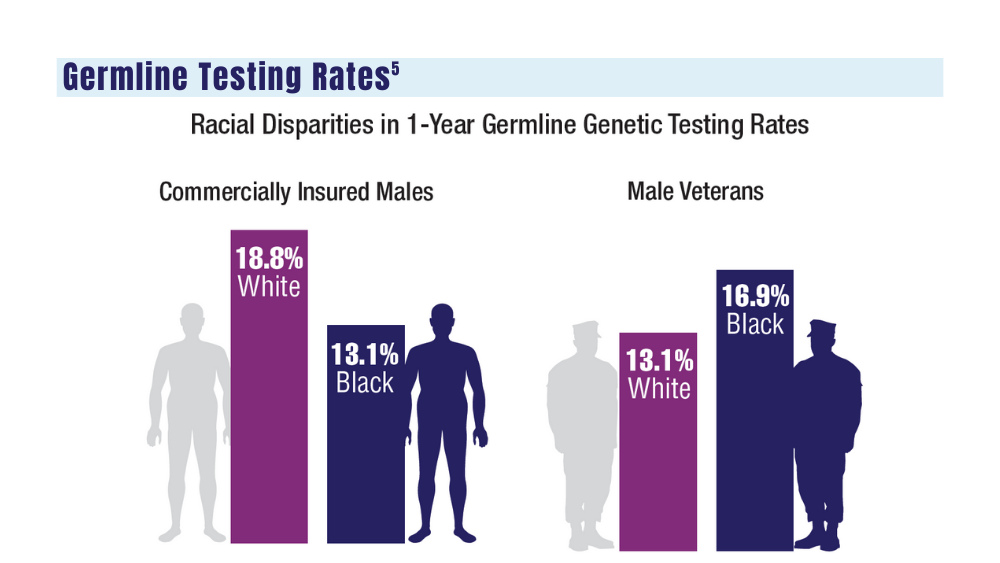
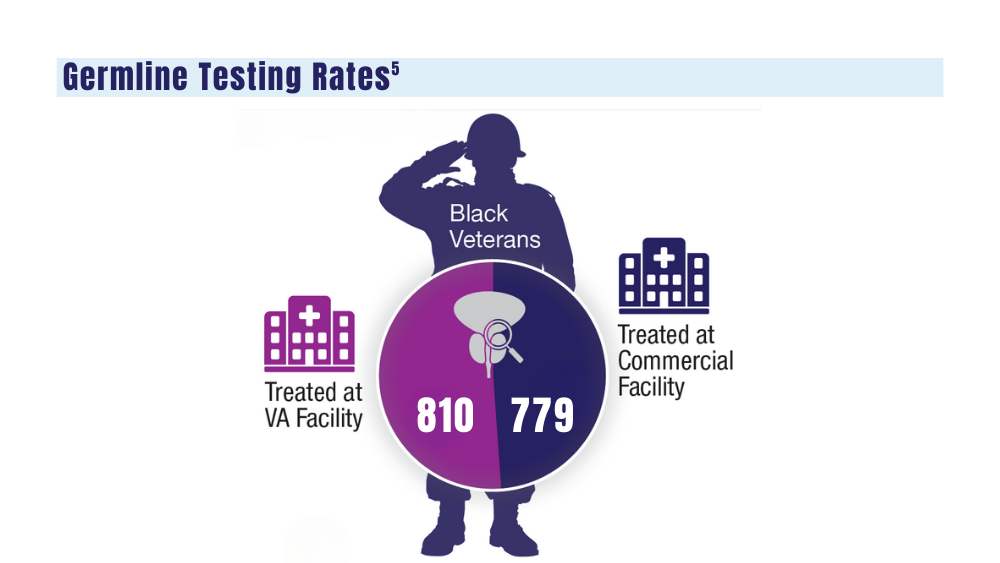
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
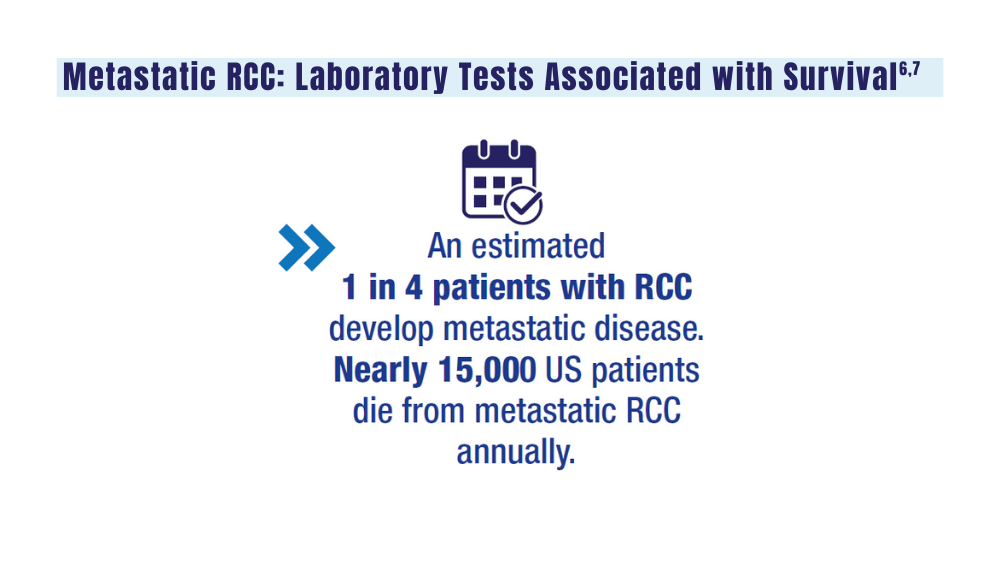
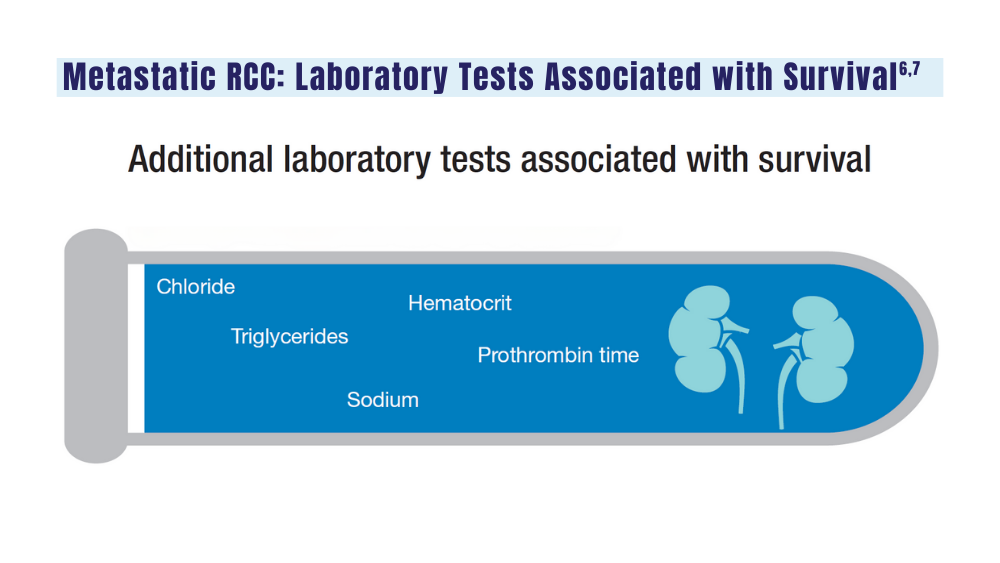
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
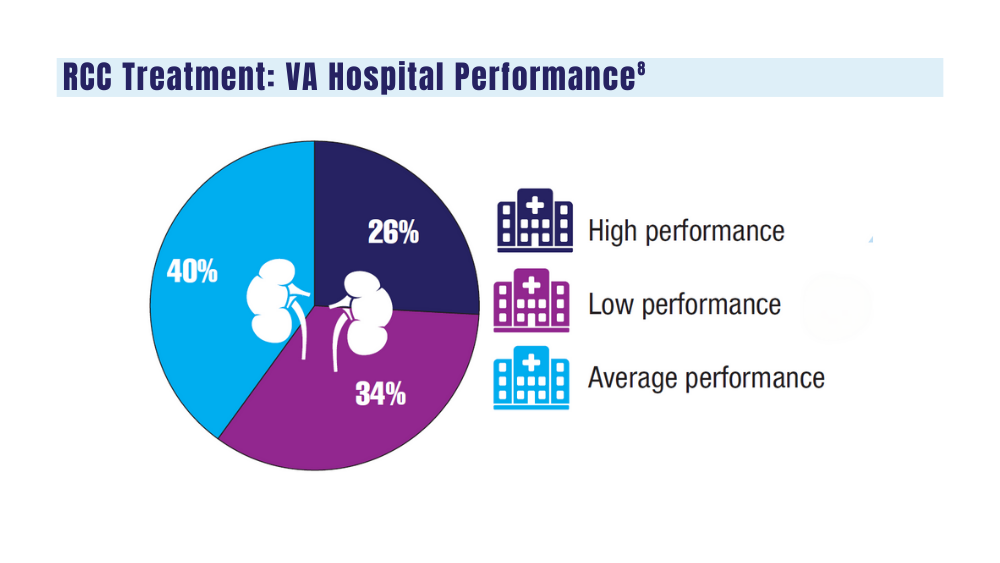
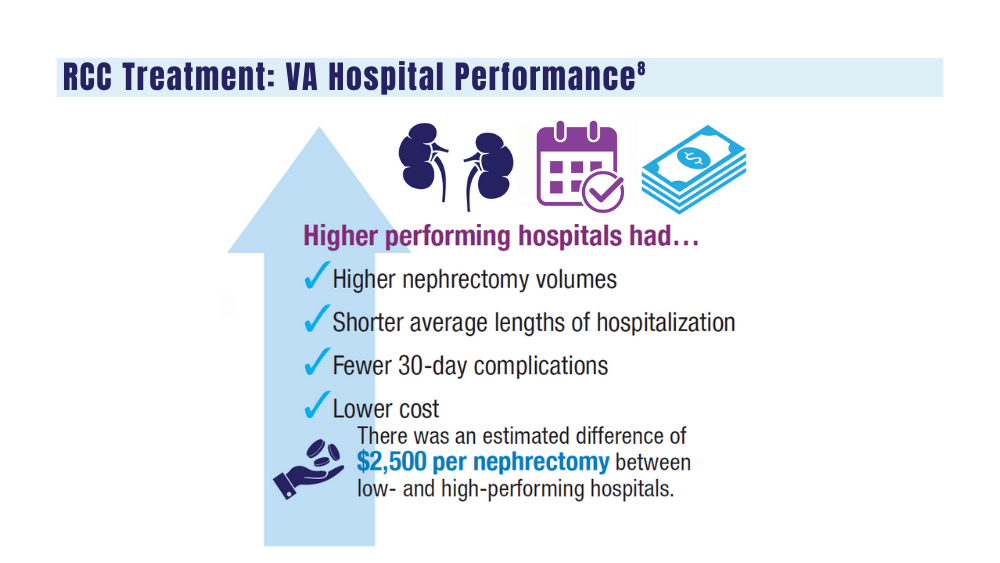
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
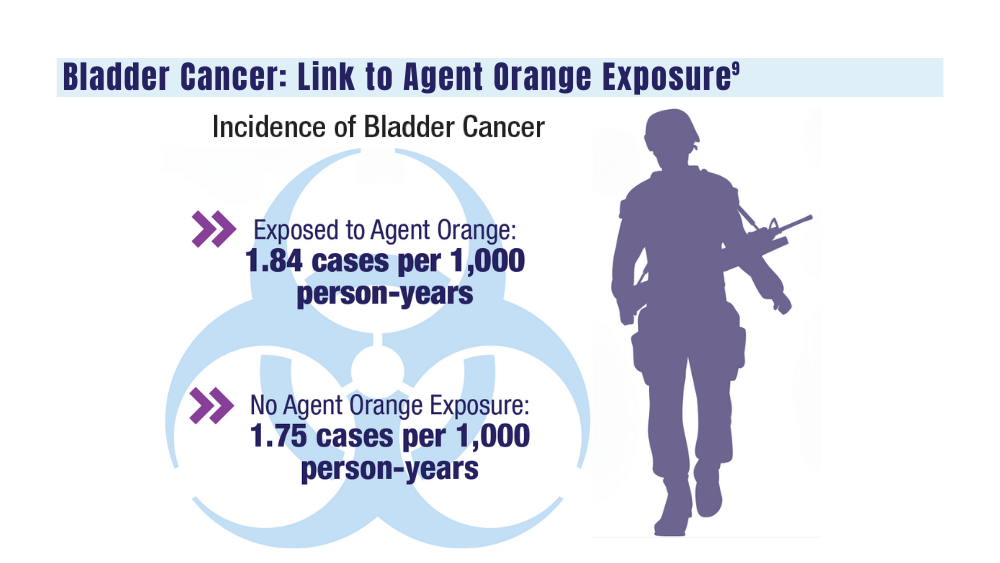
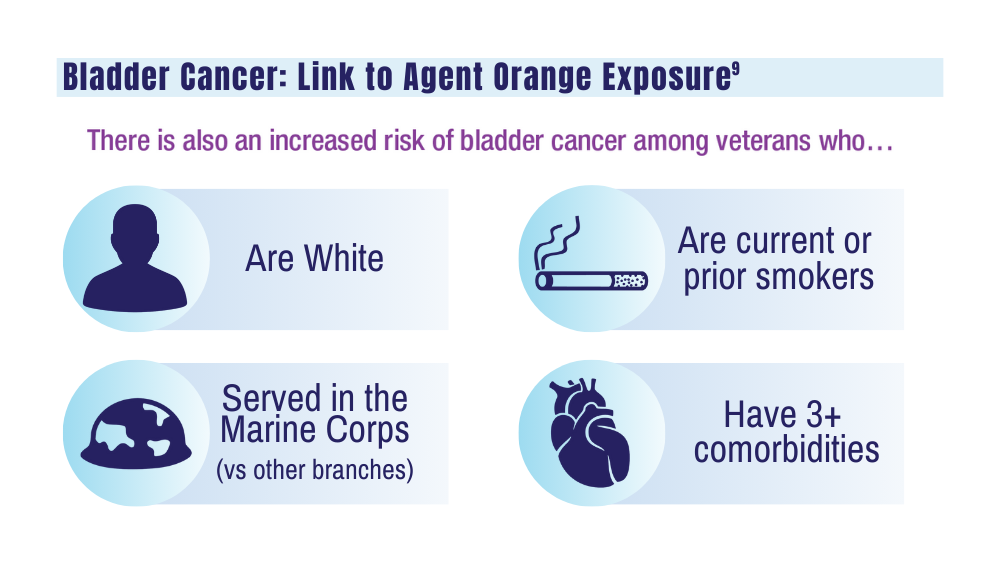
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
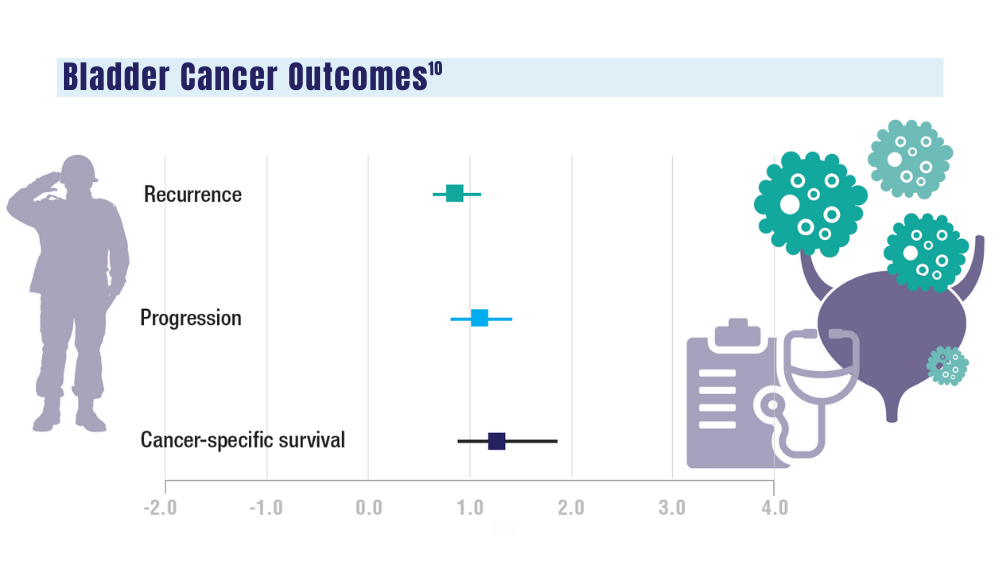
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
Higher Prostate Cancer Rates Seen in Black Men, but Advanced Cases Similar to White Men
There was a substantial difference in prostate cancer diagnosis across ethnic groups: 25% of Black men with a raised PSA were diagnosed with prostate cancer within 1 year of being tested, compared with 20% of White men and 13% of Asian men, in the analysis of a large primary care cohort in the United Kingdom.
Incidence of advanced prostate cancer for Asian men with a raised PSA result was 4.5%, compared with 7.5% for White men and 7.0% for Black men.
Men included in the study were aged 40 and older and had no prior cancer diagnosis. Their ethnicity and PSA test result were logged in a national dataset between 2010 and 2017.
The study of more than 730,000 men, published in BMC Medicine, didn’t explore reasons for the differences, but experts offer their thoughts on what led to the findings and what these results imply.
Why the Higher Diagnosis Rates but Not More Advanced Disease in Black Men?
Lead author Liz Down, a graduate research assistant at the University of Exeter, Exeter, England, suggests the higher diagnosis rates but not more advanced disease in Black men may be linked to genetic variations.
Her team’s studies have shown that Black men in the United Kingdom and United States have higher levels of PSA. The PSA value is used to identify patients who might benefit from specialist investigation, and current guidelines in the UK and US don’t distinguish between ethnic groups.
As most men have slow-growing prostate cancer, this may lead to a disproportionately higher number of Black men being diagnosed with prostate cancer, she said.
“One possible interpretation,” Ms. Down notes, “is that prostate cancer follows a similar trajectory in Black and White men. What is different, however, is that Black men have higher PSA levels.”
As to why the advanced-cancer incidence is similar in Black and White patients in the study, Daniel George, MD, director of genitourinary oncology at Duke Cancer Institute in Durham, North Carolina, says it’s important to understand that the Black men in this study “are not necessarily representative of the Black population at large.”
In this study, “they’re a little bit more healthcare inclined,” Dr. George notes. The study population is actively seeking the PSA test. Their socioeconomic profile might be closer to their White counterparts’, and that may make results more similar, he said.
“It’s possible that because this is a screening and not just men coming in for symptoms or cause, that we’re not seeing as much advanced disease,” he continued.
Amar Kishan, MD, chief of the genitourinary oncology service at University of California Los Angeles (UCLA) Health, says the genomic factors and environmental stressors that lead to elevated PSA counts don’t necessarily translate into aggressiveness of disease.
Why do Different Races have Different Prostate Cancer Risk?
Dr. George points out that the study also highlights that Asian men were significantly less likely to be diagnosed with prostate cancer within 1 year of the test.
The reasons for differences in prostate risk by race are complex, he notes. There are some clues that biologic factors may be at work. For instance, early puberty has a link to prostate cancer as it does to breast cancer, and height is also associated with a greater risk of prostate cancer, Dr. George said.
It’s not necessarily a racial association but there are some biological factors associated with prostate cancer later in life, he explained. “These may be enriched in certain populations, including northern Europeans and patients with African ancestries.”
The study also notes that Black men are more likely to die from prostate cancer than are White men, and Asian men are less likely than White or Black men to die from it.
Ms. Down said the difference in prostate cancer mortality between Black vs White men, in particular, may be related to a number of factors, and age, and lifetime risk of prostate cancer may play a major role, at least in the UK.
Should There Be Different ‘Normal’ PSA Levels for Different Races?
Dr. George says there is likely a need to change the system because a PSA level in one race may not signal the same risk it does in another. So medicine probably needs to standardize what a “normal” PSA is by race, he says, adding that he is a coauthor of an upcoming paper regarding that issue.
The lowest instances of prostate cancer were in Asian patients so this isn’t just a Black and White issue, Dr. George notes. “Being able to establish benchmarks by race and ethnicity is something that is probably needed in the field,” he says.
Dr. Kishan, on the other hand, says data from this study are not enough to support differentiating PSA levels based on race. He noted a limitation of the study is that it was not able to calculate the false-negative rate of PSA tests.
What are the Implications for Treating and Screening for Prostate Cancer
Dr. Kishan says there may be a role for increased intensity of screening, whether at an earlier age or with different intervals, but prostate cancer treatment should not differ by race.
“Our prior study, as well as others,” he says, “have shown that when you balance Black and White patients for every factor that might impact prognosis other than race — such as age, disease aggressiveness, etc. — Black men actually tend to have better outcomes than White men. Thus, it would mean potentially overtreating (i.e., causing unnecessary side effects) to increase treatment intensity purely based on race with the available data.”
According to the paper, prostate cancer incidence in men with higher PSA levels increases with increasing age, even when using age-adjusted thresholds.
Dr. George says we know from this study and other studies as well that Black men are more likely to be diagnosed with prostate at a younger age. “Therefore, we probably need to be thinking about screening Black men starting at a younger age. These are the men who are most likely to benefit from an intervention — patients who have life expectancies of 20 years or more.”
What are the Downsides to Overdiagnosing Prostate Cancer in Men?
“It’s one of the biggest concerns that men have in proactively seeking healthcare,” Dr. George says. “They’re more likely to undergo treatment for this disease if they’re getting screened because (clinicians are) more likely to find it.”
Some of those men, he says, are going to undergo treatment for disease that won’t ultimately kill them, but may cause complications the men shouldn’t have had at all or otherwise may have had later.
“Overtreatment is a real concern. That’s why active surveillance is so important to minimize overtreatment of patients by finding out which cancers are low risk for progression and which are becoming more aggressive,” Dr. George says.
Authors of the study write that “the potential for overdiagnosis and the subsequent psychological and physical impact of diagnosis and treatment is an important consideration.”
All authors of the new paper received financial support from Cancer Research UK, the National Institute for Health and Care Research (NIHR), and the Higgins family for the submitted work.
Dr. George reports no relevant financial relationships.
Dr. Kishan reports consulting fees and speaking honoraria from Varian Medical Systems, Janssen, and Boston Scientific; research funding from PointBioPharma, Lantheus, and Janssen; and serving on advisory boards for Lantheus, Janssen and Boston Scientific.
There was a substantial difference in prostate cancer diagnosis across ethnic groups: 25% of Black men with a raised PSA were diagnosed with prostate cancer within 1 year of being tested, compared with 20% of White men and 13% of Asian men, in the analysis of a large primary care cohort in the United Kingdom.
Incidence of advanced prostate cancer for Asian men with a raised PSA result was 4.5%, compared with 7.5% for White men and 7.0% for Black men.
Men included in the study were aged 40 and older and had no prior cancer diagnosis. Their ethnicity and PSA test result were logged in a national dataset between 2010 and 2017.
The study of more than 730,000 men, published in BMC Medicine, didn’t explore reasons for the differences, but experts offer their thoughts on what led to the findings and what these results imply.
Why the Higher Diagnosis Rates but Not More Advanced Disease in Black Men?
Lead author Liz Down, a graduate research assistant at the University of Exeter, Exeter, England, suggests the higher diagnosis rates but not more advanced disease in Black men may be linked to genetic variations.
Her team’s studies have shown that Black men in the United Kingdom and United States have higher levels of PSA. The PSA value is used to identify patients who might benefit from specialist investigation, and current guidelines in the UK and US don’t distinguish between ethnic groups.
As most men have slow-growing prostate cancer, this may lead to a disproportionately higher number of Black men being diagnosed with prostate cancer, she said.
“One possible interpretation,” Ms. Down notes, “is that prostate cancer follows a similar trajectory in Black and White men. What is different, however, is that Black men have higher PSA levels.”
As to why the advanced-cancer incidence is similar in Black and White patients in the study, Daniel George, MD, director of genitourinary oncology at Duke Cancer Institute in Durham, North Carolina, says it’s important to understand that the Black men in this study “are not necessarily representative of the Black population at large.”
In this study, “they’re a little bit more healthcare inclined,” Dr. George notes. The study population is actively seeking the PSA test. Their socioeconomic profile might be closer to their White counterparts’, and that may make results more similar, he said.
“It’s possible that because this is a screening and not just men coming in for symptoms or cause, that we’re not seeing as much advanced disease,” he continued.
Amar Kishan, MD, chief of the genitourinary oncology service at University of California Los Angeles (UCLA) Health, says the genomic factors and environmental stressors that lead to elevated PSA counts don’t necessarily translate into aggressiveness of disease.
Why do Different Races have Different Prostate Cancer Risk?
Dr. George points out that the study also highlights that Asian men were significantly less likely to be diagnosed with prostate cancer within 1 year of the test.
The reasons for differences in prostate risk by race are complex, he notes. There are some clues that biologic factors may be at work. For instance, early puberty has a link to prostate cancer as it does to breast cancer, and height is also associated with a greater risk of prostate cancer, Dr. George said.
It’s not necessarily a racial association but there are some biological factors associated with prostate cancer later in life, he explained. “These may be enriched in certain populations, including northern Europeans and patients with African ancestries.”
The study also notes that Black men are more likely to die from prostate cancer than are White men, and Asian men are less likely than White or Black men to die from it.
Ms. Down said the difference in prostate cancer mortality between Black vs White men, in particular, may be related to a number of factors, and age, and lifetime risk of prostate cancer may play a major role, at least in the UK.
Should There Be Different ‘Normal’ PSA Levels for Different Races?
Dr. George says there is likely a need to change the system because a PSA level in one race may not signal the same risk it does in another. So medicine probably needs to standardize what a “normal” PSA is by race, he says, adding that he is a coauthor of an upcoming paper regarding that issue.
The lowest instances of prostate cancer were in Asian patients so this isn’t just a Black and White issue, Dr. George notes. “Being able to establish benchmarks by race and ethnicity is something that is probably needed in the field,” he says.
Dr. Kishan, on the other hand, says data from this study are not enough to support differentiating PSA levels based on race. He noted a limitation of the study is that it was not able to calculate the false-negative rate of PSA tests.
What are the Implications for Treating and Screening for Prostate Cancer
Dr. Kishan says there may be a role for increased intensity of screening, whether at an earlier age or with different intervals, but prostate cancer treatment should not differ by race.
“Our prior study, as well as others,” he says, “have shown that when you balance Black and White patients for every factor that might impact prognosis other than race — such as age, disease aggressiveness, etc. — Black men actually tend to have better outcomes than White men. Thus, it would mean potentially overtreating (i.e., causing unnecessary side effects) to increase treatment intensity purely based on race with the available data.”
According to the paper, prostate cancer incidence in men with higher PSA levels increases with increasing age, even when using age-adjusted thresholds.
Dr. George says we know from this study and other studies as well that Black men are more likely to be diagnosed with prostate at a younger age. “Therefore, we probably need to be thinking about screening Black men starting at a younger age. These are the men who are most likely to benefit from an intervention — patients who have life expectancies of 20 years or more.”
What are the Downsides to Overdiagnosing Prostate Cancer in Men?
“It’s one of the biggest concerns that men have in proactively seeking healthcare,” Dr. George says. “They’re more likely to undergo treatment for this disease if they’re getting screened because (clinicians are) more likely to find it.”
Some of those men, he says, are going to undergo treatment for disease that won’t ultimately kill them, but may cause complications the men shouldn’t have had at all or otherwise may have had later.
“Overtreatment is a real concern. That’s why active surveillance is so important to minimize overtreatment of patients by finding out which cancers are low risk for progression and which are becoming more aggressive,” Dr. George says.
Authors of the study write that “the potential for overdiagnosis and the subsequent psychological and physical impact of diagnosis and treatment is an important consideration.”
All authors of the new paper received financial support from Cancer Research UK, the National Institute for Health and Care Research (NIHR), and the Higgins family for the submitted work.
Dr. George reports no relevant financial relationships.
Dr. Kishan reports consulting fees and speaking honoraria from Varian Medical Systems, Janssen, and Boston Scientific; research funding from PointBioPharma, Lantheus, and Janssen; and serving on advisory boards for Lantheus, Janssen and Boston Scientific.
There was a substantial difference in prostate cancer diagnosis across ethnic groups: 25% of Black men with a raised PSA were diagnosed with prostate cancer within 1 year of being tested, compared with 20% of White men and 13% of Asian men, in the analysis of a large primary care cohort in the United Kingdom.
Incidence of advanced prostate cancer for Asian men with a raised PSA result was 4.5%, compared with 7.5% for White men and 7.0% for Black men.
Men included in the study were aged 40 and older and had no prior cancer diagnosis. Their ethnicity and PSA test result were logged in a national dataset between 2010 and 2017.
The study of more than 730,000 men, published in BMC Medicine, didn’t explore reasons for the differences, but experts offer their thoughts on what led to the findings and what these results imply.
Why the Higher Diagnosis Rates but Not More Advanced Disease in Black Men?
Lead author Liz Down, a graduate research assistant at the University of Exeter, Exeter, England, suggests the higher diagnosis rates but not more advanced disease in Black men may be linked to genetic variations.
Her team’s studies have shown that Black men in the United Kingdom and United States have higher levels of PSA. The PSA value is used to identify patients who might benefit from specialist investigation, and current guidelines in the UK and US don’t distinguish between ethnic groups.
As most men have slow-growing prostate cancer, this may lead to a disproportionately higher number of Black men being diagnosed with prostate cancer, she said.
“One possible interpretation,” Ms. Down notes, “is that prostate cancer follows a similar trajectory in Black and White men. What is different, however, is that Black men have higher PSA levels.”
As to why the advanced-cancer incidence is similar in Black and White patients in the study, Daniel George, MD, director of genitourinary oncology at Duke Cancer Institute in Durham, North Carolina, says it’s important to understand that the Black men in this study “are not necessarily representative of the Black population at large.”
In this study, “they’re a little bit more healthcare inclined,” Dr. George notes. The study population is actively seeking the PSA test. Their socioeconomic profile might be closer to their White counterparts’, and that may make results more similar, he said.
“It’s possible that because this is a screening and not just men coming in for symptoms or cause, that we’re not seeing as much advanced disease,” he continued.
Amar Kishan, MD, chief of the genitourinary oncology service at University of California Los Angeles (UCLA) Health, says the genomic factors and environmental stressors that lead to elevated PSA counts don’t necessarily translate into aggressiveness of disease.
Why do Different Races have Different Prostate Cancer Risk?
Dr. George points out that the study also highlights that Asian men were significantly less likely to be diagnosed with prostate cancer within 1 year of the test.
The reasons for differences in prostate risk by race are complex, he notes. There are some clues that biologic factors may be at work. For instance, early puberty has a link to prostate cancer as it does to breast cancer, and height is also associated with a greater risk of prostate cancer, Dr. George said.
It’s not necessarily a racial association but there are some biological factors associated with prostate cancer later in life, he explained. “These may be enriched in certain populations, including northern Europeans and patients with African ancestries.”
The study also notes that Black men are more likely to die from prostate cancer than are White men, and Asian men are less likely than White or Black men to die from it.
Ms. Down said the difference in prostate cancer mortality between Black vs White men, in particular, may be related to a number of factors, and age, and lifetime risk of prostate cancer may play a major role, at least in the UK.
Should There Be Different ‘Normal’ PSA Levels for Different Races?
Dr. George says there is likely a need to change the system because a PSA level in one race may not signal the same risk it does in another. So medicine probably needs to standardize what a “normal” PSA is by race, he says, adding that he is a coauthor of an upcoming paper regarding that issue.
The lowest instances of prostate cancer were in Asian patients so this isn’t just a Black and White issue, Dr. George notes. “Being able to establish benchmarks by race and ethnicity is something that is probably needed in the field,” he says.
Dr. Kishan, on the other hand, says data from this study are not enough to support differentiating PSA levels based on race. He noted a limitation of the study is that it was not able to calculate the false-negative rate of PSA tests.
What are the Implications for Treating and Screening for Prostate Cancer
Dr. Kishan says there may be a role for increased intensity of screening, whether at an earlier age or with different intervals, but prostate cancer treatment should not differ by race.
“Our prior study, as well as others,” he says, “have shown that when you balance Black and White patients for every factor that might impact prognosis other than race — such as age, disease aggressiveness, etc. — Black men actually tend to have better outcomes than White men. Thus, it would mean potentially overtreating (i.e., causing unnecessary side effects) to increase treatment intensity purely based on race with the available data.”
According to the paper, prostate cancer incidence in men with higher PSA levels increases with increasing age, even when using age-adjusted thresholds.
Dr. George says we know from this study and other studies as well that Black men are more likely to be diagnosed with prostate at a younger age. “Therefore, we probably need to be thinking about screening Black men starting at a younger age. These are the men who are most likely to benefit from an intervention — patients who have life expectancies of 20 years or more.”
What are the Downsides to Overdiagnosing Prostate Cancer in Men?
“It’s one of the biggest concerns that men have in proactively seeking healthcare,” Dr. George says. “They’re more likely to undergo treatment for this disease if they’re getting screened because (clinicians are) more likely to find it.”
Some of those men, he says, are going to undergo treatment for disease that won’t ultimately kill them, but may cause complications the men shouldn’t have had at all or otherwise may have had later.
“Overtreatment is a real concern. That’s why active surveillance is so important to minimize overtreatment of patients by finding out which cancers are low risk for progression and which are becoming more aggressive,” Dr. George says.
Authors of the study write that “the potential for overdiagnosis and the subsequent psychological and physical impact of diagnosis and treatment is an important consideration.”
All authors of the new paper received financial support from Cancer Research UK, the National Institute for Health and Care Research (NIHR), and the Higgins family for the submitted work.
Dr. George reports no relevant financial relationships.
Dr. Kishan reports consulting fees and speaking honoraria from Varian Medical Systems, Janssen, and Boston Scientific; research funding from PointBioPharma, Lantheus, and Janssen; and serving on advisory boards for Lantheus, Janssen and Boston Scientific.
FROM BMC MEDICINE
Outside the Guidelines: Denosumab Overuse in Prostate Cancer
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Is MRI Screening Unnecessarily High in Prostate Cancer?
TOPLINE:
METHODOLOGY:
- New initiatives are focusing on organizing prostate cancer screening using MRI to reduce overdiagnosis, as current evidence does not support the effectiveness of a single PSA test, with guidelines now recommending repeated testing every 1-4 years.
- In the STHLM3-MRI trial, men, aged 50-74 years, living in Stockholm County, Sweden, were invited to participate in prostate cancer screening and randomly assigned to traditional screening with systematic or an MRI-based strategy.
- Blood samples were analyzed for PSA levels and Stockholm3 risk score; men with elevated risk underwent targeted MRI and biopsy procedures.
- In this follow-up analysis, 2,078 men with PSA levels of 1.5 ng/mL or higher and a Stockholm3 risk score less than 0.11 were re-invited for screening 2-3 years after their initial screening.
- The primary outcome was clinically significant prostate cancer (Gleason score of 3 + 4 or greater). A Gleason score of 6 was detected in 0.7% of patients, and a score of 4 + 3 or greater was detected in 19 (1.3%) men.
TAKEAWAY:
- Of 1,500 men (median age of 67 years) who underwent a blood test, the median PSA level was 2.8 ng/mL and 26.0% changed risk classification groups (PSA levels < 3 vs 3 ng/mL).
- Out of 667 men with PSA levels of 3 ng/mL or higher, 617 (92.5%) had an MRI. Of the 617, 51 (7.6%) had equivocal lesions (a Prostate Imaging-Reporting and Data System score of 3) and 33 (4.9%) had suspicious lesions.
- Of the 1,500 rescreened men, clinically significant prostate cancer was detected in 48 men (3.2%); this corresponds to 59.2% of the biopsied men.
- Out of 383 men who had previously received a negative MRI result, only 10 (2.6%) exhibited a lesion with a Prostate Imaging-Reporting and Data System score of 4 or higher.
IN PRACTICE:
In an accompanying editorial, Ola Bratt, MD, PhD, noted that the “most important finding was the very high proportion of nonsuspicious repeat MRI scans,” but also emphasizes the necessity of observing a decrease in overall prostate cancer incidence before asserting that the current cancer diagnostics effectively reduce overdiagnosis.
SOURCE:
This study, led by Tobias Nordström, MD, PhD, from Karolinska Institute, Stockholm, Sweden, was published on February 7, 2024, in JAMA Network Open.
LIMITATIONS:
Long-term outcomes like prostate cancer mortality were not evaluated. Information on cancer detection in men with a negative MRI result at rescreening was not available. Authors noted that a subset of individuals may still be at risk despite lower PSA levels.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare, Karolinska Institute, Prostatacancerförbundet, Region Stockholm, and Åke Wibergs Stiftelse. The authors reported financial relationships outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- New initiatives are focusing on organizing prostate cancer screening using MRI to reduce overdiagnosis, as current evidence does not support the effectiveness of a single PSA test, with guidelines now recommending repeated testing every 1-4 years.
- In the STHLM3-MRI trial, men, aged 50-74 years, living in Stockholm County, Sweden, were invited to participate in prostate cancer screening and randomly assigned to traditional screening with systematic or an MRI-based strategy.
- Blood samples were analyzed for PSA levels and Stockholm3 risk score; men with elevated risk underwent targeted MRI and biopsy procedures.
- In this follow-up analysis, 2,078 men with PSA levels of 1.5 ng/mL or higher and a Stockholm3 risk score less than 0.11 were re-invited for screening 2-3 years after their initial screening.
- The primary outcome was clinically significant prostate cancer (Gleason score of 3 + 4 or greater). A Gleason score of 6 was detected in 0.7% of patients, and a score of 4 + 3 or greater was detected in 19 (1.3%) men.
TAKEAWAY:
- Of 1,500 men (median age of 67 years) who underwent a blood test, the median PSA level was 2.8 ng/mL and 26.0% changed risk classification groups (PSA levels < 3 vs 3 ng/mL).
- Out of 667 men with PSA levels of 3 ng/mL or higher, 617 (92.5%) had an MRI. Of the 617, 51 (7.6%) had equivocal lesions (a Prostate Imaging-Reporting and Data System score of 3) and 33 (4.9%) had suspicious lesions.
- Of the 1,500 rescreened men, clinically significant prostate cancer was detected in 48 men (3.2%); this corresponds to 59.2% of the biopsied men.
- Out of 383 men who had previously received a negative MRI result, only 10 (2.6%) exhibited a lesion with a Prostate Imaging-Reporting and Data System score of 4 or higher.
IN PRACTICE:
In an accompanying editorial, Ola Bratt, MD, PhD, noted that the “most important finding was the very high proportion of nonsuspicious repeat MRI scans,” but also emphasizes the necessity of observing a decrease in overall prostate cancer incidence before asserting that the current cancer diagnostics effectively reduce overdiagnosis.
SOURCE:
This study, led by Tobias Nordström, MD, PhD, from Karolinska Institute, Stockholm, Sweden, was published on February 7, 2024, in JAMA Network Open.
LIMITATIONS:
Long-term outcomes like prostate cancer mortality were not evaluated. Information on cancer detection in men with a negative MRI result at rescreening was not available. Authors noted that a subset of individuals may still be at risk despite lower PSA levels.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare, Karolinska Institute, Prostatacancerförbundet, Region Stockholm, and Åke Wibergs Stiftelse. The authors reported financial relationships outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- New initiatives are focusing on organizing prostate cancer screening using MRI to reduce overdiagnosis, as current evidence does not support the effectiveness of a single PSA test, with guidelines now recommending repeated testing every 1-4 years.
- In the STHLM3-MRI trial, men, aged 50-74 years, living in Stockholm County, Sweden, were invited to participate in prostate cancer screening and randomly assigned to traditional screening with systematic or an MRI-based strategy.
- Blood samples were analyzed for PSA levels and Stockholm3 risk score; men with elevated risk underwent targeted MRI and biopsy procedures.
- In this follow-up analysis, 2,078 men with PSA levels of 1.5 ng/mL or higher and a Stockholm3 risk score less than 0.11 were re-invited for screening 2-3 years after their initial screening.
- The primary outcome was clinically significant prostate cancer (Gleason score of 3 + 4 or greater). A Gleason score of 6 was detected in 0.7% of patients, and a score of 4 + 3 or greater was detected in 19 (1.3%) men.
TAKEAWAY:
- Of 1,500 men (median age of 67 years) who underwent a blood test, the median PSA level was 2.8 ng/mL and 26.0% changed risk classification groups (PSA levels < 3 vs 3 ng/mL).
- Out of 667 men with PSA levels of 3 ng/mL or higher, 617 (92.5%) had an MRI. Of the 617, 51 (7.6%) had equivocal lesions (a Prostate Imaging-Reporting and Data System score of 3) and 33 (4.9%) had suspicious lesions.
- Of the 1,500 rescreened men, clinically significant prostate cancer was detected in 48 men (3.2%); this corresponds to 59.2% of the biopsied men.
- Out of 383 men who had previously received a negative MRI result, only 10 (2.6%) exhibited a lesion with a Prostate Imaging-Reporting and Data System score of 4 or higher.
IN PRACTICE:
In an accompanying editorial, Ola Bratt, MD, PhD, noted that the “most important finding was the very high proportion of nonsuspicious repeat MRI scans,” but also emphasizes the necessity of observing a decrease in overall prostate cancer incidence before asserting that the current cancer diagnostics effectively reduce overdiagnosis.
SOURCE:
This study, led by Tobias Nordström, MD, PhD, from Karolinska Institute, Stockholm, Sweden, was published on February 7, 2024, in JAMA Network Open.
LIMITATIONS:
Long-term outcomes like prostate cancer mortality were not evaluated. Information on cancer detection in men with a negative MRI result at rescreening was not available. Authors noted that a subset of individuals may still be at risk despite lower PSA levels.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare, Karolinska Institute, Prostatacancerförbundet, Region Stockholm, and Åke Wibergs Stiftelse. The authors reported financial relationships outside this work.
A version of this article appeared on Medscape.com.
Focal Therapy for Prostate Cancer: Evidence-Based or Oversold?
In 2013, a prostate-specific antigen (PSA) blood test revealed that Richard LaFrate’s levels had jumped.
Previously in a normal range, his PSA was now above 6 ng/mL, indicating an elevated likelihood for prostate cancer. The jazz guitarist from Leesburg, Florida, then 70 years old, underwent a biopsy, which found two Gleason 6 lesions.
Mr. LaFrate had low-risk prostate cancer.
Guidelines now recommend active surveillance for patients like Mr. LaFrate, who have low-risk disease. This strategy would mean monitoring the cancer until LaFrate required treatment, with the upside being he might never need therapy.
Mr. LaFrate’s urologist, however, was pushing whole gland surgery — an invasive and unnecessary procedure given his diagnosis and age.
Mr. LaFrate decided to look for another doctor. He filled out a form online that pointed him to a new urologist who offered him one option: An investigational procedure known as high-intensity focused ultrasound.
At the time, high-intensity focused ultrasound — a form of focal therapy — was being studied in the United States to treat men with low or intermediate-risk prostate cancer, but it was still relatively early days.
Mr. LaFrate’s urologist asked him to pay $25,000 out of pocket to undergo the focal procedure at a clinic in the Bahamas. He refused and, ultimately, landed on active surveillance as the best strategy to manage for his low-risk disease.
That urologist was “a shyster in my opinion,” Mr. LaFrate said.
— Gleason 3+4 (grade group 2) tumors — as an alternative to invasive surgery and active surveillance. Prestigious medical centers, such as Cleveland Clinic, Mayo Clinic, Memorial Sloan Kettering, UCLA, and the University of Chicago, routinely offer focal therapy.
But use of the techniques remains controversial and costly.
As the Cleveland Clinic’s website acknowledges, although “the use of focal therapy for localized prostate cancer appears to be a promising development in a number of ways, it is still considered investigational and not yet part of standard therapy.” Major caveats to focal therapy include unknown long-term effectiveness, the possibility of leaving behind untreated cancer, and higher overall costs.
No major national guidelines endorse the use of focal therapy, unless offered in a research or clinical trial setting. Insurance companies, such as Aetna, Blue Cross Blue Shield, and United, also consider focal therapy for prostate cancer investigational and don’t cover it.
Without a stamp of approval from guideline bodies and insurance companies, patients, like Mr. LaFrate, remain vulnerable to the high out-of-pocket costs for these focal techniques.
“Almost every place charges $15,000-$30,000 in cash,” said Daniel Spratt, MD, radiation oncology chair at University Hospitals Seidman Cancer Center and Case Western Reserve University in Cleveland.
Dr. Spratt has seen hundreds of patients after focal therapy, some from prominent centers, who have emptied their bank accounts to undergo treatment with the promise of great results and ultimately felt misled when the cancer has recurred.
“It pains me that there are doctors willing to ignore the Hippocratic oath of ‘Do No Harm’ simply to jump on this fad to bring in revenue,” Dr. Spratt said.
Evidence-Based or Oversold?
Focal therapy gained a foothold in the United Kingdom well before the United States.
Hashim Ahmed, FRCS, urology chair at Imperial College London, has used focal therapy for 15 years, treated over 1000 patients, and taught dozens of surgeons how to use the leading focal therapies — focal cryoablation, in which surgeons use a needle-thin probe to target, freeze, and kill prostate tumors, as well as high-intensity focused ultrasound, which uses sound wave energy to superheat and kill tumors.
“Certainly, in the United Kingdom, focal therapy has been prime time in a number of centers for a number of years,” Dr. Ahmed said.
In the United States, focal therapy has become an attractive option for men with prostate cancer who want to avoid radiation or radical prostatectomy but don’t feel comfortable simply monitoring their disease with active surveillance. Experts from specialized focal therapy centers touting the promise of this “innovative technique” predict its routine use in the next few years.
But the excitement surrounding the use of focal therapy in prostate cancer has outpaced broader acceptance.
In 2015, the FDA approved high-intensity focused ultrasound to treat prostatic disease, but not prostate cancer specifically. Although the approval language “means that companies cannot advertise that their devices can be used for prostate cancer,” physicians can still determine how to use the technology, which includes treating prostate cancer, Dr. Ahmed said.
The evidence is starting to catch up to the demand. The latest research suggests that the partial-gland techniques may stand up well to radical prostatectomy.
A 2022 prospective database study comparing radical prostatectomies to focal therapy — mostly high-intensity focused ultrasound — in more than 800 men found similar rates of failure-free survival in the two groups at the 8-year follow-up. A 2019 registry study found that failure-free survival at 3 years was just over 90% in high and intermediate-risk patients receiving focal cryotherapy, with the rate rising to about 93% for the intermediate-risk group. And a 2018 prospective study of 625 patients with intermediate or high-risk prostate cancer who underwent high-intensity focused ultrasound had 5-year metastasis-free survival of 98% and overall survival rates of 100%.
One of the biggest draws of focal therapy vs more aggressive treatments is the “massive differences in side-effect profiles,” said Dr. Ahmed.
In a 2021 meta-analysis, researchers found that 6 months after high-intensity focused ultrasound, 98% of patients remained continent and 80% retained erectile function, while erectile dysfunction can occur in 30% to as many as 85% of patients following prostatectomy or radiotherapy and urinary incontinence can occur in as many as 40% of patients.
Despite these potential advantages of focal therapy, the long-term efficacy of the techniques remains uncertain.
A recent study from a team at MSK, for instance, reported that 40% of men with intermediate (grade 2) or high-risk (grade 3) disease had residual cancer following MRI-guided focused ultrasound. A 2020 prospective registry study found that almost 20% of patients undergoing high-intensity focal ultrasound required a second round following a recurrence.
Dr. Spratt worries that patients who recur after focal therapy may go on to receive a second round — often offered at half price — and will still ultimately need surgery or radiation therapy later. By that point, however, patients may have spent as much as $45,000 — ie, $30,000 on the initial and another $15,000 on the follow-up procedure.
When patients see Dr. Spratt after a recurrence, he informs them that their side effects will be worse if he gives them radiation or surgery now vs if he had given them curative therapy upfront. “But this is what we’re left with,” he tells them.
Another big concern in the field is “the quality of data for focal therapy is overwhelmingly poor,” said Jonathan Shoag, MD, a urologic oncologist at University Hospitals and an associate professor of urology at Case Western Reserve University School of Medicine in Cleveland. “Essentially, the bulk of the data is from single-institution retrospective series without defined follow-up protocols or endpoints.”
The American Urological Association (AUA) has even cautioned experts and patients about the lack of high-quality data comparing focal therapy techniques to radiation therapy, surgery, and active surveillance. According to the AUA, focal options should only be considered in intermediate-risk prostate cancer in a clinical trial setting.
“The lack of randomized clinical trials poses a major stumbling block for the field,” said Dr. Ahmed.
Although randomized trials would be ideal, the results would take many years to mature, and growing patient demand for these less invasive focal procedures has made randomized trials difficult to complete, explained Arvin George, MD, associate professor at Johns Hopkins School of Medicine in Baltimore. Several randomized trials attempted in Norway and the United Kingdom, for instance, fell apart when patients refused to be randomized between focal and radical therapy, Dr. George said.
Focal therapy is now in the same position that active surveillance was a few years ago, according to Dr. George.
“We are hearing the same concerns about focal therapy now as we did about active surveillance,” he said. The initial evidence supporting active surveillance largely came from real-world experience and retrospective studies. The randomized data came later, and skeptics of active surveillance “were proven wrong,” he added.
But Dr. Shoag has a different take on the trajectory of focal therapy research and care in the United States.
“I think there’s this emerging kind of tragedy happening in our field now, where you have even academic institutions offering focal therapy to patients off-trial with essentially no data to suggest it is oncologically effective,” Dr. Shoag said.
William Catalona, MD, Northwestern University Feinberg School of Medicine, Chicago, agreed, noting that too many low-risk patients are undergoing focal treatment who should be on active surveillance. “Many men are attracted to focal because they just are uncomfortable having a cancer in their body that’s not treated,” Dr. Catalona said. But “giving these patients focal therapy is really overtreatment.”
Patients with higher-risk disease who want to avoid aggressive treatment are also being lured into focal without guidelines or clear evidence to back up that option, Dr. Catalona explained.
Although it’s not clear how many men in the United States are receiving focal therapy who shouldn’t, even proponents of focal therapy, like George, have expressed concern.
Dr. George agreed that focal therapy marketing geared towards patients is drawing in some men who are not good candidates for these techniques, and feels there’s not enough objective material from medical societies or academic centers giving patients a realistic picture of focal therapy.
“There is concern that patients may be receiving biased information,” Dr. George said, adding that it’s ultimately up to the physician to reconcile the best available evidence, understand the outcomes, and discuss these options with the patient to guide them to what’s best.
At the end of the day, Dr. Spratt said, physicians giving focal therapy off a clinical trial need to pause and ask themselves “why are they giving a treatment that remains investigational by payers, not recommended by any major guideline, and that lacks any randomized evidence?”
Mr. LaFrate does not regret his decision to forgo focal therapy in 2013. He has been on active surveillance for about a decade now.
Following an MRI in 2022, Mr. LaFrate’s radiology report found that “clinically significant cancer is very unlikely to be present.”
Still, his PSA has risen two points in the past year to 14. His current urologist feels that the PSA is going up because there’s cancer present and is suggesting focal therapy for Mr. LaFrate.
Mr. LaFrate, who has prostate enlargement issues, remains skeptical of focal therapy and is still resisting the sales pitch.
“My doctor is not aggressively pushing it. He’s just giving me that as one of my options,” he said. “I just have a hunch I don’t need it at this point.”
A version of this article appeared on Medscape.com.
In 2013, a prostate-specific antigen (PSA) blood test revealed that Richard LaFrate’s levels had jumped.
Previously in a normal range, his PSA was now above 6 ng/mL, indicating an elevated likelihood for prostate cancer. The jazz guitarist from Leesburg, Florida, then 70 years old, underwent a biopsy, which found two Gleason 6 lesions.
Mr. LaFrate had low-risk prostate cancer.
Guidelines now recommend active surveillance for patients like Mr. LaFrate, who have low-risk disease. This strategy would mean monitoring the cancer until LaFrate required treatment, with the upside being he might never need therapy.
Mr. LaFrate’s urologist, however, was pushing whole gland surgery — an invasive and unnecessary procedure given his diagnosis and age.
Mr. LaFrate decided to look for another doctor. He filled out a form online that pointed him to a new urologist who offered him one option: An investigational procedure known as high-intensity focused ultrasound.
At the time, high-intensity focused ultrasound — a form of focal therapy — was being studied in the United States to treat men with low or intermediate-risk prostate cancer, but it was still relatively early days.
Mr. LaFrate’s urologist asked him to pay $25,000 out of pocket to undergo the focal procedure at a clinic in the Bahamas. He refused and, ultimately, landed on active surveillance as the best strategy to manage for his low-risk disease.
That urologist was “a shyster in my opinion,” Mr. LaFrate said.
— Gleason 3+4 (grade group 2) tumors — as an alternative to invasive surgery and active surveillance. Prestigious medical centers, such as Cleveland Clinic, Mayo Clinic, Memorial Sloan Kettering, UCLA, and the University of Chicago, routinely offer focal therapy.
But use of the techniques remains controversial and costly.
As the Cleveland Clinic’s website acknowledges, although “the use of focal therapy for localized prostate cancer appears to be a promising development in a number of ways, it is still considered investigational and not yet part of standard therapy.” Major caveats to focal therapy include unknown long-term effectiveness, the possibility of leaving behind untreated cancer, and higher overall costs.
No major national guidelines endorse the use of focal therapy, unless offered in a research or clinical trial setting. Insurance companies, such as Aetna, Blue Cross Blue Shield, and United, also consider focal therapy for prostate cancer investigational and don’t cover it.
Without a stamp of approval from guideline bodies and insurance companies, patients, like Mr. LaFrate, remain vulnerable to the high out-of-pocket costs for these focal techniques.
“Almost every place charges $15,000-$30,000 in cash,” said Daniel Spratt, MD, radiation oncology chair at University Hospitals Seidman Cancer Center and Case Western Reserve University in Cleveland.
Dr. Spratt has seen hundreds of patients after focal therapy, some from prominent centers, who have emptied their bank accounts to undergo treatment with the promise of great results and ultimately felt misled when the cancer has recurred.
“It pains me that there are doctors willing to ignore the Hippocratic oath of ‘Do No Harm’ simply to jump on this fad to bring in revenue,” Dr. Spratt said.
Evidence-Based or Oversold?
Focal therapy gained a foothold in the United Kingdom well before the United States.
Hashim Ahmed, FRCS, urology chair at Imperial College London, has used focal therapy for 15 years, treated over 1000 patients, and taught dozens of surgeons how to use the leading focal therapies — focal cryoablation, in which surgeons use a needle-thin probe to target, freeze, and kill prostate tumors, as well as high-intensity focused ultrasound, which uses sound wave energy to superheat and kill tumors.
“Certainly, in the United Kingdom, focal therapy has been prime time in a number of centers for a number of years,” Dr. Ahmed said.
In the United States, focal therapy has become an attractive option for men with prostate cancer who want to avoid radiation or radical prostatectomy but don’t feel comfortable simply monitoring their disease with active surveillance. Experts from specialized focal therapy centers touting the promise of this “innovative technique” predict its routine use in the next few years.
But the excitement surrounding the use of focal therapy in prostate cancer has outpaced broader acceptance.
In 2015, the FDA approved high-intensity focused ultrasound to treat prostatic disease, but not prostate cancer specifically. Although the approval language “means that companies cannot advertise that their devices can be used for prostate cancer,” physicians can still determine how to use the technology, which includes treating prostate cancer, Dr. Ahmed said.
The evidence is starting to catch up to the demand. The latest research suggests that the partial-gland techniques may stand up well to radical prostatectomy.
A 2022 prospective database study comparing radical prostatectomies to focal therapy — mostly high-intensity focused ultrasound — in more than 800 men found similar rates of failure-free survival in the two groups at the 8-year follow-up. A 2019 registry study found that failure-free survival at 3 years was just over 90% in high and intermediate-risk patients receiving focal cryotherapy, with the rate rising to about 93% for the intermediate-risk group. And a 2018 prospective study of 625 patients with intermediate or high-risk prostate cancer who underwent high-intensity focused ultrasound had 5-year metastasis-free survival of 98% and overall survival rates of 100%.
One of the biggest draws of focal therapy vs more aggressive treatments is the “massive differences in side-effect profiles,” said Dr. Ahmed.
In a 2021 meta-analysis, researchers found that 6 months after high-intensity focused ultrasound, 98% of patients remained continent and 80% retained erectile function, while erectile dysfunction can occur in 30% to as many as 85% of patients following prostatectomy or radiotherapy and urinary incontinence can occur in as many as 40% of patients.
Despite these potential advantages of focal therapy, the long-term efficacy of the techniques remains uncertain.
A recent study from a team at MSK, for instance, reported that 40% of men with intermediate (grade 2) or high-risk (grade 3) disease had residual cancer following MRI-guided focused ultrasound. A 2020 prospective registry study found that almost 20% of patients undergoing high-intensity focal ultrasound required a second round following a recurrence.
Dr. Spratt worries that patients who recur after focal therapy may go on to receive a second round — often offered at half price — and will still ultimately need surgery or radiation therapy later. By that point, however, patients may have spent as much as $45,000 — ie, $30,000 on the initial and another $15,000 on the follow-up procedure.
When patients see Dr. Spratt after a recurrence, he informs them that their side effects will be worse if he gives them radiation or surgery now vs if he had given them curative therapy upfront. “But this is what we’re left with,” he tells them.
Another big concern in the field is “the quality of data for focal therapy is overwhelmingly poor,” said Jonathan Shoag, MD, a urologic oncologist at University Hospitals and an associate professor of urology at Case Western Reserve University School of Medicine in Cleveland. “Essentially, the bulk of the data is from single-institution retrospective series without defined follow-up protocols or endpoints.”
The American Urological Association (AUA) has even cautioned experts and patients about the lack of high-quality data comparing focal therapy techniques to radiation therapy, surgery, and active surveillance. According to the AUA, focal options should only be considered in intermediate-risk prostate cancer in a clinical trial setting.
“The lack of randomized clinical trials poses a major stumbling block for the field,” said Dr. Ahmed.
Although randomized trials would be ideal, the results would take many years to mature, and growing patient demand for these less invasive focal procedures has made randomized trials difficult to complete, explained Arvin George, MD, associate professor at Johns Hopkins School of Medicine in Baltimore. Several randomized trials attempted in Norway and the United Kingdom, for instance, fell apart when patients refused to be randomized between focal and radical therapy, Dr. George said.
Focal therapy is now in the same position that active surveillance was a few years ago, according to Dr. George.
“We are hearing the same concerns about focal therapy now as we did about active surveillance,” he said. The initial evidence supporting active surveillance largely came from real-world experience and retrospective studies. The randomized data came later, and skeptics of active surveillance “were proven wrong,” he added.
But Dr. Shoag has a different take on the trajectory of focal therapy research and care in the United States.
“I think there’s this emerging kind of tragedy happening in our field now, where you have even academic institutions offering focal therapy to patients off-trial with essentially no data to suggest it is oncologically effective,” Dr. Shoag said.
William Catalona, MD, Northwestern University Feinberg School of Medicine, Chicago, agreed, noting that too many low-risk patients are undergoing focal treatment who should be on active surveillance. “Many men are attracted to focal because they just are uncomfortable having a cancer in their body that’s not treated,” Dr. Catalona said. But “giving these patients focal therapy is really overtreatment.”
Patients with higher-risk disease who want to avoid aggressive treatment are also being lured into focal without guidelines or clear evidence to back up that option, Dr. Catalona explained.
Although it’s not clear how many men in the United States are receiving focal therapy who shouldn’t, even proponents of focal therapy, like George, have expressed concern.
Dr. George agreed that focal therapy marketing geared towards patients is drawing in some men who are not good candidates for these techniques, and feels there’s not enough objective material from medical societies or academic centers giving patients a realistic picture of focal therapy.
“There is concern that patients may be receiving biased information,” Dr. George said, adding that it’s ultimately up to the physician to reconcile the best available evidence, understand the outcomes, and discuss these options with the patient to guide them to what’s best.
At the end of the day, Dr. Spratt said, physicians giving focal therapy off a clinical trial need to pause and ask themselves “why are they giving a treatment that remains investigational by payers, not recommended by any major guideline, and that lacks any randomized evidence?”
Mr. LaFrate does not regret his decision to forgo focal therapy in 2013. He has been on active surveillance for about a decade now.
Following an MRI in 2022, Mr. LaFrate’s radiology report found that “clinically significant cancer is very unlikely to be present.”
Still, his PSA has risen two points in the past year to 14. His current urologist feels that the PSA is going up because there’s cancer present and is suggesting focal therapy for Mr. LaFrate.
Mr. LaFrate, who has prostate enlargement issues, remains skeptical of focal therapy and is still resisting the sales pitch.
“My doctor is not aggressively pushing it. He’s just giving me that as one of my options,” he said. “I just have a hunch I don’t need it at this point.”
A version of this article appeared on Medscape.com.
In 2013, a prostate-specific antigen (PSA) blood test revealed that Richard LaFrate’s levels had jumped.
Previously in a normal range, his PSA was now above 6 ng/mL, indicating an elevated likelihood for prostate cancer. The jazz guitarist from Leesburg, Florida, then 70 years old, underwent a biopsy, which found two Gleason 6 lesions.
Mr. LaFrate had low-risk prostate cancer.
Guidelines now recommend active surveillance for patients like Mr. LaFrate, who have low-risk disease. This strategy would mean monitoring the cancer until LaFrate required treatment, with the upside being he might never need therapy.
Mr. LaFrate’s urologist, however, was pushing whole gland surgery — an invasive and unnecessary procedure given his diagnosis and age.
Mr. LaFrate decided to look for another doctor. He filled out a form online that pointed him to a new urologist who offered him one option: An investigational procedure known as high-intensity focused ultrasound.
At the time, high-intensity focused ultrasound — a form of focal therapy — was being studied in the United States to treat men with low or intermediate-risk prostate cancer, but it was still relatively early days.
Mr. LaFrate’s urologist asked him to pay $25,000 out of pocket to undergo the focal procedure at a clinic in the Bahamas. He refused and, ultimately, landed on active surveillance as the best strategy to manage for his low-risk disease.
That urologist was “a shyster in my opinion,” Mr. LaFrate said.
— Gleason 3+4 (grade group 2) tumors — as an alternative to invasive surgery and active surveillance. Prestigious medical centers, such as Cleveland Clinic, Mayo Clinic, Memorial Sloan Kettering, UCLA, and the University of Chicago, routinely offer focal therapy.
But use of the techniques remains controversial and costly.
As the Cleveland Clinic’s website acknowledges, although “the use of focal therapy for localized prostate cancer appears to be a promising development in a number of ways, it is still considered investigational and not yet part of standard therapy.” Major caveats to focal therapy include unknown long-term effectiveness, the possibility of leaving behind untreated cancer, and higher overall costs.
No major national guidelines endorse the use of focal therapy, unless offered in a research or clinical trial setting. Insurance companies, such as Aetna, Blue Cross Blue Shield, and United, also consider focal therapy for prostate cancer investigational and don’t cover it.
Without a stamp of approval from guideline bodies and insurance companies, patients, like Mr. LaFrate, remain vulnerable to the high out-of-pocket costs for these focal techniques.
“Almost every place charges $15,000-$30,000 in cash,” said Daniel Spratt, MD, radiation oncology chair at University Hospitals Seidman Cancer Center and Case Western Reserve University in Cleveland.
Dr. Spratt has seen hundreds of patients after focal therapy, some from prominent centers, who have emptied their bank accounts to undergo treatment with the promise of great results and ultimately felt misled when the cancer has recurred.
“It pains me that there are doctors willing to ignore the Hippocratic oath of ‘Do No Harm’ simply to jump on this fad to bring in revenue,” Dr. Spratt said.
Evidence-Based or Oversold?
Focal therapy gained a foothold in the United Kingdom well before the United States.
Hashim Ahmed, FRCS, urology chair at Imperial College London, has used focal therapy for 15 years, treated over 1000 patients, and taught dozens of surgeons how to use the leading focal therapies — focal cryoablation, in which surgeons use a needle-thin probe to target, freeze, and kill prostate tumors, as well as high-intensity focused ultrasound, which uses sound wave energy to superheat and kill tumors.
“Certainly, in the United Kingdom, focal therapy has been prime time in a number of centers for a number of years,” Dr. Ahmed said.
In the United States, focal therapy has become an attractive option for men with prostate cancer who want to avoid radiation or radical prostatectomy but don’t feel comfortable simply monitoring their disease with active surveillance. Experts from specialized focal therapy centers touting the promise of this “innovative technique” predict its routine use in the next few years.
But the excitement surrounding the use of focal therapy in prostate cancer has outpaced broader acceptance.
In 2015, the FDA approved high-intensity focused ultrasound to treat prostatic disease, but not prostate cancer specifically. Although the approval language “means that companies cannot advertise that their devices can be used for prostate cancer,” physicians can still determine how to use the technology, which includes treating prostate cancer, Dr. Ahmed said.
The evidence is starting to catch up to the demand. The latest research suggests that the partial-gland techniques may stand up well to radical prostatectomy.
A 2022 prospective database study comparing radical prostatectomies to focal therapy — mostly high-intensity focused ultrasound — in more than 800 men found similar rates of failure-free survival in the two groups at the 8-year follow-up. A 2019 registry study found that failure-free survival at 3 years was just over 90% in high and intermediate-risk patients receiving focal cryotherapy, with the rate rising to about 93% for the intermediate-risk group. And a 2018 prospective study of 625 patients with intermediate or high-risk prostate cancer who underwent high-intensity focused ultrasound had 5-year metastasis-free survival of 98% and overall survival rates of 100%.
One of the biggest draws of focal therapy vs more aggressive treatments is the “massive differences in side-effect profiles,” said Dr. Ahmed.
In a 2021 meta-analysis, researchers found that 6 months after high-intensity focused ultrasound, 98% of patients remained continent and 80% retained erectile function, while erectile dysfunction can occur in 30% to as many as 85% of patients following prostatectomy or radiotherapy and urinary incontinence can occur in as many as 40% of patients.
Despite these potential advantages of focal therapy, the long-term efficacy of the techniques remains uncertain.
A recent study from a team at MSK, for instance, reported that 40% of men with intermediate (grade 2) or high-risk (grade 3) disease had residual cancer following MRI-guided focused ultrasound. A 2020 prospective registry study found that almost 20% of patients undergoing high-intensity focal ultrasound required a second round following a recurrence.
Dr. Spratt worries that patients who recur after focal therapy may go on to receive a second round — often offered at half price — and will still ultimately need surgery or radiation therapy later. By that point, however, patients may have spent as much as $45,000 — ie, $30,000 on the initial and another $15,000 on the follow-up procedure.
When patients see Dr. Spratt after a recurrence, he informs them that their side effects will be worse if he gives them radiation or surgery now vs if he had given them curative therapy upfront. “But this is what we’re left with,” he tells them.
Another big concern in the field is “the quality of data for focal therapy is overwhelmingly poor,” said Jonathan Shoag, MD, a urologic oncologist at University Hospitals and an associate professor of urology at Case Western Reserve University School of Medicine in Cleveland. “Essentially, the bulk of the data is from single-institution retrospective series without defined follow-up protocols or endpoints.”
The American Urological Association (AUA) has even cautioned experts and patients about the lack of high-quality data comparing focal therapy techniques to radiation therapy, surgery, and active surveillance. According to the AUA, focal options should only be considered in intermediate-risk prostate cancer in a clinical trial setting.
“The lack of randomized clinical trials poses a major stumbling block for the field,” said Dr. Ahmed.
Although randomized trials would be ideal, the results would take many years to mature, and growing patient demand for these less invasive focal procedures has made randomized trials difficult to complete, explained Arvin George, MD, associate professor at Johns Hopkins School of Medicine in Baltimore. Several randomized trials attempted in Norway and the United Kingdom, for instance, fell apart when patients refused to be randomized between focal and radical therapy, Dr. George said.
Focal therapy is now in the same position that active surveillance was a few years ago, according to Dr. George.
“We are hearing the same concerns about focal therapy now as we did about active surveillance,” he said. The initial evidence supporting active surveillance largely came from real-world experience and retrospective studies. The randomized data came later, and skeptics of active surveillance “were proven wrong,” he added.
But Dr. Shoag has a different take on the trajectory of focal therapy research and care in the United States.
“I think there’s this emerging kind of tragedy happening in our field now, where you have even academic institutions offering focal therapy to patients off-trial with essentially no data to suggest it is oncologically effective,” Dr. Shoag said.
William Catalona, MD, Northwestern University Feinberg School of Medicine, Chicago, agreed, noting that too many low-risk patients are undergoing focal treatment who should be on active surveillance. “Many men are attracted to focal because they just are uncomfortable having a cancer in their body that’s not treated,” Dr. Catalona said. But “giving these patients focal therapy is really overtreatment.”
Patients with higher-risk disease who want to avoid aggressive treatment are also being lured into focal without guidelines or clear evidence to back up that option, Dr. Catalona explained.
Although it’s not clear how many men in the United States are receiving focal therapy who shouldn’t, even proponents of focal therapy, like George, have expressed concern.
Dr. George agreed that focal therapy marketing geared towards patients is drawing in some men who are not good candidates for these techniques, and feels there’s not enough objective material from medical societies or academic centers giving patients a realistic picture of focal therapy.
“There is concern that patients may be receiving biased information,” Dr. George said, adding that it’s ultimately up to the physician to reconcile the best available evidence, understand the outcomes, and discuss these options with the patient to guide them to what’s best.
At the end of the day, Dr. Spratt said, physicians giving focal therapy off a clinical trial need to pause and ask themselves “why are they giving a treatment that remains investigational by payers, not recommended by any major guideline, and that lacks any randomized evidence?”
Mr. LaFrate does not regret his decision to forgo focal therapy in 2013. He has been on active surveillance for about a decade now.
Following an MRI in 2022, Mr. LaFrate’s radiology report found that “clinically significant cancer is very unlikely to be present.”
Still, his PSA has risen two points in the past year to 14. His current urologist feels that the PSA is going up because there’s cancer present and is suggesting focal therapy for Mr. LaFrate.
Mr. LaFrate, who has prostate enlargement issues, remains skeptical of focal therapy and is still resisting the sales pitch.
“My doctor is not aggressively pushing it. He’s just giving me that as one of my options,” he said. “I just have a hunch I don’t need it at this point.”
A version of this article appeared on Medscape.com.
Plant-Based Diet a Boon for Men With Prostate Cancer
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
Cardiorespiratory Fitness May Cut Prostate Cancer Risk
Men with cardiorespiratory fitness (CRF) who increased their CRF by more than 3% had a significantly lower risk of prostate cancer incidence, a large Swedish study found.
The prospective analysis, published in the British Journal of Sports Medicine, done in a cohort of nearly 58,000, was conducted by Kate A. Bolam, PhD, a clinical exercise physiologist at the Swedish School of Sport and Health Sciences in Stockholm.
“The findings suggest that physicians could work toward supporting patients to understand what types of activities could improve their fitness and ways they can incorporate these activities into their lives in an enjoyable way, or at the very least refer patients on to an exercise specialist,” Dr. Bolam said in an interview.
Grouped by baseline CRF, the association between change in absolute CRF and prostate cancer incidence was significant only for participants with a moderate baseline CRF. Moreover, changes in both absolute and relative CRF were not associated with prostate cancer mortality.
The lack of mortality significance may be due to the relatively few deaths from prostate cancer in the cohort, Dr. Bolam said. “It may be we weren’t powered to detect anything with such low numbers. And it’s not likely men will die from prostate cancer but more likely from more common chronic diseases such as heart disease.” The authors noted that unlike the case with other common cancers, there are relatively few preventable risk factors with strong evidence for reducing overall prostate cancer risk. “Aside from developmental factors, being diagnosed with overweight or obesity are the main risk factors for developing advanced prostate cancer, but insufficient evidence exists to extend this conclusion to non-advanced prostate cancer,” they wrote.
There is evidence, however, that exercise reduces all-cause mortality risk across many cancer types, including prostate.
Study details
The cohort was drawn from Swedish national health-profile database figures from 1982 to 2019. Participants completed an occupational health profile assessment including at least two valid CRF tests on a cycle ergometer. During a mean follow-up of 6.7 years, 592 (1%) of 57,652 men (mean age 41.3 years, standard deviation 10.55) were diagnosed with prostate cancer, and in 46 (.08%) prostate cancer was the primary cause of death.
An increase in absolute CRF (as a percentage of liters per minute of cardiac output) was associated with a reduced incidence risk, with a hazard ratio of 0.98 (95% CI, 0.96-0.99). Grouping participants as having increased (+3%), stable (±3%), or decreased (−3%) CRF, the investigators found increased fitness was associated with an HR for prostate cancer incidence of 0.65 (95% CI, 0.49-0.86), vs decreased fitness.
According to the authors, this and similar investigations of mechanisms behind physical activity benefits will lead to more targeted prevention recommendations. The results highlight the importance of encouraging the general public to increase CRF or reach moderate fitness levels, Dr. Bolam’s group wrote. The group is planning a similar study in breast cancer.
This study was funded by the Swedish Cancer Society. The authors declared no competing interests.
Men with cardiorespiratory fitness (CRF) who increased their CRF by more than 3% had a significantly lower risk of prostate cancer incidence, a large Swedish study found.
The prospective analysis, published in the British Journal of Sports Medicine, done in a cohort of nearly 58,000, was conducted by Kate A. Bolam, PhD, a clinical exercise physiologist at the Swedish School of Sport and Health Sciences in Stockholm.
“The findings suggest that physicians could work toward supporting patients to understand what types of activities could improve their fitness and ways they can incorporate these activities into their lives in an enjoyable way, or at the very least refer patients on to an exercise specialist,” Dr. Bolam said in an interview.
Grouped by baseline CRF, the association between change in absolute CRF and prostate cancer incidence was significant only for participants with a moderate baseline CRF. Moreover, changes in both absolute and relative CRF were not associated with prostate cancer mortality.
The lack of mortality significance may be due to the relatively few deaths from prostate cancer in the cohort, Dr. Bolam said. “It may be we weren’t powered to detect anything with such low numbers. And it’s not likely men will die from prostate cancer but more likely from more common chronic diseases such as heart disease.” The authors noted that unlike the case with other common cancers, there are relatively few preventable risk factors with strong evidence for reducing overall prostate cancer risk. “Aside from developmental factors, being diagnosed with overweight or obesity are the main risk factors for developing advanced prostate cancer, but insufficient evidence exists to extend this conclusion to non-advanced prostate cancer,” they wrote.
There is evidence, however, that exercise reduces all-cause mortality risk across many cancer types, including prostate.
Study details
The cohort was drawn from Swedish national health-profile database figures from 1982 to 2019. Participants completed an occupational health profile assessment including at least two valid CRF tests on a cycle ergometer. During a mean follow-up of 6.7 years, 592 (1%) of 57,652 men (mean age 41.3 years, standard deviation 10.55) were diagnosed with prostate cancer, and in 46 (.08%) prostate cancer was the primary cause of death.
An increase in absolute CRF (as a percentage of liters per minute of cardiac output) was associated with a reduced incidence risk, with a hazard ratio of 0.98 (95% CI, 0.96-0.99). Grouping participants as having increased (+3%), stable (±3%), or decreased (−3%) CRF, the investigators found increased fitness was associated with an HR for prostate cancer incidence of 0.65 (95% CI, 0.49-0.86), vs decreased fitness.
According to the authors, this and similar investigations of mechanisms behind physical activity benefits will lead to more targeted prevention recommendations. The results highlight the importance of encouraging the general public to increase CRF or reach moderate fitness levels, Dr. Bolam’s group wrote. The group is planning a similar study in breast cancer.
This study was funded by the Swedish Cancer Society. The authors declared no competing interests.
Men with cardiorespiratory fitness (CRF) who increased their CRF by more than 3% had a significantly lower risk of prostate cancer incidence, a large Swedish study found.
The prospective analysis, published in the British Journal of Sports Medicine, done in a cohort of nearly 58,000, was conducted by Kate A. Bolam, PhD, a clinical exercise physiologist at the Swedish School of Sport and Health Sciences in Stockholm.
“The findings suggest that physicians could work toward supporting patients to understand what types of activities could improve their fitness and ways they can incorporate these activities into their lives in an enjoyable way, or at the very least refer patients on to an exercise specialist,” Dr. Bolam said in an interview.
Grouped by baseline CRF, the association between change in absolute CRF and prostate cancer incidence was significant only for participants with a moderate baseline CRF. Moreover, changes in both absolute and relative CRF were not associated with prostate cancer mortality.
The lack of mortality significance may be due to the relatively few deaths from prostate cancer in the cohort, Dr. Bolam said. “It may be we weren’t powered to detect anything with such low numbers. And it’s not likely men will die from prostate cancer but more likely from more common chronic diseases such as heart disease.” The authors noted that unlike the case with other common cancers, there are relatively few preventable risk factors with strong evidence for reducing overall prostate cancer risk. “Aside from developmental factors, being diagnosed with overweight or obesity are the main risk factors for developing advanced prostate cancer, but insufficient evidence exists to extend this conclusion to non-advanced prostate cancer,” they wrote.
There is evidence, however, that exercise reduces all-cause mortality risk across many cancer types, including prostate.
Study details
The cohort was drawn from Swedish national health-profile database figures from 1982 to 2019. Participants completed an occupational health profile assessment including at least two valid CRF tests on a cycle ergometer. During a mean follow-up of 6.7 years, 592 (1%) of 57,652 men (mean age 41.3 years, standard deviation 10.55) were diagnosed with prostate cancer, and in 46 (.08%) prostate cancer was the primary cause of death.
An increase in absolute CRF (as a percentage of liters per minute of cardiac output) was associated with a reduced incidence risk, with a hazard ratio of 0.98 (95% CI, 0.96-0.99). Grouping participants as having increased (+3%), stable (±3%), or decreased (−3%) CRF, the investigators found increased fitness was associated with an HR for prostate cancer incidence of 0.65 (95% CI, 0.49-0.86), vs decreased fitness.
According to the authors, this and similar investigations of mechanisms behind physical activity benefits will lead to more targeted prevention recommendations. The results highlight the importance of encouraging the general public to increase CRF or reach moderate fitness levels, Dr. Bolam’s group wrote. The group is planning a similar study in breast cancer.
This study was funded by the Swedish Cancer Society. The authors declared no competing interests.
FROM BRITISH JOURNAL OF SPORTS MEDICINE
Small PFS gain in metastatic prostate cancer with TKI and ICI
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
The combination of the tyrosine kinase inhibitor (TKI), cabozantinib (Cabometyx), and the immune checkpoint inhibitor (ICI), atezolizumab (Tecentriq), was associated with a median PFS of 6.3 months vs 4.2 months for patients assigned to second hormonal therapy with either abiraterone (Zytiga) and prednisone, or enzalutamide (Xtandi) in the CONTACT-02 trial, Neeraj Agarwal, MD, reported at the ASCO Genitourinary Cancers Symposium.
“CONTACT 2 is the first phase 3 trial of the TKI/ICI combination to show statistically significant improvement in PFS in patients with mCRPC,” said Dr. Agarwal, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
The data support the combination of cabozantinib and atezolizumab as a potential new treatment option for patients with mCRPC that has progressed on novel hormonal therapy, he said.
Study Design Questioned
That opinion, however, was not shared by Kim N. Chi, MD, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant.
Dr. Chi acknowledged that the study results as presented were positive, but also pointed to several limitations, including the small difference between the treatment groups in radiographic progression-free survival (rPFS).
“I would say the rPFS benefit is modest, and in the absence of other improvements the difference in the median rPFS is equivalent from one scan to the next in the scanning cycle. I would argue about the clinical significance of that,” he said.
He also noted that there was no improvement in the investigational arm in patient-reported outcomes, and that pain progression and quality-of-life deterioration occurred within 2 to 4 months, which is “quite quick.”
Additionally, he questioned the choice of an androgen receptor pathway inhibitor (ARPI) switch as the control arm of the study.
“I’d also argue that ARPI switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases; there are better options,” he said.
For example, in phase 3 trials, docetaxel and cabazitaxel (Jevtana) have consistently demonstrated radiographic PFS of 8 to 9 months. In addition, lutetium-177–PSMA-617, a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells and the tumor microenvironment, has also been shown to have PFS and overall survival benefits, he said.
“Irrespective of regulatory decisions, I personally could not recommend this at this time, given the data that we’ve seen and the better options that are available for this patient population,” Dr. Chi said.
Real-World Practice
“Kim Chi offered a pretty fair critique and summary of the control arm, but in real world practice, ARPI switch, from abi [abiraterone] to enza [enzalutamide] or enza to abi continues to be used in routine clinical practice for various reasons,” Xin Gao, MD, a genitourinary oncologist at Mass General Cancer Center in Boston, said in an interview.
“There are patients who can’t tolerate chemotherapy or don’t want chemotherapy, and we do know also that there are patients who can benefit from an ARPI switch, especially some patients with more indolent disease,” said Dr. Gao, who attended the presentation but was not involved in the study.
He noted that some patients being switched from abiraterone to enzalutamide have clinical responses, and that the ARPIs are generally more tolerable than chemotherapy.
In addition, CONTACT-02 is one of a series of trials in which ARPI switch was used as the control arm, and many of these trials were initiated before there were data confirming the superior efficacy of some newer therapeutic options, Dr. Gao noted.
He agreed, however that there is growing evidence to show that ARPI switch may not be the optimal choice for patients with more measurable disease, especially visceral metastases, and other more aggressive forms of mCRPC.
CONTACT-02 Details
Investigators in the phase 3 study screened 866 men with mCRPC and after stratification by liver metastases, prior docetaxel use for castration-sensitive prostate cancer, and disease stage for which the first novel hormonal therapy was given. About 500 patients (507) were randomized to receive either oral cabozantinib 40 mg daily plus intravenous atezolizumab 1200 mg every 3 weeks or second hormonal therapy with either abiraterone 1000 mg with oral prednisone 5 mg twice daily, or oral enzalutamide 160 mg daily.
After a median follow-up of 14.3 months in the PFS intention-to-treat population, the median PFS by blinded central review was 6.3 months with cabozantinib/atezolizumab and 4.2 months with second hormonal therapy. This translated into a hazard ratio of 0.64 (P = .0002). The results were similar for a PFS analysis according to Prostate Cancer Working Group 3 criteria.
The combination was also associated with modest improvements in PFS in prespecified subgroups, including patients who had liver or bone metastases and those who had previously received docetaxel.
There were no significant differences in overall survival at the time of data cutoff. Overall survival data were not mature and will be reported at a later date.
Disease control rates, a composite of complete and partial responses and stable disease, were 73% with the combination and 55% with second hormonal therapy (P value not shown).
Safety Data
The safety analysis indicated that patients found the ARPI switch easier to tolerate than the combination.
Adverse events leading to dose reductions occurred in 40% of patients on the combination, vs 3% of patients on second hormonal therapy, and treatment-related adverse events leading to discontinuation occurred in 13% and 2%, respectively.
Grade 3 or 4 adverse events occurred in 48% of patients assigned to the combination vs. 23% of patients assigned to the ARPI switch.
In all, 8% of patients on the combination and 12% on second hormonal therapy died on study, but none of the deaths were deemed to be treatment related.
CONTACT-02 was sponsored by Exelixis in partnerships with Ipsen and Takeda.
Dr. Agarwal disclosed institutional research funding from Exelixis, Roche, Takeda, and others, and travel expenses from Pfizer. Dr. Chi disclosed honoraria, a consulting/advisory role and institutional research funding with Roche and others. Dr. Gao has served as a consultant or advisor to several companies, not including the sponsors of the study, and has served as principal investigator at his institution, which has received research funding from Exelixis, Takeda, and others.
FROM ASCO GU 2024
Combo Tx Best in Metastatic Prostate Cancer with HRR Mutations
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
FROM ASCO GU 2024
More Side Effects With Local Therapies for Prostate Cancer
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN