User login
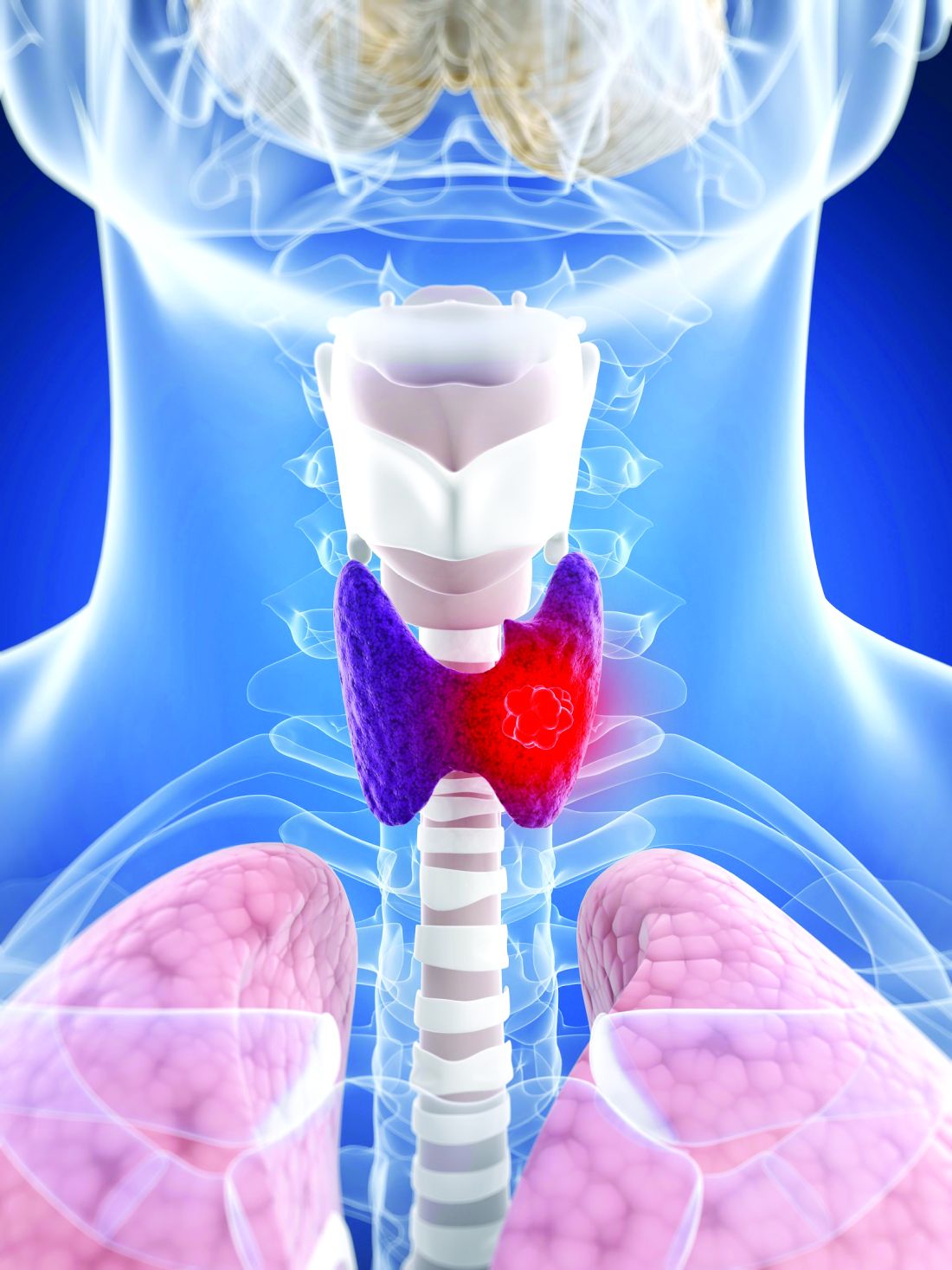
Lifestyle choices could curb genetic risk for thyroid cancer
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
‘Game changer’: Thyroid cancer recurrence no higher with lobectomy
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Thyroid nodule volume reduction correlates with energy in ablation
MONTREAL – In the treatment of thyroid nodules with radiofrequency ablation (RFA), the amount of energy delivered per unit volume of the nodule strongly correlates with the extent of nodule volume reduction after 6 and 12 months, suggesting an important indicator of treatment success.
The findings “provide an objective measure or goal energy input to achieve during the [RFA] procedure rather than relying only on the subjective judgment of sonographic changes, and in turn, produce more reliable outcomes for our patients,” first author Samantha A. Wolfe, MD, said in an interview.
Dr. Wolfe, of the department of otolaryngology – head and neck surgery at Johns Hopkins University, Baltimore, presented the findings at the American Thyroid Association annual meeting.
Commenting on the study, Insoo Suh, MD, an associate professor and associate vice chair of Surgical Innovation at New York University Langone Health, agreed that “an accounting of the total amount of energy delivered can be a useful additional data point for the operator when they are determining whether an ablation is successful.”
He noted, however, that the location of a nodule can be an important factor when deciding upon amounts of RF energy.
“Some target areas are too close for comfort to critical structures, such as the trachea or the recurrent laryngeal nerve, so sound judgment would dictate that the energy be dialed down in those areas, even if the price you pay is a slightly lower volume reduction,” he explained.
Analysis of patients given RFA at Johns Hopkins
RFA utilizes RF energy for the reduction of nodule compression and aesthetic symptoms, avoiding the need for thyroid hormone replacement or surgery.
And while decisions regarding RFA treatment location and duration are commonly guided by the operator’s judgment of sonographic changes, those assessments can potentially result in inconsistent outcomes.
In observing a relationship between higher amounts of RF energy and nodule volume reduction, Dr. Wolfe and associates conducted their prospective study of nodules treated by two experienced endocrine surgeons at Johns Hopkins between June 2019 and May 2022 at 6 and 12 months in relation to the amount of total energy delivered during the treatment.
The analysis included 101 nodules, which had a median initial volume of 12.9 mL.
After 6 months, the median volume reduction ratio was 60%, and at 12 months, the median reduction was 64%.
In terms of the goal of achieving 50% or more volume reduction at 6 months, the median energy delivered was significantly higher for nodules that did reach that goal compared with those that had a volume reduction of less than 50% (2,317 vs. 1,912 J/mL, respectively; P = .01).
The figures were similar at 12 months (2,031 vs. 1254 J/mL; P < .01).
In a logistic regression analysis, the amount of energy delivered strongly increased the odds of obtaining a volume reduction ratio of at least 50% (odds ratio, 2.58; P = .048).
“Every twofold increase in energy delivered increases the odds of achieving a 50% volume ratio reduction by 2.58 times,” Dr. Wolfe explained.
Likewise, the same twofold increase in energy delivered also increased the odds of achieving a greater than 80% volume ratio reduction by 2.55 times (OR, 2.55; P = .038), she added.
Information may help to decide who needs multiple ablations
Of note, the effect was stronger with smaller nodules. Those with an initial volume of less than 20 mL had a significantly greater volume ratio reduction than nodules that were 20 mL or larger (61% vs. 48%, respectively; P = .05).
The initial volume of nodules that did, and did not, achieve a 50% volume ratio reduction at 6 months were 10.9 mL versus 19.1 mL, and the initial volumes of those that did, and did not, have at least a 50% reduction at 12 months were 10.5 mL and 41.5 mL.
“At 6 and 12 months, the successfully treated nodules had a significantly smaller immediate initial volume than those that did not,” Dr. Wolfe said.
“This information may aid in identifying patients with large nodules that are less likely to achieve a greater than 50% volume reduction ratio and may require multiple treatments,” she added.
Other factors – including the probe tip size and total energy delivered – did not significantly correlate with volume ratio reduction at 6 or 12 months.
There was also no significant difference in terms of thyroid-stimulating hormone levels among nodules that achieved at least a 50% volume reduction and those that did not.
Nodules that did not have a satisfactory volume reduction at 12 months had a relatively large median total energy value delivered during ablation (103,463 J, compared with 25,969 J among those achieving more than 50% volume ratio reduction), which Dr. Wolfe said likely reflects that those nodules had a large initial volume.
“This speaks to the importance of describing the energy utilized per unit of nodule volume rather than just a gross measurement,” she said during her presentation.
Dr. Wolfe added that in terms of strategies for getting more energy into the nodule, a key approach is time.
“Sometimes you will see sonographic changes very quickly in the nodule, and it could be tempting to consider that area ablated and move on if you only rely on sonographic changes,” she said in an interview. “However, our research shows that, by spending more time, and thus inputting more energy into the nodule, we had better volume reduction.”
Dr. Wolfe and Dr. Suh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – In the treatment of thyroid nodules with radiofrequency ablation (RFA), the amount of energy delivered per unit volume of the nodule strongly correlates with the extent of nodule volume reduction after 6 and 12 months, suggesting an important indicator of treatment success.
The findings “provide an objective measure or goal energy input to achieve during the [RFA] procedure rather than relying only on the subjective judgment of sonographic changes, and in turn, produce more reliable outcomes for our patients,” first author Samantha A. Wolfe, MD, said in an interview.
Dr. Wolfe, of the department of otolaryngology – head and neck surgery at Johns Hopkins University, Baltimore, presented the findings at the American Thyroid Association annual meeting.
Commenting on the study, Insoo Suh, MD, an associate professor and associate vice chair of Surgical Innovation at New York University Langone Health, agreed that “an accounting of the total amount of energy delivered can be a useful additional data point for the operator when they are determining whether an ablation is successful.”
He noted, however, that the location of a nodule can be an important factor when deciding upon amounts of RF energy.
“Some target areas are too close for comfort to critical structures, such as the trachea or the recurrent laryngeal nerve, so sound judgment would dictate that the energy be dialed down in those areas, even if the price you pay is a slightly lower volume reduction,” he explained.
Analysis of patients given RFA at Johns Hopkins
RFA utilizes RF energy for the reduction of nodule compression and aesthetic symptoms, avoiding the need for thyroid hormone replacement or surgery.
And while decisions regarding RFA treatment location and duration are commonly guided by the operator’s judgment of sonographic changes, those assessments can potentially result in inconsistent outcomes.
In observing a relationship between higher amounts of RF energy and nodule volume reduction, Dr. Wolfe and associates conducted their prospective study of nodules treated by two experienced endocrine surgeons at Johns Hopkins between June 2019 and May 2022 at 6 and 12 months in relation to the amount of total energy delivered during the treatment.
The analysis included 101 nodules, which had a median initial volume of 12.9 mL.
After 6 months, the median volume reduction ratio was 60%, and at 12 months, the median reduction was 64%.
In terms of the goal of achieving 50% or more volume reduction at 6 months, the median energy delivered was significantly higher for nodules that did reach that goal compared with those that had a volume reduction of less than 50% (2,317 vs. 1,912 J/mL, respectively; P = .01).
The figures were similar at 12 months (2,031 vs. 1254 J/mL; P < .01).
In a logistic regression analysis, the amount of energy delivered strongly increased the odds of obtaining a volume reduction ratio of at least 50% (odds ratio, 2.58; P = .048).
“Every twofold increase in energy delivered increases the odds of achieving a 50% volume ratio reduction by 2.58 times,” Dr. Wolfe explained.
Likewise, the same twofold increase in energy delivered also increased the odds of achieving a greater than 80% volume ratio reduction by 2.55 times (OR, 2.55; P = .038), she added.
Information may help to decide who needs multiple ablations
Of note, the effect was stronger with smaller nodules. Those with an initial volume of less than 20 mL had a significantly greater volume ratio reduction than nodules that were 20 mL or larger (61% vs. 48%, respectively; P = .05).
The initial volume of nodules that did, and did not, achieve a 50% volume ratio reduction at 6 months were 10.9 mL versus 19.1 mL, and the initial volumes of those that did, and did not, have at least a 50% reduction at 12 months were 10.5 mL and 41.5 mL.
“At 6 and 12 months, the successfully treated nodules had a significantly smaller immediate initial volume than those that did not,” Dr. Wolfe said.
“This information may aid in identifying patients with large nodules that are less likely to achieve a greater than 50% volume reduction ratio and may require multiple treatments,” she added.
Other factors – including the probe tip size and total energy delivered – did not significantly correlate with volume ratio reduction at 6 or 12 months.
There was also no significant difference in terms of thyroid-stimulating hormone levels among nodules that achieved at least a 50% volume reduction and those that did not.
Nodules that did not have a satisfactory volume reduction at 12 months had a relatively large median total energy value delivered during ablation (103,463 J, compared with 25,969 J among those achieving more than 50% volume ratio reduction), which Dr. Wolfe said likely reflects that those nodules had a large initial volume.
“This speaks to the importance of describing the energy utilized per unit of nodule volume rather than just a gross measurement,” she said during her presentation.
Dr. Wolfe added that in terms of strategies for getting more energy into the nodule, a key approach is time.
“Sometimes you will see sonographic changes very quickly in the nodule, and it could be tempting to consider that area ablated and move on if you only rely on sonographic changes,” she said in an interview. “However, our research shows that, by spending more time, and thus inputting more energy into the nodule, we had better volume reduction.”
Dr. Wolfe and Dr. Suh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – In the treatment of thyroid nodules with radiofrequency ablation (RFA), the amount of energy delivered per unit volume of the nodule strongly correlates with the extent of nodule volume reduction after 6 and 12 months, suggesting an important indicator of treatment success.
The findings “provide an objective measure or goal energy input to achieve during the [RFA] procedure rather than relying only on the subjective judgment of sonographic changes, and in turn, produce more reliable outcomes for our patients,” first author Samantha A. Wolfe, MD, said in an interview.
Dr. Wolfe, of the department of otolaryngology – head and neck surgery at Johns Hopkins University, Baltimore, presented the findings at the American Thyroid Association annual meeting.
Commenting on the study, Insoo Suh, MD, an associate professor and associate vice chair of Surgical Innovation at New York University Langone Health, agreed that “an accounting of the total amount of energy delivered can be a useful additional data point for the operator when they are determining whether an ablation is successful.”
He noted, however, that the location of a nodule can be an important factor when deciding upon amounts of RF energy.
“Some target areas are too close for comfort to critical structures, such as the trachea or the recurrent laryngeal nerve, so sound judgment would dictate that the energy be dialed down in those areas, even if the price you pay is a slightly lower volume reduction,” he explained.
Analysis of patients given RFA at Johns Hopkins
RFA utilizes RF energy for the reduction of nodule compression and aesthetic symptoms, avoiding the need for thyroid hormone replacement or surgery.
And while decisions regarding RFA treatment location and duration are commonly guided by the operator’s judgment of sonographic changes, those assessments can potentially result in inconsistent outcomes.
In observing a relationship between higher amounts of RF energy and nodule volume reduction, Dr. Wolfe and associates conducted their prospective study of nodules treated by two experienced endocrine surgeons at Johns Hopkins between June 2019 and May 2022 at 6 and 12 months in relation to the amount of total energy delivered during the treatment.
The analysis included 101 nodules, which had a median initial volume of 12.9 mL.
After 6 months, the median volume reduction ratio was 60%, and at 12 months, the median reduction was 64%.
In terms of the goal of achieving 50% or more volume reduction at 6 months, the median energy delivered was significantly higher for nodules that did reach that goal compared with those that had a volume reduction of less than 50% (2,317 vs. 1,912 J/mL, respectively; P = .01).
The figures were similar at 12 months (2,031 vs. 1254 J/mL; P < .01).
In a logistic regression analysis, the amount of energy delivered strongly increased the odds of obtaining a volume reduction ratio of at least 50% (odds ratio, 2.58; P = .048).
“Every twofold increase in energy delivered increases the odds of achieving a 50% volume ratio reduction by 2.58 times,” Dr. Wolfe explained.
Likewise, the same twofold increase in energy delivered also increased the odds of achieving a greater than 80% volume ratio reduction by 2.55 times (OR, 2.55; P = .038), she added.
Information may help to decide who needs multiple ablations
Of note, the effect was stronger with smaller nodules. Those with an initial volume of less than 20 mL had a significantly greater volume ratio reduction than nodules that were 20 mL or larger (61% vs. 48%, respectively; P = .05).
The initial volume of nodules that did, and did not, achieve a 50% volume ratio reduction at 6 months were 10.9 mL versus 19.1 mL, and the initial volumes of those that did, and did not, have at least a 50% reduction at 12 months were 10.5 mL and 41.5 mL.
“At 6 and 12 months, the successfully treated nodules had a significantly smaller immediate initial volume than those that did not,” Dr. Wolfe said.
“This information may aid in identifying patients with large nodules that are less likely to achieve a greater than 50% volume reduction ratio and may require multiple treatments,” she added.
Other factors – including the probe tip size and total energy delivered – did not significantly correlate with volume ratio reduction at 6 or 12 months.
There was also no significant difference in terms of thyroid-stimulating hormone levels among nodules that achieved at least a 50% volume reduction and those that did not.
Nodules that did not have a satisfactory volume reduction at 12 months had a relatively large median total energy value delivered during ablation (103,463 J, compared with 25,969 J among those achieving more than 50% volume ratio reduction), which Dr. Wolfe said likely reflects that those nodules had a large initial volume.
“This speaks to the importance of describing the energy utilized per unit of nodule volume rather than just a gross measurement,” she said during her presentation.
Dr. Wolfe added that in terms of strategies for getting more energy into the nodule, a key approach is time.
“Sometimes you will see sonographic changes very quickly in the nodule, and it could be tempting to consider that area ablated and move on if you only rely on sonographic changes,” she said in an interview. “However, our research shows that, by spending more time, and thus inputting more energy into the nodule, we had better volume reduction.”
Dr. Wolfe and Dr. Suh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022
USPSTF holds firm on postmenopausal hormone recommendations
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
New EU guidelines: Individualize care for thyroid cancer in kids
Comprehensive evaluation, multidisciplinary care, individualized treatment, and ongoing follow-up are all key to the management of pediatric thyroid nodules and differentiated thyroid carcinoma (DTC), according to the first European guidelines for this rare disease.
The guidelines were recently published in the European Thyroid Journal.
Lead author Chantal A. Lebbink told this news organization one of the key takeaways for clinicians is that management of pediatric thyroid nodules and DTC is «challenging and cannot be captured in a one-size-fits-all model.”
She also underlined the need for a “multidisciplinary approach in pediatric thyroid cancer expertise centers.”
Above all, Ms. Lebbink, who is a PhD student in the department of pediatric endocrinology, Wilhelmina Children’s Hospital, Utrecht, the Netherlands, said that pediatric DTC “is not adult DTC in a small person; it has different genetics and a different clinical behavior.”
The authors noted that DTC may be a rare disease, but its worldwide incidence is rising. It has several histologic subtypes, although the “vast majority” of cases are papillary thyroid carcinoma.
Crucially, there are “important differences” between adult and pediatric DTC in terms of their clinical, molecular, and pathologic characteristics, with pediatric patients commonly presenting with more advanced disease with greater lymph node involvement, distant metastases, and multifocal disease.
“However, despite the aggressive presentation, the overall survival rates are excellent,” Ms. Lebbink said.
There are also differences in genetic alterations between adult and pediatric patients. RET-PTC and NTRK fusions are more common in pediatric patients, while mutations in BRAF V600E and RAS point mutations are less frequent.
First European guidelines on thyroid cancer, thyroid nodules in children
Despite these differences, and the existence of U.S. guidelines, until now there have been no European recommendations on the management of pediatric thyroid nodules and DTC.
The European Thyroid Association therefore convened a panel of experts in pediatric and adult endocrinology, pathology, endocrine surgery, nuclear medicine, clinical genetics, and oncology, and tasked them with looking at diagnostics and staging, treatment, and follow-up.
The 2015 American Thyroid Association pediatric guideline was used as framework for the European guideline, with the expert panel identifying areas of discordance and outstanding clinical questions (Thyroid. 2015 Jul;25[7]:716-59).
To answer these questions, they searched PubMed and identified 3,251 studies, of which 45 studies met the inclusion criteria. From this they developed a comprehensive set of recommendations. These include that a child with suspected or proven cancer be referred to an experienced multidisciplinary team and their likely benefit from higher- versus lower-intensity treatment be established.
In addition, children should undergo a preoperative evaluation, with neck palpation, comprehensive neck ultrasonography, and laboratory work-up as a minimum, with further testing suggested in case of a family history or extensive disease.
Total thyroidectomy is the recommended treatment, although the authors call for further studies to assess the impact of limited surgery, and they suggest that prophylactic central lymph node dissection be reserved for advanced cases.
Crucially, all children “should be operated on by high-volume pediatric thyroid cancer surgeons with experience in pediatric thyroid cancer and who are embedded in a center with expertise in the management of DTC,” they wrote.
RAI therapy recommended for all children, in contrast to ATA guidelines
Radioactive iodine (I-131) therapy is recommended for all children following total thyroidectomy, with treatment following an individual patient-based approach.
This differs slightly from the ATA guidelines, which recommend against radioactive iodine (RAI) therapy for children with low-risk differentiated thyroid cancer that is mostly confined to the thyroid (N0 or minimal N1a disease). A study presented at the recent 2022 annual meeting of the ATA found that such children who were spared RAI showed no increases in risk of remission compared with those who did receive it.
The ETA guidelines then go on to recommend that children should be followed up with thyroid-stimulating hormone monitoring and suppression to low-normal levels, as well as serum thyroglobulin measurement and neck ultrasound, although other imaging modalities are not recommended.
In children with persistent or recurrent cervical disease, “surgery or I-131 therapy are indicated depending on the size, tumor load, and degree of progression,” and the authors said that cases of radioactive refractory disease should be “thoroughly investigated.”
Patients should also be counseled on the risk of the late effects of treatment for DTC and undergo monitoring, with follow-up continued for at least 10 years. Any subsequent follow-up should be “the result of shared decision-making between the physician and the patient.”
Evidence for molecular testing is scarce
Ms. Lebbink said that developing the guidelines nevertheless revealed a series of gaps in current knowledge, notably that the evidence for molecular testing “and the clinical implications in the preoperative stage are scarce.”
Specifically, the “positive and negative predictive value of molecular testing in fine needle biopsy specimen for the presence of DTC in a thyroid nodule must be further investigated.»
She also said that there has been a shift towards less aggressive treatment, due to a reluctance to performed prophylactic central neck dissection, and to offer I-131 therapy after surgery.
“However, before less aggressive treatment could be recommended,” Ms. Lebbink said, “it first must be investigated if there are differences in outcomes,” such as recurrence rates, disease-free survival rates, and survival rates between patients who do and do not receive the treatments.
No funding was declared. One author has reported relationships with Sanofi, AstraZeneca, Bayer, and GE. No other relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Comprehensive evaluation, multidisciplinary care, individualized treatment, and ongoing follow-up are all key to the management of pediatric thyroid nodules and differentiated thyroid carcinoma (DTC), according to the first European guidelines for this rare disease.
The guidelines were recently published in the European Thyroid Journal.
Lead author Chantal A. Lebbink told this news organization one of the key takeaways for clinicians is that management of pediatric thyroid nodules and DTC is «challenging and cannot be captured in a one-size-fits-all model.”
She also underlined the need for a “multidisciplinary approach in pediatric thyroid cancer expertise centers.”
Above all, Ms. Lebbink, who is a PhD student in the department of pediatric endocrinology, Wilhelmina Children’s Hospital, Utrecht, the Netherlands, said that pediatric DTC “is not adult DTC in a small person; it has different genetics and a different clinical behavior.”
The authors noted that DTC may be a rare disease, but its worldwide incidence is rising. It has several histologic subtypes, although the “vast majority” of cases are papillary thyroid carcinoma.
Crucially, there are “important differences” between adult and pediatric DTC in terms of their clinical, molecular, and pathologic characteristics, with pediatric patients commonly presenting with more advanced disease with greater lymph node involvement, distant metastases, and multifocal disease.
“However, despite the aggressive presentation, the overall survival rates are excellent,” Ms. Lebbink said.
There are also differences in genetic alterations between adult and pediatric patients. RET-PTC and NTRK fusions are more common in pediatric patients, while mutations in BRAF V600E and RAS point mutations are less frequent.
First European guidelines on thyroid cancer, thyroid nodules in children
Despite these differences, and the existence of U.S. guidelines, until now there have been no European recommendations on the management of pediatric thyroid nodules and DTC.
The European Thyroid Association therefore convened a panel of experts in pediatric and adult endocrinology, pathology, endocrine surgery, nuclear medicine, clinical genetics, and oncology, and tasked them with looking at diagnostics and staging, treatment, and follow-up.
The 2015 American Thyroid Association pediatric guideline was used as framework for the European guideline, with the expert panel identifying areas of discordance and outstanding clinical questions (Thyroid. 2015 Jul;25[7]:716-59).
To answer these questions, they searched PubMed and identified 3,251 studies, of which 45 studies met the inclusion criteria. From this they developed a comprehensive set of recommendations. These include that a child with suspected or proven cancer be referred to an experienced multidisciplinary team and their likely benefit from higher- versus lower-intensity treatment be established.
In addition, children should undergo a preoperative evaluation, with neck palpation, comprehensive neck ultrasonography, and laboratory work-up as a minimum, with further testing suggested in case of a family history or extensive disease.
Total thyroidectomy is the recommended treatment, although the authors call for further studies to assess the impact of limited surgery, and they suggest that prophylactic central lymph node dissection be reserved for advanced cases.
Crucially, all children “should be operated on by high-volume pediatric thyroid cancer surgeons with experience in pediatric thyroid cancer and who are embedded in a center with expertise in the management of DTC,” they wrote.
RAI therapy recommended for all children, in contrast to ATA guidelines
Radioactive iodine (I-131) therapy is recommended for all children following total thyroidectomy, with treatment following an individual patient-based approach.
This differs slightly from the ATA guidelines, which recommend against radioactive iodine (RAI) therapy for children with low-risk differentiated thyroid cancer that is mostly confined to the thyroid (N0 or minimal N1a disease). A study presented at the recent 2022 annual meeting of the ATA found that such children who were spared RAI showed no increases in risk of remission compared with those who did receive it.
The ETA guidelines then go on to recommend that children should be followed up with thyroid-stimulating hormone monitoring and suppression to low-normal levels, as well as serum thyroglobulin measurement and neck ultrasound, although other imaging modalities are not recommended.
In children with persistent or recurrent cervical disease, “surgery or I-131 therapy are indicated depending on the size, tumor load, and degree of progression,” and the authors said that cases of radioactive refractory disease should be “thoroughly investigated.”
Patients should also be counseled on the risk of the late effects of treatment for DTC and undergo monitoring, with follow-up continued for at least 10 years. Any subsequent follow-up should be “the result of shared decision-making between the physician and the patient.”
Evidence for molecular testing is scarce
Ms. Lebbink said that developing the guidelines nevertheless revealed a series of gaps in current knowledge, notably that the evidence for molecular testing “and the clinical implications in the preoperative stage are scarce.”
Specifically, the “positive and negative predictive value of molecular testing in fine needle biopsy specimen for the presence of DTC in a thyroid nodule must be further investigated.»
She also said that there has been a shift towards less aggressive treatment, due to a reluctance to performed prophylactic central neck dissection, and to offer I-131 therapy after surgery.
“However, before less aggressive treatment could be recommended,” Ms. Lebbink said, “it first must be investigated if there are differences in outcomes,” such as recurrence rates, disease-free survival rates, and survival rates between patients who do and do not receive the treatments.
No funding was declared. One author has reported relationships with Sanofi, AstraZeneca, Bayer, and GE. No other relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Comprehensive evaluation, multidisciplinary care, individualized treatment, and ongoing follow-up are all key to the management of pediatric thyroid nodules and differentiated thyroid carcinoma (DTC), according to the first European guidelines for this rare disease.
The guidelines were recently published in the European Thyroid Journal.
Lead author Chantal A. Lebbink told this news organization one of the key takeaways for clinicians is that management of pediatric thyroid nodules and DTC is «challenging and cannot be captured in a one-size-fits-all model.”
She also underlined the need for a “multidisciplinary approach in pediatric thyroid cancer expertise centers.”
Above all, Ms. Lebbink, who is a PhD student in the department of pediatric endocrinology, Wilhelmina Children’s Hospital, Utrecht, the Netherlands, said that pediatric DTC “is not adult DTC in a small person; it has different genetics and a different clinical behavior.”
The authors noted that DTC may be a rare disease, but its worldwide incidence is rising. It has several histologic subtypes, although the “vast majority” of cases are papillary thyroid carcinoma.
Crucially, there are “important differences” between adult and pediatric DTC in terms of their clinical, molecular, and pathologic characteristics, with pediatric patients commonly presenting with more advanced disease with greater lymph node involvement, distant metastases, and multifocal disease.
“However, despite the aggressive presentation, the overall survival rates are excellent,” Ms. Lebbink said.
There are also differences in genetic alterations between adult and pediatric patients. RET-PTC and NTRK fusions are more common in pediatric patients, while mutations in BRAF V600E and RAS point mutations are less frequent.
First European guidelines on thyroid cancer, thyroid nodules in children
Despite these differences, and the existence of U.S. guidelines, until now there have been no European recommendations on the management of pediatric thyroid nodules and DTC.
The European Thyroid Association therefore convened a panel of experts in pediatric and adult endocrinology, pathology, endocrine surgery, nuclear medicine, clinical genetics, and oncology, and tasked them with looking at diagnostics and staging, treatment, and follow-up.
The 2015 American Thyroid Association pediatric guideline was used as framework for the European guideline, with the expert panel identifying areas of discordance and outstanding clinical questions (Thyroid. 2015 Jul;25[7]:716-59).
To answer these questions, they searched PubMed and identified 3,251 studies, of which 45 studies met the inclusion criteria. From this they developed a comprehensive set of recommendations. These include that a child with suspected or proven cancer be referred to an experienced multidisciplinary team and their likely benefit from higher- versus lower-intensity treatment be established.
In addition, children should undergo a preoperative evaluation, with neck palpation, comprehensive neck ultrasonography, and laboratory work-up as a minimum, with further testing suggested in case of a family history or extensive disease.
Total thyroidectomy is the recommended treatment, although the authors call for further studies to assess the impact of limited surgery, and they suggest that prophylactic central lymph node dissection be reserved for advanced cases.
Crucially, all children “should be operated on by high-volume pediatric thyroid cancer surgeons with experience in pediatric thyroid cancer and who are embedded in a center with expertise in the management of DTC,” they wrote.
RAI therapy recommended for all children, in contrast to ATA guidelines
Radioactive iodine (I-131) therapy is recommended for all children following total thyroidectomy, with treatment following an individual patient-based approach.
This differs slightly from the ATA guidelines, which recommend against radioactive iodine (RAI) therapy for children with low-risk differentiated thyroid cancer that is mostly confined to the thyroid (N0 or minimal N1a disease). A study presented at the recent 2022 annual meeting of the ATA found that such children who were spared RAI showed no increases in risk of remission compared with those who did receive it.
The ETA guidelines then go on to recommend that children should be followed up with thyroid-stimulating hormone monitoring and suppression to low-normal levels, as well as serum thyroglobulin measurement and neck ultrasound, although other imaging modalities are not recommended.
In children with persistent or recurrent cervical disease, “surgery or I-131 therapy are indicated depending on the size, tumor load, and degree of progression,” and the authors said that cases of radioactive refractory disease should be “thoroughly investigated.”
Patients should also be counseled on the risk of the late effects of treatment for DTC and undergo monitoring, with follow-up continued for at least 10 years. Any subsequent follow-up should be “the result of shared decision-making between the physician and the patient.”
Evidence for molecular testing is scarce
Ms. Lebbink said that developing the guidelines nevertheless revealed a series of gaps in current knowledge, notably that the evidence for molecular testing “and the clinical implications in the preoperative stage are scarce.”
Specifically, the “positive and negative predictive value of molecular testing in fine needle biopsy specimen for the presence of DTC in a thyroid nodule must be further investigated.»
She also said that there has been a shift towards less aggressive treatment, due to a reluctance to performed prophylactic central neck dissection, and to offer I-131 therapy after surgery.
“However, before less aggressive treatment could be recommended,” Ms. Lebbink said, “it first must be investigated if there are differences in outcomes,” such as recurrence rates, disease-free survival rates, and survival rates between patients who do and do not receive the treatments.
No funding was declared. One author has reported relationships with Sanofi, AstraZeneca, Bayer, and GE. No other relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN THYROID JOURNAL
Safe to expand limits of active surveillance in thyroid cancer?
Expanding eligibility for active surveillance in low-risk papillary thyroid cancer appears to be safe, a new prospective trial indicates.
Researchers found that doubling the limits for tumor size to 2 cm and nearly doubling the limits for tumor growth in low-risk papillary thyroid cancer showed no increased risk of adverse outcomes or mortality for patients undergoing active surveillance versus surgery.
“The results of this nonrandomized controlled trial suggest the basis of a more permissive strategy for thyroid cancer management, strengthening the evidence for active surveillance and broadening potential candidacy to most diagnosed thyroid cancers,” the authors conclude. “By extending [tumor] size/growth limits, these study results potentially broaden the potential candidacy for active surveillance and reduce the likelihood of surgery by lengthening the window of observation.”
However, “the expanded parameters are quite controversial,” first author Allen S. Ho, MD, of Cedars-Sinai Medical Center, Los Angeles, told this news organization. Prior studies have only examined tumor size limits up to 1 cm and “clinicians rarely recommend active surveillance up to 2 cm,” Dr. Ho noted. “As far as we know, Cedars-Sinai is the only place that will consider it.”
In addition, the ultimate decision surrounding active surveillance versus surgery may depend on the patient’s level of anxiety, researchers found.
The research was published in JAMA Oncology.
The potential to expand criteria for thyroid cancer active surveillance comes amid ongoing concerns surrounding overtreatment. Advances in technology have led to increased detection of small, often indolent thyroid cancers that can likely be monitored safely through active surveillance but may present decision-making challenges for clinicians about whether to treat or watch and wait.
Similar challenges in prostate cancer have been addressed with tiered risk stratification, but such guidelines have not been as firmly established in thyroid cancer.
Guidelines from the American Thyroid Association in 2015 suggest active surveillance as an alternative for very low-risk tumors; however, studies in general have recommended the approach for initial tumor sizes of only up to 1 cm and with growth of less than 3 mm. And overall, active surveillance has not been broadly adopted as an option in thyroid cancer, the authors explained.
To determine if criteria for active surveillance can be safely expanded to tumors up to 2 cm and for those with growth up to 5 mm, Dr. Ho and colleagues compared outcomes among 222 patients with Bethesda 5 or 6 nodules of 2 cm or smaller who received either active surveillance or immediate surgery.
The patients were recruited from Cedars-Sinai Medical Center between 2014 and 2021. Patients were a median 46.8 years old; 76% were female.
The median size of tumors was 11 mm, with about 60% representing larger tumors (10.1 to 20 mm) and 20.6% measuring 15.1 to 20 mm.
About half of patients (n = 112) chose active surveillance. The median size of tumors in this group was smaller than those in the surgery group (10.1 mm vs. 12 mm). Tumor growth exceeded 5 mm in 3.6% of cases, and tumor volume increases of more than 100% occurred in 7% of cases.
With a mean follow-up of 37 months, 90% (101) of those on active surveillance continued with that approach. Notably, 41% of these patients demonstrated a decrease in tumor size, and no cases of metastatic lymph nodes or distant metastases emerged.
Of the 110 patients who elected to undergo immediate surgery, 19% (21) had equivocal-risk or undetermined features on final pathology, but the disease severity for these patients remained classified as stage I thyroid cancer.
The disease-specific survival and overall survival rates were the same in both groups, at 100%.
Although a general concern is that larger tumors may be more likely to grow, it’s important to note that “papillary thyroid cancer exists in a spectrum,” Dr. Ho explained. What that means is “some smaller cancers grow quickly, while some larger cancers are stable for decades.”
“We believe that a 1 cm cutoff is arbitrary,” Dr. Ho said, adding that 2 cm cancers that grow will still be within the therapeutic window for safe surgery.
However, a key factor in treatment decisions is patient fear. The authors also looked at the anxiety levels in both groups, using the 18-item Thyroid Cancer Modified Anxiety Scale.
Among the 59 patients who participated, those who chose immediate surgery had significantly higher baseline anxiety levels, compared with those who opted for active surveillance. Notably, these higher rates of anxiety endured over time, including after the intervention.
“It is unsurprising that patients choosing surgery possess a higher baseline level of worry,” Dr. Ho said. “However, we were astonished to find that such patients retained high levels of worry, even after surgery and presumed cure of their cancer.”
The role of the anxiety, however, underscores the need for clinicians to be mindful of the often profound psychological impacts of cancer, even low-risk disease.
“We always encourage clinicians to educate patients on active surveillance, especially as it gets highlighted more in official guidelines,” Dr. Ho noted. “However, we certainly acknowledge that cancer is a life-changing diagnosis, and the term can carry enormous psychological weight.”
The authors also acknowledged several study limitations, including the single-center, nonrandomized design and small sample size, and urge follow-up analyses to “independently verify our findings.”
In an accompanying editorial, Andrea L. Merrill, MD, from Boston Medical Center, and Priya H. Dedhia, MD, PhD, with Ohio State University Wexner Medical Center, said the findings have important clinical implications.
“This provocative study not only lays the groundwork for expanding active surveillance criteria for low-risk papillary thyroid cancer but may also improve use of current American Thyroid Association guidelines for active surveillance by demonstrating that use of active surveillance for Bethesda 5 or 6 nodules 20 mm or smaller was not associated with an increase in staging or disease-specific mortality,” they write.
The study is also notable for being among the first to assess the role of patient anxiety in the selection of immediate surgery versus active surveillance, Dr. Merrill and Dr. Dedhia added.
“These findings imply that patient anxiety should be an essential component of shared decision-making and selection of strategies for low-risk papillary thyroid cancer,” they say.
The study authors and editorial authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Expanding eligibility for active surveillance in low-risk papillary thyroid cancer appears to be safe, a new prospective trial indicates.
Researchers found that doubling the limits for tumor size to 2 cm and nearly doubling the limits for tumor growth in low-risk papillary thyroid cancer showed no increased risk of adverse outcomes or mortality for patients undergoing active surveillance versus surgery.
“The results of this nonrandomized controlled trial suggest the basis of a more permissive strategy for thyroid cancer management, strengthening the evidence for active surveillance and broadening potential candidacy to most diagnosed thyroid cancers,” the authors conclude. “By extending [tumor] size/growth limits, these study results potentially broaden the potential candidacy for active surveillance and reduce the likelihood of surgery by lengthening the window of observation.”
However, “the expanded parameters are quite controversial,” first author Allen S. Ho, MD, of Cedars-Sinai Medical Center, Los Angeles, told this news organization. Prior studies have only examined tumor size limits up to 1 cm and “clinicians rarely recommend active surveillance up to 2 cm,” Dr. Ho noted. “As far as we know, Cedars-Sinai is the only place that will consider it.”
In addition, the ultimate decision surrounding active surveillance versus surgery may depend on the patient’s level of anxiety, researchers found.
The research was published in JAMA Oncology.
The potential to expand criteria for thyroid cancer active surveillance comes amid ongoing concerns surrounding overtreatment. Advances in technology have led to increased detection of small, often indolent thyroid cancers that can likely be monitored safely through active surveillance but may present decision-making challenges for clinicians about whether to treat or watch and wait.
Similar challenges in prostate cancer have been addressed with tiered risk stratification, but such guidelines have not been as firmly established in thyroid cancer.
Guidelines from the American Thyroid Association in 2015 suggest active surveillance as an alternative for very low-risk tumors; however, studies in general have recommended the approach for initial tumor sizes of only up to 1 cm and with growth of less than 3 mm. And overall, active surveillance has not been broadly adopted as an option in thyroid cancer, the authors explained.
To determine if criteria for active surveillance can be safely expanded to tumors up to 2 cm and for those with growth up to 5 mm, Dr. Ho and colleagues compared outcomes among 222 patients with Bethesda 5 or 6 nodules of 2 cm or smaller who received either active surveillance or immediate surgery.
The patients were recruited from Cedars-Sinai Medical Center between 2014 and 2021. Patients were a median 46.8 years old; 76% were female.
The median size of tumors was 11 mm, with about 60% representing larger tumors (10.1 to 20 mm) and 20.6% measuring 15.1 to 20 mm.
About half of patients (n = 112) chose active surveillance. The median size of tumors in this group was smaller than those in the surgery group (10.1 mm vs. 12 mm). Tumor growth exceeded 5 mm in 3.6% of cases, and tumor volume increases of more than 100% occurred in 7% of cases.
With a mean follow-up of 37 months, 90% (101) of those on active surveillance continued with that approach. Notably, 41% of these patients demonstrated a decrease in tumor size, and no cases of metastatic lymph nodes or distant metastases emerged.
Of the 110 patients who elected to undergo immediate surgery, 19% (21) had equivocal-risk or undetermined features on final pathology, but the disease severity for these patients remained classified as stage I thyroid cancer.
The disease-specific survival and overall survival rates were the same in both groups, at 100%.
Although a general concern is that larger tumors may be more likely to grow, it’s important to note that “papillary thyroid cancer exists in a spectrum,” Dr. Ho explained. What that means is “some smaller cancers grow quickly, while some larger cancers are stable for decades.”
“We believe that a 1 cm cutoff is arbitrary,” Dr. Ho said, adding that 2 cm cancers that grow will still be within the therapeutic window for safe surgery.
However, a key factor in treatment decisions is patient fear. The authors also looked at the anxiety levels in both groups, using the 18-item Thyroid Cancer Modified Anxiety Scale.
Among the 59 patients who participated, those who chose immediate surgery had significantly higher baseline anxiety levels, compared with those who opted for active surveillance. Notably, these higher rates of anxiety endured over time, including after the intervention.
“It is unsurprising that patients choosing surgery possess a higher baseline level of worry,” Dr. Ho said. “However, we were astonished to find that such patients retained high levels of worry, even after surgery and presumed cure of their cancer.”
The role of the anxiety, however, underscores the need for clinicians to be mindful of the often profound psychological impacts of cancer, even low-risk disease.
“We always encourage clinicians to educate patients on active surveillance, especially as it gets highlighted more in official guidelines,” Dr. Ho noted. “However, we certainly acknowledge that cancer is a life-changing diagnosis, and the term can carry enormous psychological weight.”
The authors also acknowledged several study limitations, including the single-center, nonrandomized design and small sample size, and urge follow-up analyses to “independently verify our findings.”
In an accompanying editorial, Andrea L. Merrill, MD, from Boston Medical Center, and Priya H. Dedhia, MD, PhD, with Ohio State University Wexner Medical Center, said the findings have important clinical implications.
“This provocative study not only lays the groundwork for expanding active surveillance criteria for low-risk papillary thyroid cancer but may also improve use of current American Thyroid Association guidelines for active surveillance by demonstrating that use of active surveillance for Bethesda 5 or 6 nodules 20 mm or smaller was not associated with an increase in staging or disease-specific mortality,” they write.
The study is also notable for being among the first to assess the role of patient anxiety in the selection of immediate surgery versus active surveillance, Dr. Merrill and Dr. Dedhia added.
“These findings imply that patient anxiety should be an essential component of shared decision-making and selection of strategies for low-risk papillary thyroid cancer,” they say.
The study authors and editorial authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Expanding eligibility for active surveillance in low-risk papillary thyroid cancer appears to be safe, a new prospective trial indicates.
Researchers found that doubling the limits for tumor size to 2 cm and nearly doubling the limits for tumor growth in low-risk papillary thyroid cancer showed no increased risk of adverse outcomes or mortality for patients undergoing active surveillance versus surgery.
“The results of this nonrandomized controlled trial suggest the basis of a more permissive strategy for thyroid cancer management, strengthening the evidence for active surveillance and broadening potential candidacy to most diagnosed thyroid cancers,” the authors conclude. “By extending [tumor] size/growth limits, these study results potentially broaden the potential candidacy for active surveillance and reduce the likelihood of surgery by lengthening the window of observation.”
However, “the expanded parameters are quite controversial,” first author Allen S. Ho, MD, of Cedars-Sinai Medical Center, Los Angeles, told this news organization. Prior studies have only examined tumor size limits up to 1 cm and “clinicians rarely recommend active surveillance up to 2 cm,” Dr. Ho noted. “As far as we know, Cedars-Sinai is the only place that will consider it.”
In addition, the ultimate decision surrounding active surveillance versus surgery may depend on the patient’s level of anxiety, researchers found.
The research was published in JAMA Oncology.
The potential to expand criteria for thyroid cancer active surveillance comes amid ongoing concerns surrounding overtreatment. Advances in technology have led to increased detection of small, often indolent thyroid cancers that can likely be monitored safely through active surveillance but may present decision-making challenges for clinicians about whether to treat or watch and wait.
Similar challenges in prostate cancer have been addressed with tiered risk stratification, but such guidelines have not been as firmly established in thyroid cancer.
Guidelines from the American Thyroid Association in 2015 suggest active surveillance as an alternative for very low-risk tumors; however, studies in general have recommended the approach for initial tumor sizes of only up to 1 cm and with growth of less than 3 mm. And overall, active surveillance has not been broadly adopted as an option in thyroid cancer, the authors explained.
To determine if criteria for active surveillance can be safely expanded to tumors up to 2 cm and for those with growth up to 5 mm, Dr. Ho and colleagues compared outcomes among 222 patients with Bethesda 5 or 6 nodules of 2 cm or smaller who received either active surveillance or immediate surgery.
The patients were recruited from Cedars-Sinai Medical Center between 2014 and 2021. Patients were a median 46.8 years old; 76% were female.
The median size of tumors was 11 mm, with about 60% representing larger tumors (10.1 to 20 mm) and 20.6% measuring 15.1 to 20 mm.
About half of patients (n = 112) chose active surveillance. The median size of tumors in this group was smaller than those in the surgery group (10.1 mm vs. 12 mm). Tumor growth exceeded 5 mm in 3.6% of cases, and tumor volume increases of more than 100% occurred in 7% of cases.
With a mean follow-up of 37 months, 90% (101) of those on active surveillance continued with that approach. Notably, 41% of these patients demonstrated a decrease in tumor size, and no cases of metastatic lymph nodes or distant metastases emerged.
Of the 110 patients who elected to undergo immediate surgery, 19% (21) had equivocal-risk or undetermined features on final pathology, but the disease severity for these patients remained classified as stage I thyroid cancer.
The disease-specific survival and overall survival rates were the same in both groups, at 100%.
Although a general concern is that larger tumors may be more likely to grow, it’s important to note that “papillary thyroid cancer exists in a spectrum,” Dr. Ho explained. What that means is “some smaller cancers grow quickly, while some larger cancers are stable for decades.”
“We believe that a 1 cm cutoff is arbitrary,” Dr. Ho said, adding that 2 cm cancers that grow will still be within the therapeutic window for safe surgery.
However, a key factor in treatment decisions is patient fear. The authors also looked at the anxiety levels in both groups, using the 18-item Thyroid Cancer Modified Anxiety Scale.
Among the 59 patients who participated, those who chose immediate surgery had significantly higher baseline anxiety levels, compared with those who opted for active surveillance. Notably, these higher rates of anxiety endured over time, including after the intervention.
“It is unsurprising that patients choosing surgery possess a higher baseline level of worry,” Dr. Ho said. “However, we were astonished to find that such patients retained high levels of worry, even after surgery and presumed cure of their cancer.”
The role of the anxiety, however, underscores the need for clinicians to be mindful of the often profound psychological impacts of cancer, even low-risk disease.
“We always encourage clinicians to educate patients on active surveillance, especially as it gets highlighted more in official guidelines,” Dr. Ho noted. “However, we certainly acknowledge that cancer is a life-changing diagnosis, and the term can carry enormous psychological weight.”
The authors also acknowledged several study limitations, including the single-center, nonrandomized design and small sample size, and urge follow-up analyses to “independently verify our findings.”
In an accompanying editorial, Andrea L. Merrill, MD, from Boston Medical Center, and Priya H. Dedhia, MD, PhD, with Ohio State University Wexner Medical Center, said the findings have important clinical implications.
“This provocative study not only lays the groundwork for expanding active surveillance criteria for low-risk papillary thyroid cancer but may also improve use of current American Thyroid Association guidelines for active surveillance by demonstrating that use of active surveillance for Bethesda 5 or 6 nodules 20 mm or smaller was not associated with an increase in staging or disease-specific mortality,” they write.
The study is also notable for being among the first to assess the role of patient anxiety in the selection of immediate surgery versus active surveillance, Dr. Merrill and Dr. Dedhia added.
“These findings imply that patient anxiety should be an essential component of shared decision-making and selection of strategies for low-risk papillary thyroid cancer,” they say.
The study authors and editorial authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with low-risk thyroid cancer can skip radioactive iodine
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022
Stopping levothyroxine in subclinical hypothyroidism safe, feasible
MONTREAL – Patients who discontinue levothyroxine for subclinical hypothyroidism may gravitate towards becoming mildly hypothyroid again, but they importantly show no differences in terms of symptoms and quality of life – and sometimes show even improvement – compared with those who continue treatment, new research shows.
“Our results show feasibility of patient enrollment and safety of discontinuing levothyroxine in patients with subclinical hypothyroidism,” said first author Spyridoula Maraka, MD, when presenting the findings at the American Thyroid Association annual meeting.
With evidence showing widespread overtreatment with levothyroxine for a variety of reasons, “a discontinuation study like this is important to understand the true need for life-long thyroxine therapy,” commented James V. Hennessey, MD, director of clinical endocrinology at Beth Israel Deaconess Medical Center, Boston.
Recommendations against levothyroxine for subclinical hypothyroidism
Subclinical hypothyroidism is commonly over-diagnosed, and treatment with thyroid hormone replacement, levothyroxine, has been shown to provide little, if any, benefit in terms of quality of life or relief of thyroid-related symptoms for these patients.
The treatment is meanwhile associated with burdens including cost and lifestyle adjustments, and one guideline panel recently issued a strong recommendation against routine levothyroxine use in most adults with subclinical hypothyroidism.
Nevertheless, levothyroxine treatment has soared in popularity and become one of the most commonly prescribed drugs in the United States.
With research lacking on one key solution of discontinuation of the therapy, Dr. Maraka, who is part of the Division of Endocrinology and Metabolism at the University of Arkansas for Medical Sciences, Little Rock, and colleagues conducted a double-blind, placebo-controlled trial at the Central Arkansas Veterans Healthcare System. In total, 50 patients treated for subclinical hypothyroidism were randomized 1:1 to continue receiving levothyroxine (25-75 mcg daily) or to discontinue treatment and receive a placebo instead, with a planned 6-month follow-up.
In the current interim analysis, Dr. Maraka reported results for the first 40 patients, including 20 randomized to levothyroxine and 20 to discontinuation.
There were no significant differences between the discontinuation and levothyroxine groups at baseline, which were of a similar age (66.2 vs. 70.8 years) and gender (75% women vs. 85% men).
The groups had similar baseline thyroid-stimulating hormone (TSH) levels (3.0 vs. 2.6 mIU/L), free T4 (both 0.9 ng/dL), thyroid peroxidase antibody positivity (17% vs. 11%), and similar clinical symptoms. All patients had at least one elevated TSH reading prior to starting levothyroxine.
With a follow-up of 6-8 weeks, 36.8% of patients in the discontinuation group had subclinical hypothyroidism, compared with 10% of patients who remained on levothyroxine (P = .0648), TSH levels were 5.5 versus 2.7 mIU/L (P = .001) and free T4 levels were 0.8 versus 0.9 ng/dL (P = .011).
No differences in symptoms, quality of life between groups
Importantly, there were no significant differences between the discontinuation versus levothyroxine groups in terms of symptoms, and even some improvements with discontinuation, including Thyroid-Specific Quality of Life Patient-Reported Outcome (ThyPRO)-Hypothyroid Symptoms score (4.6 reduction vs. 2.2 increase), tiredness (2.6 reduction vs. 1.1 increase), and EuroQoL 5-Dimension Self-Report Questionnaire (EQ-5D) quality of life score, for which there were no differences between groups.
There were no reports of overt hypothyroidism; hyperthyroidism; cardiovascular events including atrial fibrillation, stroke, or heart failure; osteoporotic fractures; or deaths.
One patient in the discontinuation group had a TSH level of 11 mIU/L at 6-8 weeks and switched to open-label levothyroxine 75 mcg daily. Another patient in the discontinuation group switched to open-label levothyroxine 75 mcg daily at 10 weeks due to fatigue; however, the patient was diagnosed with metastatic colon cancer 1 month later.
The finding that only about a third of patients who discontinued levothyroxine developed subclinical hypothyroidism was lower than expected, Dr. Maraka noted.
“This was ... unexpected ... for us,” she said. “We were expecting a larger number of patients to develop hypothyroidism, but to our surprise, that was not the case.”
“But what is more important is that there was no difference in the quality of life measures,” she added. “If anything, the placebo group was a little better, though the [differences] were not statistically significant.”
Dr. Maraka also noted that in further research and a final 6-month analysis, the authors will look at factors associated with developing subclinical hypothyroidism after treatment discontinuation, among other issues.
Discontinuation of levothyroxine is manageable
The results are encouraging, as they provide assurance that discontinuation of levothyroxine is manageable.
“This research will pave the way for initiatives to promote levothyroxine deprescription and implementation of evidence-based care for patients with subclinical hypothyroidism,” she said.
In further comments, Dr. Hennessey noted that the dilemma of having patients on levothyroxine who may not be benefitting from treatment is “significant,” with patients sometimes reluctant to discontinue treatment due to concerns of developing hypothyroidism-associated symptoms such as brain fog and weight gain.
He noted, however, that “many with mildly elevated TSH actually go on to normalize with time, so they are not really hypothyroid, [and] if we remove thyroxine from people with normal thyroid function, they will remain normal.”
Dr. Maraka has reported no relevant financial relationships. Dr. Hennessey has reported consulting for pharmaceutical companies to design clinical studies for thyroid medications.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients who discontinue levothyroxine for subclinical hypothyroidism may gravitate towards becoming mildly hypothyroid again, but they importantly show no differences in terms of symptoms and quality of life – and sometimes show even improvement – compared with those who continue treatment, new research shows.
“Our results show feasibility of patient enrollment and safety of discontinuing levothyroxine in patients with subclinical hypothyroidism,” said first author Spyridoula Maraka, MD, when presenting the findings at the American Thyroid Association annual meeting.
With evidence showing widespread overtreatment with levothyroxine for a variety of reasons, “a discontinuation study like this is important to understand the true need for life-long thyroxine therapy,” commented James V. Hennessey, MD, director of clinical endocrinology at Beth Israel Deaconess Medical Center, Boston.
Recommendations against levothyroxine for subclinical hypothyroidism
Subclinical hypothyroidism is commonly over-diagnosed, and treatment with thyroid hormone replacement, levothyroxine, has been shown to provide little, if any, benefit in terms of quality of life or relief of thyroid-related symptoms for these patients.
The treatment is meanwhile associated with burdens including cost and lifestyle adjustments, and one guideline panel recently issued a strong recommendation against routine levothyroxine use in most adults with subclinical hypothyroidism.
Nevertheless, levothyroxine treatment has soared in popularity and become one of the most commonly prescribed drugs in the United States.
With research lacking on one key solution of discontinuation of the therapy, Dr. Maraka, who is part of the Division of Endocrinology and Metabolism at the University of Arkansas for Medical Sciences, Little Rock, and colleagues conducted a double-blind, placebo-controlled trial at the Central Arkansas Veterans Healthcare System. In total, 50 patients treated for subclinical hypothyroidism were randomized 1:1 to continue receiving levothyroxine (25-75 mcg daily) or to discontinue treatment and receive a placebo instead, with a planned 6-month follow-up.
In the current interim analysis, Dr. Maraka reported results for the first 40 patients, including 20 randomized to levothyroxine and 20 to discontinuation.
There were no significant differences between the discontinuation and levothyroxine groups at baseline, which were of a similar age (66.2 vs. 70.8 years) and gender (75% women vs. 85% men).
The groups had similar baseline thyroid-stimulating hormone (TSH) levels (3.0 vs. 2.6 mIU/L), free T4 (both 0.9 ng/dL), thyroid peroxidase antibody positivity (17% vs. 11%), and similar clinical symptoms. All patients had at least one elevated TSH reading prior to starting levothyroxine.
With a follow-up of 6-8 weeks, 36.8% of patients in the discontinuation group had subclinical hypothyroidism, compared with 10% of patients who remained on levothyroxine (P = .0648), TSH levels were 5.5 versus 2.7 mIU/L (P = .001) and free T4 levels were 0.8 versus 0.9 ng/dL (P = .011).
No differences in symptoms, quality of life between groups
Importantly, there were no significant differences between the discontinuation versus levothyroxine groups in terms of symptoms, and even some improvements with discontinuation, including Thyroid-Specific Quality of Life Patient-Reported Outcome (ThyPRO)-Hypothyroid Symptoms score (4.6 reduction vs. 2.2 increase), tiredness (2.6 reduction vs. 1.1 increase), and EuroQoL 5-Dimension Self-Report Questionnaire (EQ-5D) quality of life score, for which there were no differences between groups.
There were no reports of overt hypothyroidism; hyperthyroidism; cardiovascular events including atrial fibrillation, stroke, or heart failure; osteoporotic fractures; or deaths.
One patient in the discontinuation group had a TSH level of 11 mIU/L at 6-8 weeks and switched to open-label levothyroxine 75 mcg daily. Another patient in the discontinuation group switched to open-label levothyroxine 75 mcg daily at 10 weeks due to fatigue; however, the patient was diagnosed with metastatic colon cancer 1 month later.
The finding that only about a third of patients who discontinued levothyroxine developed subclinical hypothyroidism was lower than expected, Dr. Maraka noted.
“This was ... unexpected ... for us,” she said. “We were expecting a larger number of patients to develop hypothyroidism, but to our surprise, that was not the case.”
“But what is more important is that there was no difference in the quality of life measures,” she added. “If anything, the placebo group was a little better, though the [differences] were not statistically significant.”
Dr. Maraka also noted that in further research and a final 6-month analysis, the authors will look at factors associated with developing subclinical hypothyroidism after treatment discontinuation, among other issues.
Discontinuation of levothyroxine is manageable
The results are encouraging, as they provide assurance that discontinuation of levothyroxine is manageable.
“This research will pave the way for initiatives to promote levothyroxine deprescription and implementation of evidence-based care for patients with subclinical hypothyroidism,” she said.
In further comments, Dr. Hennessey noted that the dilemma of having patients on levothyroxine who may not be benefitting from treatment is “significant,” with patients sometimes reluctant to discontinue treatment due to concerns of developing hypothyroidism-associated symptoms such as brain fog and weight gain.
He noted, however, that “many with mildly elevated TSH actually go on to normalize with time, so they are not really hypothyroid, [and] if we remove thyroxine from people with normal thyroid function, they will remain normal.”
Dr. Maraka has reported no relevant financial relationships. Dr. Hennessey has reported consulting for pharmaceutical companies to design clinical studies for thyroid medications.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients who discontinue levothyroxine for subclinical hypothyroidism may gravitate towards becoming mildly hypothyroid again, but they importantly show no differences in terms of symptoms and quality of life – and sometimes show even improvement – compared with those who continue treatment, new research shows.
“Our results show feasibility of patient enrollment and safety of discontinuing levothyroxine in patients with subclinical hypothyroidism,” said first author Spyridoula Maraka, MD, when presenting the findings at the American Thyroid Association annual meeting.
With evidence showing widespread overtreatment with levothyroxine for a variety of reasons, “a discontinuation study like this is important to understand the true need for life-long thyroxine therapy,” commented James V. Hennessey, MD, director of clinical endocrinology at Beth Israel Deaconess Medical Center, Boston.
Recommendations against levothyroxine for subclinical hypothyroidism
Subclinical hypothyroidism is commonly over-diagnosed, and treatment with thyroid hormone replacement, levothyroxine, has been shown to provide little, if any, benefit in terms of quality of life or relief of thyroid-related symptoms for these patients.
The treatment is meanwhile associated with burdens including cost and lifestyle adjustments, and one guideline panel recently issued a strong recommendation against routine levothyroxine use in most adults with subclinical hypothyroidism.
Nevertheless, levothyroxine treatment has soared in popularity and become one of the most commonly prescribed drugs in the United States.
With research lacking on one key solution of discontinuation of the therapy, Dr. Maraka, who is part of the Division of Endocrinology and Metabolism at the University of Arkansas for Medical Sciences, Little Rock, and colleagues conducted a double-blind, placebo-controlled trial at the Central Arkansas Veterans Healthcare System. In total, 50 patients treated for subclinical hypothyroidism were randomized 1:1 to continue receiving levothyroxine (25-75 mcg daily) or to discontinue treatment and receive a placebo instead, with a planned 6-month follow-up.
In the current interim analysis, Dr. Maraka reported results for the first 40 patients, including 20 randomized to levothyroxine and 20 to discontinuation.
There were no significant differences between the discontinuation and levothyroxine groups at baseline, which were of a similar age (66.2 vs. 70.8 years) and gender (75% women vs. 85% men).
The groups had similar baseline thyroid-stimulating hormone (TSH) levels (3.0 vs. 2.6 mIU/L), free T4 (both 0.9 ng/dL), thyroid peroxidase antibody positivity (17% vs. 11%), and similar clinical symptoms. All patients had at least one elevated TSH reading prior to starting levothyroxine.
With a follow-up of 6-8 weeks, 36.8% of patients in the discontinuation group had subclinical hypothyroidism, compared with 10% of patients who remained on levothyroxine (P = .0648), TSH levels were 5.5 versus 2.7 mIU/L (P = .001) and free T4 levels were 0.8 versus 0.9 ng/dL (P = .011).
No differences in symptoms, quality of life between groups
Importantly, there were no significant differences between the discontinuation versus levothyroxine groups in terms of symptoms, and even some improvements with discontinuation, including Thyroid-Specific Quality of Life Patient-Reported Outcome (ThyPRO)-Hypothyroid Symptoms score (4.6 reduction vs. 2.2 increase), tiredness (2.6 reduction vs. 1.1 increase), and EuroQoL 5-Dimension Self-Report Questionnaire (EQ-5D) quality of life score, for which there were no differences between groups.
There were no reports of overt hypothyroidism; hyperthyroidism; cardiovascular events including atrial fibrillation, stroke, or heart failure; osteoporotic fractures; or deaths.
One patient in the discontinuation group had a TSH level of 11 mIU/L at 6-8 weeks and switched to open-label levothyroxine 75 mcg daily. Another patient in the discontinuation group switched to open-label levothyroxine 75 mcg daily at 10 weeks due to fatigue; however, the patient was diagnosed with metastatic colon cancer 1 month later.
The finding that only about a third of patients who discontinued levothyroxine developed subclinical hypothyroidism was lower than expected, Dr. Maraka noted.
“This was ... unexpected ... for us,” she said. “We were expecting a larger number of patients to develop hypothyroidism, but to our surprise, that was not the case.”
“But what is more important is that there was no difference in the quality of life measures,” she added. “If anything, the placebo group was a little better, though the [differences] were not statistically significant.”
Dr. Maraka also noted that in further research and a final 6-month analysis, the authors will look at factors associated with developing subclinical hypothyroidism after treatment discontinuation, among other issues.
Discontinuation of levothyroxine is manageable
The results are encouraging, as they provide assurance that discontinuation of levothyroxine is manageable.
“This research will pave the way for initiatives to promote levothyroxine deprescription and implementation of evidence-based care for patients with subclinical hypothyroidism,” she said.
In further comments, Dr. Hennessey noted that the dilemma of having patients on levothyroxine who may not be benefitting from treatment is “significant,” with patients sometimes reluctant to discontinue treatment due to concerns of developing hypothyroidism-associated symptoms such as brain fog and weight gain.
He noted, however, that “many with mildly elevated TSH actually go on to normalize with time, so they are not really hypothyroid, [and] if we remove thyroxine from people with normal thyroid function, they will remain normal.”
Dr. Maraka has reported no relevant financial relationships. Dr. Hennessey has reported consulting for pharmaceutical companies to design clinical studies for thyroid medications.
A version of this article first appeared on Medscape.com.
AT ATA 2022
Don’t be afraid of weight gain with hyperthyroid treatment
MONTREAL – Amid common patient concerns about weight gain in the treatment of hyperthyroidism, findings from a large study suggest the therapy with the most favorable survival rate – radioiodine – is not associated with an increased risk of weight gain or obesity.
“EGRET is the first large study using population-based linked community and hospital data to elucidate the long-term consequences of treatment modalities for hyperthyroidism,” said co-author Kristien Boelaert, MD, PhD, while presenting the research at the American Thyroid Association annual meeting.
“The administration of [radioiodine] for hyperthyroidism is associated with a survival benefit for patients with hyperthyroidism and is not associated with increased risks of becoming obese,” Dr. Boelaert, a professor of endocrinology and consultant endocrinologist with the Institute of Applied Health Research, University of Birmingham, England, told this news organization.
However, “overall, there was a nearly 10% risk of major adverse cardiac events [MACE] in patients with hyperthyroidism regardless of the treatment modality used,” she noted.
Commenting on the findings, Jonathon O. Russell, MD, said the study offers surprising – but encouraging – results.
The discovery that radioiodine shows no increase in weight gain “contradicts numerous previous studies which have consistently demonstrated weight gain following definitive radioiodine,” Dr. Russell told this news organization.
Overall, however, “these findings reinforce our knowledge that definitive treatment of an overactive thyroid leads to a longer life – even if there is some weight gain,” added Dr. Russell, who is chief of the Division of Head and Neck Endocrine Surgery at Johns Hopkins, Baltimore.
Hyperthyroidism associated with serious long-term cardiometabolic issues
Hyperthyroidism is associated with serious long-term cardiovascular and metabolic morbidity and mortality, and treatment is therefore essential. However, the swing to hypothyroidism that often occurs afterward commonly results in regaining the weight lost due to the hyperthyroidism, if not more, potentially leading to obesity and its attendant health risks.
To investigate those risks in relation to the three key hyperthyroidism treatments, the authors conducted the EGRET trial. They identified 62,474 patients in the United Kingdom population-based electronic health record database who had newly diagnosed hyperthyroidism and were treated with antithyroid drugs (73.4%), radioiodine (19.5%), or thyroidectomy (7.1%) between April 1997 and December 2015.
Exclusion criteria included those with less than 6 months of antithyroid drugs as the only form of treatment, thyroid cancer, or pregnancy during the first episode.
With a median follow-up of about 8 years, those who were treated with thyroidectomy had a significantly increased risk of gaining weight, compared with the general population (P < .001), and of developing obesity (body mass index > 30 kg/m2; P = .003), while the corresponding increases with antithyroid drugs and radioiodine were not significantly different, compared with the general population over the same period.
In terms of survival, with an average follow-up of about 11 years per person, about 14% of the cohort died, with rates of 14.4% in the antithyroid drug group, 15.8% in the radioiodine group, and 9.2% in the thyroidectomy group.
Mortality rates were further assessed based on an average treatment effects analysis in which the average change was estimated, compared with the index of antithyroid drugs – for instance, if all were treated instead with radioiodine. In that extension of life analysis, those treated with radioiodine could be expected to die, on average, 1.2 years later than those taking antithyroid drugs (P < .001), while those treated with thyroidectomy would be expected to die 0.6 years later, which was not statistically significant.
Using the same average treatment effects analysis, Dr. Boelaert noted, “we found a slightly increased risk of major adverse cardiovascular events following radioiodine, compared with antithyroid drugs; [however], the risk was very small and may not be clinically relevant.”
“Previous data from our and other groups have shown reduced risks of mortality and cardiovascular death following radioiodine-induced hypothyroidism, although this is not confirmed in all studies.”
Weight gain after hyperthyroid treatment drives concerns
The findings are important because weight gain associated with hyperthyroidism treatment is no small matter for many patients, even prompting a lack of adherence to therapy for some, despite its importance, Dr. Boelaert noted.
“Since the majority of patients lose weight as a consequence of being hyperthyroid, it can be expected that they will at least regain the lost weight and possibly even have a weight overshoot,” she explained. “Indeed, many patients are reluctant to accept definitive treatment with surgery or radioiodine out of fear of weight gain.”
“This may cause difficulties to some patients who occasionally may even stop taking antithyroid drugs to prevent this weight regain. Such lack of adherence may have dire consequences and is likely a contributing factor to the increased mortality in these patients,” she observed.
In a previous study of 1,373 patients, Dr. Boelaert and colleagues found that men treated for hyperthyroidism gained an average of 8.0 kg (17.6 lb), and women gained an average of 5.5 kg (12.1 lb).
Compared with the background population, men were significantly more likely to gain weight over the study period (odds ratio, 1.7; P < .001) as were women (OR, 1.3; P < .001). Also in that study, radioiodine was associated with greater weight gain (0.6 kg; P < .001), compared with antithyroid drug treatment alone.
Dr. Russell added that even when weight gain does occur, the payoff of having treated the potentially serious state of hyperthyroidism is a highly beneficial trade-off.
Ultimately, “the goal of treating any patient with Graves’ should be to get them to become hypothyroid as quickly as possible,” he said. “Patients have options, and all of these options can be safe in the right situation.”
“It is unrealistic to think that going from a hyperthyroid state to a low thyroid state will not result in weight gain for many patients,” Dr. Russell added. “But the key point is that overall health is better despite this weight gain.”
Dr. Boelaert has disclosed consulting fees paid to the University of Birmingham by Lilly and Eisai. Dr. Russell has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Amid common patient concerns about weight gain in the treatment of hyperthyroidism, findings from a large study suggest the therapy with the most favorable survival rate – radioiodine – is not associated with an increased risk of weight gain or obesity.
“EGRET is the first large study using population-based linked community and hospital data to elucidate the long-term consequences of treatment modalities for hyperthyroidism,” said co-author Kristien Boelaert, MD, PhD, while presenting the research at the American Thyroid Association annual meeting.
“The administration of [radioiodine] for hyperthyroidism is associated with a survival benefit for patients with hyperthyroidism and is not associated with increased risks of becoming obese,” Dr. Boelaert, a professor of endocrinology and consultant endocrinologist with the Institute of Applied Health Research, University of Birmingham, England, told this news organization.
However, “overall, there was a nearly 10% risk of major adverse cardiac events [MACE] in patients with hyperthyroidism regardless of the treatment modality used,” she noted.
Commenting on the findings, Jonathon O. Russell, MD, said the study offers surprising – but encouraging – results.
The discovery that radioiodine shows no increase in weight gain “contradicts numerous previous studies which have consistently demonstrated weight gain following definitive radioiodine,” Dr. Russell told this news organization.
Overall, however, “these findings reinforce our knowledge that definitive treatment of an overactive thyroid leads to a longer life – even if there is some weight gain,” added Dr. Russell, who is chief of the Division of Head and Neck Endocrine Surgery at Johns Hopkins, Baltimore.
Hyperthyroidism associated with serious long-term cardiometabolic issues
Hyperthyroidism is associated with serious long-term cardiovascular and metabolic morbidity and mortality, and treatment is therefore essential. However, the swing to hypothyroidism that often occurs afterward commonly results in regaining the weight lost due to the hyperthyroidism, if not more, potentially leading to obesity and its attendant health risks.
To investigate those risks in relation to the three key hyperthyroidism treatments, the authors conducted the EGRET trial. They identified 62,474 patients in the United Kingdom population-based electronic health record database who had newly diagnosed hyperthyroidism and were treated with antithyroid drugs (73.4%), radioiodine (19.5%), or thyroidectomy (7.1%) between April 1997 and December 2015.
Exclusion criteria included those with less than 6 months of antithyroid drugs as the only form of treatment, thyroid cancer, or pregnancy during the first episode.
With a median follow-up of about 8 years, those who were treated with thyroidectomy had a significantly increased risk of gaining weight, compared with the general population (P < .001), and of developing obesity (body mass index > 30 kg/m2; P = .003), while the corresponding increases with antithyroid drugs and radioiodine were not significantly different, compared with the general population over the same period.
In terms of survival, with an average follow-up of about 11 years per person, about 14% of the cohort died, with rates of 14.4% in the antithyroid drug group, 15.8% in the radioiodine group, and 9.2% in the thyroidectomy group.
Mortality rates were further assessed based on an average treatment effects analysis in which the average change was estimated, compared with the index of antithyroid drugs – for instance, if all were treated instead with radioiodine. In that extension of life analysis, those treated with radioiodine could be expected to die, on average, 1.2 years later than those taking antithyroid drugs (P < .001), while those treated with thyroidectomy would be expected to die 0.6 years later, which was not statistically significant.
Using the same average treatment effects analysis, Dr. Boelaert noted, “we found a slightly increased risk of major adverse cardiovascular events following radioiodine, compared with antithyroid drugs; [however], the risk was very small and may not be clinically relevant.”
“Previous data from our and other groups have shown reduced risks of mortality and cardiovascular death following radioiodine-induced hypothyroidism, although this is not confirmed in all studies.”
Weight gain after hyperthyroid treatment drives concerns
The findings are important because weight gain associated with hyperthyroidism treatment is no small matter for many patients, even prompting a lack of adherence to therapy for some, despite its importance, Dr. Boelaert noted.
“Since the majority of patients lose weight as a consequence of being hyperthyroid, it can be expected that they will at least regain the lost weight and possibly even have a weight overshoot,” she explained. “Indeed, many patients are reluctant to accept definitive treatment with surgery or radioiodine out of fear of weight gain.”
“This may cause difficulties to some patients who occasionally may even stop taking antithyroid drugs to prevent this weight regain. Such lack of adherence may have dire consequences and is likely a contributing factor to the increased mortality in these patients,” she observed.
In a previous study of 1,373 patients, Dr. Boelaert and colleagues found that men treated for hyperthyroidism gained an average of 8.0 kg (17.6 lb), and women gained an average of 5.5 kg (12.1 lb).
Compared with the background population, men were significantly more likely to gain weight over the study period (odds ratio, 1.7; P < .001) as were women (OR, 1.3; P < .001). Also in that study, radioiodine was associated with greater weight gain (0.6 kg; P < .001), compared with antithyroid drug treatment alone.
Dr. Russell added that even when weight gain does occur, the payoff of having treated the potentially serious state of hyperthyroidism is a highly beneficial trade-off.
Ultimately, “the goal of treating any patient with Graves’ should be to get them to become hypothyroid as quickly as possible,” he said. “Patients have options, and all of these options can be safe in the right situation.”
“It is unrealistic to think that going from a hyperthyroid state to a low thyroid state will not result in weight gain for many patients,” Dr. Russell added. “But the key point is that overall health is better despite this weight gain.”
Dr. Boelaert has disclosed consulting fees paid to the University of Birmingham by Lilly and Eisai. Dr. Russell has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Amid common patient concerns about weight gain in the treatment of hyperthyroidism, findings from a large study suggest the therapy with the most favorable survival rate – radioiodine – is not associated with an increased risk of weight gain or obesity.
“EGRET is the first large study using population-based linked community and hospital data to elucidate the long-term consequences of treatment modalities for hyperthyroidism,” said co-author Kristien Boelaert, MD, PhD, while presenting the research at the American Thyroid Association annual meeting.
“The administration of [radioiodine] for hyperthyroidism is associated with a survival benefit for patients with hyperthyroidism and is not associated with increased risks of becoming obese,” Dr. Boelaert, a professor of endocrinology and consultant endocrinologist with the Institute of Applied Health Research, University of Birmingham, England, told this news organization.
However, “overall, there was a nearly 10% risk of major adverse cardiac events [MACE] in patients with hyperthyroidism regardless of the treatment modality used,” she noted.
Commenting on the findings, Jonathon O. Russell, MD, said the study offers surprising – but encouraging – results.
The discovery that radioiodine shows no increase in weight gain “contradicts numerous previous studies which have consistently demonstrated weight gain following definitive radioiodine,” Dr. Russell told this news organization.
Overall, however, “these findings reinforce our knowledge that definitive treatment of an overactive thyroid leads to a longer life – even if there is some weight gain,” added Dr. Russell, who is chief of the Division of Head and Neck Endocrine Surgery at Johns Hopkins, Baltimore.
Hyperthyroidism associated with serious long-term cardiometabolic issues
Hyperthyroidism is associated with serious long-term cardiovascular and metabolic morbidity and mortality, and treatment is therefore essential. However, the swing to hypothyroidism that often occurs afterward commonly results in regaining the weight lost due to the hyperthyroidism, if not more, potentially leading to obesity and its attendant health risks.
To investigate those risks in relation to the three key hyperthyroidism treatments, the authors conducted the EGRET trial. They identified 62,474 patients in the United Kingdom population-based electronic health record database who had newly diagnosed hyperthyroidism and were treated with antithyroid drugs (73.4%), radioiodine (19.5%), or thyroidectomy (7.1%) between April 1997 and December 2015.
Exclusion criteria included those with less than 6 months of antithyroid drugs as the only form of treatment, thyroid cancer, or pregnancy during the first episode.
With a median follow-up of about 8 years, those who were treated with thyroidectomy had a significantly increased risk of gaining weight, compared with the general population (P < .001), and of developing obesity (body mass index > 30 kg/m2; P = .003), while the corresponding increases with antithyroid drugs and radioiodine were not significantly different, compared with the general population over the same period.
In terms of survival, with an average follow-up of about 11 years per person, about 14% of the cohort died, with rates of 14.4% in the antithyroid drug group, 15.8% in the radioiodine group, and 9.2% in the thyroidectomy group.
Mortality rates were further assessed based on an average treatment effects analysis in which the average change was estimated, compared with the index of antithyroid drugs – for instance, if all were treated instead with radioiodine. In that extension of life analysis, those treated with radioiodine could be expected to die, on average, 1.2 years later than those taking antithyroid drugs (P < .001), while those treated with thyroidectomy would be expected to die 0.6 years later, which was not statistically significant.
Using the same average treatment effects analysis, Dr. Boelaert noted, “we found a slightly increased risk of major adverse cardiovascular events following radioiodine, compared with antithyroid drugs; [however], the risk was very small and may not be clinically relevant.”
“Previous data from our and other groups have shown reduced risks of mortality and cardiovascular death following radioiodine-induced hypothyroidism, although this is not confirmed in all studies.”
Weight gain after hyperthyroid treatment drives concerns
The findings are important because weight gain associated with hyperthyroidism treatment is no small matter for many patients, even prompting a lack of adherence to therapy for some, despite its importance, Dr. Boelaert noted.
“Since the majority of patients lose weight as a consequence of being hyperthyroid, it can be expected that they will at least regain the lost weight and possibly even have a weight overshoot,” she explained. “Indeed, many patients are reluctant to accept definitive treatment with surgery or radioiodine out of fear of weight gain.”
“This may cause difficulties to some patients who occasionally may even stop taking antithyroid drugs to prevent this weight regain. Such lack of adherence may have dire consequences and is likely a contributing factor to the increased mortality in these patients,” she observed.
In a previous study of 1,373 patients, Dr. Boelaert and colleagues found that men treated for hyperthyroidism gained an average of 8.0 kg (17.6 lb), and women gained an average of 5.5 kg (12.1 lb).
Compared with the background population, men were significantly more likely to gain weight over the study period (odds ratio, 1.7; P < .001) as were women (OR, 1.3; P < .001). Also in that study, radioiodine was associated with greater weight gain (0.6 kg; P < .001), compared with antithyroid drug treatment alone.
Dr. Russell added that even when weight gain does occur, the payoff of having treated the potentially serious state of hyperthyroidism is a highly beneficial trade-off.
Ultimately, “the goal of treating any patient with Graves’ should be to get them to become hypothyroid as quickly as possible,” he said. “Patients have options, and all of these options can be safe in the right situation.”
“It is unrealistic to think that going from a hyperthyroid state to a low thyroid state will not result in weight gain for many patients,” Dr. Russell added. “But the key point is that overall health is better despite this weight gain.”
Dr. Boelaert has disclosed consulting fees paid to the University of Birmingham by Lilly and Eisai. Dr. Russell has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022
Thyroid autoimmunity linked to cancer, but screening not advised
A new study provides more evidence that people with thyroid autoimmunity are more likely than are others to develop papillary thyroid cancer (odds ratio [OR] = 1.90, 95% confidence interval [CI], 1.33-2.70), although the overall risk remains very low.
Researchers aren't recommending routine screening in all patients with thyroid autoimmunity, but they're calling for more research into whether it's a good idea in severe cases. "This is the one circumstance where screening for subclinical disease could make sense," said Donald McLeod, MPH, PhD, an epidemiologist at Royal Brisbane & Women's Hospital in Australia and lead author of the study, published in the Journal of Clinical Oncology. "However, more research is needed because our study is the first to show this result, and we need to prove that screening would make a difference to the prognosis of these patients."
According to Dr. McLeod, "doctors and patients have been wondering about the connection between thyroid autoimmunity and thyroid cancer for many years. In fact, the first report was in 1955. While the association was plausible, all previous studies had potential for biases that could have influenced the results."
For example, he said, multiple studies didn't control for confounders, while others didn't account for the possibility that cancer could have triggered an immune response. "Other case-control studies could have been affected by selection bias, where a diagnosis of thyroid autoimmunity leads to thyroid cancer identification and entry into the study," he said. "Finally, medical surveillance of people diagnosed with thyroid autoimmunity could lead to overdiagnosis, where small, subclinical cancers are diagnosed in those patients but not identified in people who are not under medical follow-up."
For the new retrospective case-control study, researchers compared 451 active-duty members of the U.S. military who developed papillary thyroid cancer from the period of 1996-2014 to matched controls (61% of all subjects were men and the mean age was 36). Those with cancer had their serum collected 3-5 years and 7-10 years before the date of diagnosis - the index date for all subjects. Some of those considered to have thyroid autoimmunity had conditions such as Graves' disease and Hashimoto's thyroiditis.
"Eighty-five percent of cases (379 of 451) had a thyroid-related diagnosis recorded ... before their index date, compared with 5% of controls," the researchers reported. "Most cases (80%) had classical papillary thyroid cancer, with the rest having the follicular variant of papillary thyroid cancer."
After adjustment to account for various confounders, those who were positive for thyroid peroxidase antibodies 7-10 years prior to the index date were more likely to have developed thyroid cancer (OR = 1.90, 95% CI, 1.33-2.70). "The results could not be fully explained by diagnosis of thyroid autoimmunity," the researchers reported, "although when autoimmunity had been identified, thyroid cancers were diagnosed at a very early stage."
Two groups - those with the highest thyroid antibody levels and women - faced the greatest risk, Dr. McLeod said. The results regarding women were the most surprising in the study, he said. "This is the first time this has been found. We think this result needs to be confirmed. If true, it could explain why women have a three-times-higher risk of thyroid cancer than men."
The overall incidence of thyroid cancer in the U.S. was estimated at 13.49 per 100,000 person-years in 2018, with women (76% of cases) and Whites (81%) accounting for the majority. Rates have nearly doubled since 2000. The authors of a 2022 report that disclosed these numbers suggest the rise is due to overdiagnosis of small tumors.
It's not clear why thyroid autoimmunity and thyroid cancer may be linked. "Chronic inflammation from thyroid autoimmunity could cause thyroid cancer, as chronic inflammation in other organs precedes cancers at those sites," Dr. McLeod said. "Alternatively, thyroid autoimmunity could appear to be associated with thyroid cancer because of biases inherent in previous studies, including previous diagnosis of autoimmunity. Thyroid cancer could also induce an immune response, which mimics thyroid autoimmunity and could bias assessment."
As for screening of patients with thyroid autoimmunity, "the main danger is that you will commonly identify small thyroid cancers that would never become clinically apparent," he said. "This leads to unnecessary treatments that can cause complications and give people a cancer label, which can also cause harm. Diagnosis and treatment guidelines recommend against screening the general population for this reason."
Many of those with thyroid autoimmunity developed small cancers, he said, most likely "detected from ultrasound being performed because autoimmune thyroid disease was known. If all patients with thyroid autoimmunity were screened for thyroid cancer, the likelihood is that many people's cancers would be overdiagnosed."
The study was funded by the Walton Family Foundation. Dr. McLeod reports no disclosures. Some of the authors report various relationships with industry.
A new study provides more evidence that people with thyroid autoimmunity are more likely than are others to develop papillary thyroid cancer (odds ratio [OR] = 1.90, 95% confidence interval [CI], 1.33-2.70), although the overall risk remains very low.
Researchers aren't recommending routine screening in all patients with thyroid autoimmunity, but they're calling for more research into whether it's a good idea in severe cases. "This is the one circumstance where screening for subclinical disease could make sense," said Donald McLeod, MPH, PhD, an epidemiologist at Royal Brisbane & Women's Hospital in Australia and lead author of the study, published in the Journal of Clinical Oncology. "However, more research is needed because our study is the first to show this result, and we need to prove that screening would make a difference to the prognosis of these patients."
According to Dr. McLeod, "doctors and patients have been wondering about the connection between thyroid autoimmunity and thyroid cancer for many years. In fact, the first report was in 1955. While the association was plausible, all previous studies had potential for biases that could have influenced the results."
For example, he said, multiple studies didn't control for confounders, while others didn't account for the possibility that cancer could have triggered an immune response. "Other case-control studies could have been affected by selection bias, where a diagnosis of thyroid autoimmunity leads to thyroid cancer identification and entry into the study," he said. "Finally, medical surveillance of people diagnosed with thyroid autoimmunity could lead to overdiagnosis, where small, subclinical cancers are diagnosed in those patients but not identified in people who are not under medical follow-up."
For the new retrospective case-control study, researchers compared 451 active-duty members of the U.S. military who developed papillary thyroid cancer from the period of 1996-2014 to matched controls (61% of all subjects were men and the mean age was 36). Those with cancer had their serum collected 3-5 years and 7-10 years before the date of diagnosis - the index date for all subjects. Some of those considered to have thyroid autoimmunity had conditions such as Graves' disease and Hashimoto's thyroiditis.
"Eighty-five percent of cases (379 of 451) had a thyroid-related diagnosis recorded ... before their index date, compared with 5% of controls," the researchers reported. "Most cases (80%) had classical papillary thyroid cancer, with the rest having the follicular variant of papillary thyroid cancer."
After adjustment to account for various confounders, those who were positive for thyroid peroxidase antibodies 7-10 years prior to the index date were more likely to have developed thyroid cancer (OR = 1.90, 95% CI, 1.33-2.70). "The results could not be fully explained by diagnosis of thyroid autoimmunity," the researchers reported, "although when autoimmunity had been identified, thyroid cancers were diagnosed at a very early stage."
Two groups - those with the highest thyroid antibody levels and women - faced the greatest risk, Dr. McLeod said. The results regarding women were the most surprising in the study, he said. "This is the first time this has been found. We think this result needs to be confirmed. If true, it could explain why women have a three-times-higher risk of thyroid cancer than men."
The overall incidence of thyroid cancer in the U.S. was estimated at 13.49 per 100,000 person-years in 2018, with women (76% of cases) and Whites (81%) accounting for the majority. Rates have nearly doubled since 2000. The authors of a 2022 report that disclosed these numbers suggest the rise is due to overdiagnosis of small tumors.
It's not clear why thyroid autoimmunity and thyroid cancer may be linked. "Chronic inflammation from thyroid autoimmunity could cause thyroid cancer, as chronic inflammation in other organs precedes cancers at those sites," Dr. McLeod said. "Alternatively, thyroid autoimmunity could appear to be associated with thyroid cancer because of biases inherent in previous studies, including previous diagnosis of autoimmunity. Thyroid cancer could also induce an immune response, which mimics thyroid autoimmunity and could bias assessment."
As for screening of patients with thyroid autoimmunity, "the main danger is that you will commonly identify small thyroid cancers that would never become clinically apparent," he said. "This leads to unnecessary treatments that can cause complications and give people a cancer label, which can also cause harm. Diagnosis and treatment guidelines recommend against screening the general population for this reason."
Many of those with thyroid autoimmunity developed small cancers, he said, most likely "detected from ultrasound being performed because autoimmune thyroid disease was known. If all patients with thyroid autoimmunity were screened for thyroid cancer, the likelihood is that many people's cancers would be overdiagnosed."
The study was funded by the Walton Family Foundation. Dr. McLeod reports no disclosures. Some of the authors report various relationships with industry.
A new study provides more evidence that people with thyroid autoimmunity are more likely than are others to develop papillary thyroid cancer (odds ratio [OR] = 1.90, 95% confidence interval [CI], 1.33-2.70), although the overall risk remains very low.
Researchers aren't recommending routine screening in all patients with thyroid autoimmunity, but they're calling for more research into whether it's a good idea in severe cases. "This is the one circumstance where screening for subclinical disease could make sense," said Donald McLeod, MPH, PhD, an epidemiologist at Royal Brisbane & Women's Hospital in Australia and lead author of the study, published in the Journal of Clinical Oncology. "However, more research is needed because our study is the first to show this result, and we need to prove that screening would make a difference to the prognosis of these patients."
According to Dr. McLeod, "doctors and patients have been wondering about the connection between thyroid autoimmunity and thyroid cancer for many years. In fact, the first report was in 1955. While the association was plausible, all previous studies had potential for biases that could have influenced the results."
For example, he said, multiple studies didn't control for confounders, while others didn't account for the possibility that cancer could have triggered an immune response. "Other case-control studies could have been affected by selection bias, where a diagnosis of thyroid autoimmunity leads to thyroid cancer identification and entry into the study," he said. "Finally, medical surveillance of people diagnosed with thyroid autoimmunity could lead to overdiagnosis, where small, subclinical cancers are diagnosed in those patients but not identified in people who are not under medical follow-up."
For the new retrospective case-control study, researchers compared 451 active-duty members of the U.S. military who developed papillary thyroid cancer from the period of 1996-2014 to matched controls (61% of all subjects were men and the mean age was 36). Those with cancer had their serum collected 3-5 years and 7-10 years before the date of diagnosis - the index date for all subjects. Some of those considered to have thyroid autoimmunity had conditions such as Graves' disease and Hashimoto's thyroiditis.
"Eighty-five percent of cases (379 of 451) had a thyroid-related diagnosis recorded ... before their index date, compared with 5% of controls," the researchers reported. "Most cases (80%) had classical papillary thyroid cancer, with the rest having the follicular variant of papillary thyroid cancer."
After adjustment to account for various confounders, those who were positive for thyroid peroxidase antibodies 7-10 years prior to the index date were more likely to have developed thyroid cancer (OR = 1.90, 95% CI, 1.33-2.70). "The results could not be fully explained by diagnosis of thyroid autoimmunity," the researchers reported, "although when autoimmunity had been identified, thyroid cancers were diagnosed at a very early stage."
Two groups - those with the highest thyroid antibody levels and women - faced the greatest risk, Dr. McLeod said. The results regarding women were the most surprising in the study, he said. "This is the first time this has been found. We think this result needs to be confirmed. If true, it could explain why women have a three-times-higher risk of thyroid cancer than men."
The overall incidence of thyroid cancer in the U.S. was estimated at 13.49 per 100,000 person-years in 2018, with women (76% of cases) and Whites (81%) accounting for the majority. Rates have nearly doubled since 2000. The authors of a 2022 report that disclosed these numbers suggest the rise is due to overdiagnosis of small tumors.
It's not clear why thyroid autoimmunity and thyroid cancer may be linked. "Chronic inflammation from thyroid autoimmunity could cause thyroid cancer, as chronic inflammation in other organs precedes cancers at those sites," Dr. McLeod said. "Alternatively, thyroid autoimmunity could appear to be associated with thyroid cancer because of biases inherent in previous studies, including previous diagnosis of autoimmunity. Thyroid cancer could also induce an immune response, which mimics thyroid autoimmunity and could bias assessment."
As for screening of patients with thyroid autoimmunity, "the main danger is that you will commonly identify small thyroid cancers that would never become clinically apparent," he said. "This leads to unnecessary treatments that can cause complications and give people a cancer label, which can also cause harm. Diagnosis and treatment guidelines recommend against screening the general population for this reason."
Many of those with thyroid autoimmunity developed small cancers, he said, most likely "detected from ultrasound being performed because autoimmune thyroid disease was known. If all patients with thyroid autoimmunity were screened for thyroid cancer, the likelihood is that many people's cancers would be overdiagnosed."
The study was funded by the Walton Family Foundation. Dr. McLeod reports no disclosures. Some of the authors report various relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY