User login
Renal transplant improves survival in lupus nephritis patients
, according to researchers who conducted a nationwide cohort study encompassing nearly all such patients treated in the United States over a 20-year period.
Transplant conferred a 70% reduction in overall death risk in these lupus nephritis end-stage renal disease (ESRD) patients, largely due to reduced deaths caused by infection and cardiovascular disease, according to the researchers, led by April Jorge, MD, and Zachary Wallace, MD, of Massachusetts General Hospital, Harvard Medical School, Boston.
Those findings suggest that patients with lupus nephritis ESRD should routinely be considered for renal transplant in a timely manner, the investigators wrote in Annals of Internal Medicine.
“Improved access to renal transplantation for this population may considerably improve outcomes,” they said.
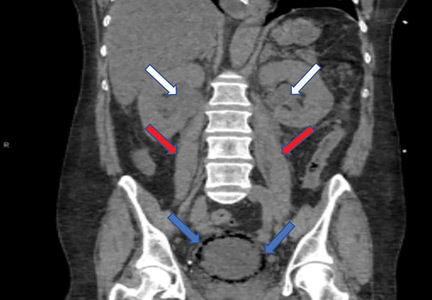
The study was based on an analysis of 9,659 patients who had lupus nephritis ESRD between 1995 and 2014 and were waitlisted for renal transplant. The data came from the United States Renal Data System, which includes most ESRD patients treated in the country. Of those 9,659 patients, 5,738 (59%) underwent kidney transplant.
Mortality rates were 22.5 per 1,000 person-years for lupus nephritis ESRD patients who underwent transplant, and 56.3 per 1,000 person-years for those patients who did not receive transplant, the investigators found.
Renal transplant reduced risk of death by 70% in results of multivariate analysis (hazard ratio, 0.30; 95% CI, 0.27-0.33).
That lower risk of all-cause mortality was consistent across racial groups and for other characteristics, such as sex, age at ESRD onset, and Medicare enrollment status.
Risk of cardiovascular death was 74% lower with renal transplant (adjusted hazard ratio, 0.26; 95% CI, 0.23-0.30), and risk of death from infection was also markedly lower among those who underwent transplant (adjusted hazard ratio, 0.41; 95% CI, 0.32-0.52), investigators found in a cause-specific mortality analysis.
While transplant has been associated with improved survival in patients with ESRD from all causes, there are “unique concerns” regarding the potential for infections or other post-transplant complications from transplant in lupus nephritis patients with ESRD, Dr. Jorge and colleagues wrote.
“To that end, our study provides evidence for a substantial survival benefit of renal transplant among patients with lupus nephritis ESRD,” they noted.
Dr. Jorge reported grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases during the conduct of the study. One co-author provided additional disclosures related to Teva Pharmaceuticals and Gilead Sciences outside of the study conduct.
SOURCE: Jorge A, et al. Ann Intern Med 2019 Jan 21. doi: 10.7326/M18-1570.
This research by Jorge et al is “strong” and has two key implications for clinical practice, said authors of an accompanying editorial in the Annals of Internal Medicine.
The first is that transplantation should be incorporated into the treatment plan for lupus nephritis patients and is particularly important before kidney failure onset, according to Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD.
“This will allow patients, families, and clinicians to devote sufficient resources to completing the transplant evaluation and searching for living donors, the preferred donor source to maximize patient and allograft survival,” they wrote.
Secondly, the evidence to date suggests wider implementation of preemptive kidney transplants would be warranted in patients with lupus nephritis, they said.
Currently, only about 9% of lupus nephritis patients with kidney failure related to lupus nephritis undergo preemptive transplants, versus 17% of patients undergoing kidney transplants for other reasons, according to the authors.
Recent studies, however, suggest preemptive transplants and early kidney transplants in lupus nephritis are indeed linked to improved patient and allograft survival, just as in other conditions, they added.
Taken together, the findings of those studies and the current study by Dr. Jorge and colleagues underscore the pronounced survival advantage attributable to kidney transplant in patients with kidney failure due to lupus nephritis, they concluded.
“It is essential that transplant be considered as promptly as possible for patients with lupus nephritis and that barriers to early transplant be surmounted,” they wrote.
The editorial was authored by Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD, of Tufts Medical Center, Boston. Dr. Goyal and Dr. Levey reported no conflicts of interest. Dr. Weiner provided disclosures related to Keryx Biopharmaceuticals, Relypsa, Inc., Janssen Biopharmaceuticals, Akebia Therapeutics, and others.
This research by Jorge et al is “strong” and has two key implications for clinical practice, said authors of an accompanying editorial in the Annals of Internal Medicine.
The first is that transplantation should be incorporated into the treatment plan for lupus nephritis patients and is particularly important before kidney failure onset, according to Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD.
“This will allow patients, families, and clinicians to devote sufficient resources to completing the transplant evaluation and searching for living donors, the preferred donor source to maximize patient and allograft survival,” they wrote.
Secondly, the evidence to date suggests wider implementation of preemptive kidney transplants would be warranted in patients with lupus nephritis, they said.
Currently, only about 9% of lupus nephritis patients with kidney failure related to lupus nephritis undergo preemptive transplants, versus 17% of patients undergoing kidney transplants for other reasons, according to the authors.
Recent studies, however, suggest preemptive transplants and early kidney transplants in lupus nephritis are indeed linked to improved patient and allograft survival, just as in other conditions, they added.
Taken together, the findings of those studies and the current study by Dr. Jorge and colleagues underscore the pronounced survival advantage attributable to kidney transplant in patients with kidney failure due to lupus nephritis, they concluded.
“It is essential that transplant be considered as promptly as possible for patients with lupus nephritis and that barriers to early transplant be surmounted,” they wrote.
The editorial was authored by Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD, of Tufts Medical Center, Boston. Dr. Goyal and Dr. Levey reported no conflicts of interest. Dr. Weiner provided disclosures related to Keryx Biopharmaceuticals, Relypsa, Inc., Janssen Biopharmaceuticals, Akebia Therapeutics, and others.
This research by Jorge et al is “strong” and has two key implications for clinical practice, said authors of an accompanying editorial in the Annals of Internal Medicine.
The first is that transplantation should be incorporated into the treatment plan for lupus nephritis patients and is particularly important before kidney failure onset, according to Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD.
“This will allow patients, families, and clinicians to devote sufficient resources to completing the transplant evaluation and searching for living donors, the preferred donor source to maximize patient and allograft survival,” they wrote.
Secondly, the evidence to date suggests wider implementation of preemptive kidney transplants would be warranted in patients with lupus nephritis, they said.
Currently, only about 9% of lupus nephritis patients with kidney failure related to lupus nephritis undergo preemptive transplants, versus 17% of patients undergoing kidney transplants for other reasons, according to the authors.
Recent studies, however, suggest preemptive transplants and early kidney transplants in lupus nephritis are indeed linked to improved patient and allograft survival, just as in other conditions, they added.
Taken together, the findings of those studies and the current study by Dr. Jorge and colleagues underscore the pronounced survival advantage attributable to kidney transplant in patients with kidney failure due to lupus nephritis, they concluded.
“It is essential that transplant be considered as promptly as possible for patients with lupus nephritis and that barriers to early transplant be surmounted,” they wrote.
The editorial was authored by Nitender Goyal, MD, Daniel E. Weiner, MD, MS, and Andrew S. Levey, MD, of Tufts Medical Center, Boston. Dr. Goyal and Dr. Levey reported no conflicts of interest. Dr. Weiner provided disclosures related to Keryx Biopharmaceuticals, Relypsa, Inc., Janssen Biopharmaceuticals, Akebia Therapeutics, and others.
, according to researchers who conducted a nationwide cohort study encompassing nearly all such patients treated in the United States over a 20-year period.
Transplant conferred a 70% reduction in overall death risk in these lupus nephritis end-stage renal disease (ESRD) patients, largely due to reduced deaths caused by infection and cardiovascular disease, according to the researchers, led by April Jorge, MD, and Zachary Wallace, MD, of Massachusetts General Hospital, Harvard Medical School, Boston.
Those findings suggest that patients with lupus nephritis ESRD should routinely be considered for renal transplant in a timely manner, the investigators wrote in Annals of Internal Medicine.
“Improved access to renal transplantation for this population may considerably improve outcomes,” they said.
The study was based on an analysis of 9,659 patients who had lupus nephritis ESRD between 1995 and 2014 and were waitlisted for renal transplant. The data came from the United States Renal Data System, which includes most ESRD patients treated in the country. Of those 9,659 patients, 5,738 (59%) underwent kidney transplant.
Mortality rates were 22.5 per 1,000 person-years for lupus nephritis ESRD patients who underwent transplant, and 56.3 per 1,000 person-years for those patients who did not receive transplant, the investigators found.
Renal transplant reduced risk of death by 70% in results of multivariate analysis (hazard ratio, 0.30; 95% CI, 0.27-0.33).
That lower risk of all-cause mortality was consistent across racial groups and for other characteristics, such as sex, age at ESRD onset, and Medicare enrollment status.
Risk of cardiovascular death was 74% lower with renal transplant (adjusted hazard ratio, 0.26; 95% CI, 0.23-0.30), and risk of death from infection was also markedly lower among those who underwent transplant (adjusted hazard ratio, 0.41; 95% CI, 0.32-0.52), investigators found in a cause-specific mortality analysis.
While transplant has been associated with improved survival in patients with ESRD from all causes, there are “unique concerns” regarding the potential for infections or other post-transplant complications from transplant in lupus nephritis patients with ESRD, Dr. Jorge and colleagues wrote.
“To that end, our study provides evidence for a substantial survival benefit of renal transplant among patients with lupus nephritis ESRD,” they noted.
Dr. Jorge reported grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases during the conduct of the study. One co-author provided additional disclosures related to Teva Pharmaceuticals and Gilead Sciences outside of the study conduct.
SOURCE: Jorge A, et al. Ann Intern Med 2019 Jan 21. doi: 10.7326/M18-1570.
, according to researchers who conducted a nationwide cohort study encompassing nearly all such patients treated in the United States over a 20-year period.
Transplant conferred a 70% reduction in overall death risk in these lupus nephritis end-stage renal disease (ESRD) patients, largely due to reduced deaths caused by infection and cardiovascular disease, according to the researchers, led by April Jorge, MD, and Zachary Wallace, MD, of Massachusetts General Hospital, Harvard Medical School, Boston.
Those findings suggest that patients with lupus nephritis ESRD should routinely be considered for renal transplant in a timely manner, the investigators wrote in Annals of Internal Medicine.
“Improved access to renal transplantation for this population may considerably improve outcomes,” they said.
The study was based on an analysis of 9,659 patients who had lupus nephritis ESRD between 1995 and 2014 and were waitlisted for renal transplant. The data came from the United States Renal Data System, which includes most ESRD patients treated in the country. Of those 9,659 patients, 5,738 (59%) underwent kidney transplant.
Mortality rates were 22.5 per 1,000 person-years for lupus nephritis ESRD patients who underwent transplant, and 56.3 per 1,000 person-years for those patients who did not receive transplant, the investigators found.
Renal transplant reduced risk of death by 70% in results of multivariate analysis (hazard ratio, 0.30; 95% CI, 0.27-0.33).
That lower risk of all-cause mortality was consistent across racial groups and for other characteristics, such as sex, age at ESRD onset, and Medicare enrollment status.
Risk of cardiovascular death was 74% lower with renal transplant (adjusted hazard ratio, 0.26; 95% CI, 0.23-0.30), and risk of death from infection was also markedly lower among those who underwent transplant (adjusted hazard ratio, 0.41; 95% CI, 0.32-0.52), investigators found in a cause-specific mortality analysis.
While transplant has been associated with improved survival in patients with ESRD from all causes, there are “unique concerns” regarding the potential for infections or other post-transplant complications from transplant in lupus nephritis patients with ESRD, Dr. Jorge and colleagues wrote.
“To that end, our study provides evidence for a substantial survival benefit of renal transplant among patients with lupus nephritis ESRD,” they noted.
Dr. Jorge reported grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases during the conduct of the study. One co-author provided additional disclosures related to Teva Pharmaceuticals and Gilead Sciences outside of the study conduct.
SOURCE: Jorge A, et al. Ann Intern Med 2019 Jan 21. doi: 10.7326/M18-1570.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Renal transplant is associated with a substantial survival benefit in patients with end-stage renal disease (ESRD) due to lupus nephritis,
Major finding: Transplant conferred a 70% reduction in overall death risk in these lupus nephritis ESRD patients, largely due to reduced deaths caused by infection and cardiovascular disease,
Study details: Analysis of 9,659 patients with lupus nephritis ESRD in the United States Renal Data System.
Disclosures: Support for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One co-author provided disclosures related to Teva Pharmaceuticals and Gilead Sciences.
Source: Jorge A, et al. Ann Intern Med. 2019 Jan 21. doi: 10.7326/M18-1570.
Metformin associated with acidosis only in patients with eGFR 30 mL/min per 1.73 m 2
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Difelikefalin shows promise for hemodialysis-associated itch
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Moderate to severe chronic itching associated with chronic kidney disease is a common and underrecognized problem with a huge quality of life impact.
Major finding: Sixty-four percent of hemodialysis patients on difelikefalin 0.5 mcg/kg experienced at least a 3-point reduction on a 0-10 worst daily itch numeric rating scale, compared with 29% of placebo-treated controls.
Study details: This phase 2, multicenter, 8-week, double-blind study comprised 174 patients with moderate to severe hemodialysis-related itching.
Disclosures: The study was sponsored by Cara Therapeutics and presented by a company officer.
Source: Menzaghi F. EADV Congress, Abstract FC0.4.7.
New diabetes drugs solidify their cardiovascular and renal benefits
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Hypertension guidelines: Treat patients, not numbers
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
KEY POINTS
- The 2017 ACC/AHA guidelines lowered the definition of hypertension to 130/80 mm Hg or higher, thereby in-creasing the number of US adults with hypertension from 31.9% to 45.6%.
- For patients with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, drug treatment “is recommended” if the average blood pressure is 130/80 mm Hg or higher. For those without cardiovascular disease and at lower risk, drug treatment is recommended if the aver-age blood pressure is 140/90 mm Hg or higher.
- A treatment goal of less than 130/80 mm Hg “is recommended” for patients with hypertension and known car-diovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, and “may be reasonable” for those without additional markers of increased cardiovascular risk.
- Intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease, at the price of causing more adverse effects.
Emphysematous cystitis
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
Anticoagulation shows promise in concurrent lupus nephritis, thrombotic microangiopathy
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: A greater proportion of patients given anticoagulation achieved partial or complete response (78.4%) versus those not given anticoagulation (50.0%).
Study details: A retrospective analysis of 97 patients with biopsy-confirmed lupus nephritis and thrombotic microangiopathy.
Disclosures: The authors reported no conflicts of interest, and no specific study funding was declared.
Source: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
CKD, and even dialysis, may not be barriers to pregnancy
SAN DIEGO – Nephrologists are often uncomfortable with the idea of advising women with chronic kidney disease (CKD) about pregnancy, a physician told colleagues. They must do better, she said, with sensitivity and insight into once-extreme possibilities like pregnancy during dialysis.
“For many women, having a child is a life goal, and our women with chronic kidney disease are not different,” said Michelle Hladunewich, MD, of Toronto’s Sunnybrook Health Sciences Center. “When we don’t know what we should do, we tend to over-aggressively counsel our women, and that can traumatize them. It’s our role as nephrologists to help them find the safest window to have their pregnancy,” she said at the meeting sponsored by the American Society of Nephrology.
According to Dr. Hladunewich, there are tens of thousands of women of child-bearing age in the United States who have CKD, end-stage renal disease (ESRD), and kidney transplants. However, she said, research presented at Kidney Week 2018 suggested that many nephrologists do not feel confident about counseling patients regarding issues such as pregnancy outcomes in CKD. “We are not that comfortable with it, but we have to become more comfortable,” she said. “We need to be prepared to talk about contraception if they don’t want to have a child or the plan about how to have a child if they do.”
It’s especially important to understand that while women can fear birth defects and the exacerbation of their disease, they may also feel “they’re not fulfilling a societal norm to have a child like everyone else,” she said.
The risks of pregnancy in CKD can affect the mother (via worse kidney function) and/or the fetus (preeclampsia, poor fetal growth, preterm delivery).
In a 2015 study, Italian researchers compared 504 pregnancies in women with CKD to 836 low-risk pregnancies in women without CKD. They found that the risks of adverse outcomes increased in women at higher stages of CKD, compared with those at lower stages: “Renal function matters, and a stepwise increase in the risk of adverse maternal-fetal outcomes is observed from stage 1 to stages 4-5.”
In addition, the researchers noted that their research suggests “the presence of a baseline risk linked to CKD per se” (J Am Soc Nephrol. 2015 Aug; 26[8]:2011-22).
Dr. Hladunewich recommended focusing on “the safest window of opportunity.” Some patients will progress to end-stage renal disease, and an earlier pregnancy during CKD is a better option, she said. As a result, encouraging an earlier pregnancy can be a wise idea.
In some cases, though, a patient may be far into the stages of CKD. Dr. Hladunewich spoke about the case of a 31-year-old patient with a 29-year history of type 1 diabetes mellitus. She’d had one miscarriage, one preterm birth, and one twin pregnancy that was terminated because of safety concerns including rapid loss of kidney function.
The patient saw Dr. Hladunewich when she had a glomerular filtration rate of 25 mL/min, 3.5 g per 24 hour of proteinuria, and hypertension. The patient had a question: “Dr. Michelle, when can I try again?”
Dr. Hladunewich joked that “I had a small stroke.” But then, she said, “I got to the business of pregnancy counseling.”
She told the woman that her progression to end-stage renal disease was likely inevitable, and “adverse pregnancy outcomes were almost guaranteed.”
The woman responded: “Not now? When?” That, Dr. Hladunewich said, “was when I had my second stroke.”
But there is a possible solution: Pregnancy during dialysis. “Historically, we’ve said absolutely no pregnancy on dialysis,” she said, “but times are changing. We believe aggressive dialysis improves fetal maternal and fetal outcomes.”
Indeed, Dr. Hladunewich led a 2014 study that linked extensive dialysis during pregnancy (compared with less dialysis) to a better likelihood of outcomes such as live birth rate and normal birth weight (JASN May 2014;25[5]:1103-9).
As she noted, “we do offer it as a reproductive option” to patients like the one she mentioned – those who are in ESRD, approaching it, or are nearing the end of their child-bearing years with no transplant in sight. In transplant cases, she said, adequate graft function is linked to good pregnancy outcomes.
Dr. Hladunewich added that it’s important to monitor and adjust treatment of patients during the postpartum period. She said it’s especially important to understand the risks of drugs during breastfeeding. Both dialysis and transplant patients can breastfeed, she said.
Dr. Hladunewich reports no disclosures.
SOURCE: Kidney Week 2018, Abstract FR-OR078.
SAN DIEGO – Nephrologists are often uncomfortable with the idea of advising women with chronic kidney disease (CKD) about pregnancy, a physician told colleagues. They must do better, she said, with sensitivity and insight into once-extreme possibilities like pregnancy during dialysis.
“For many women, having a child is a life goal, and our women with chronic kidney disease are not different,” said Michelle Hladunewich, MD, of Toronto’s Sunnybrook Health Sciences Center. “When we don’t know what we should do, we tend to over-aggressively counsel our women, and that can traumatize them. It’s our role as nephrologists to help them find the safest window to have their pregnancy,” she said at the meeting sponsored by the American Society of Nephrology.
According to Dr. Hladunewich, there are tens of thousands of women of child-bearing age in the United States who have CKD, end-stage renal disease (ESRD), and kidney transplants. However, she said, research presented at Kidney Week 2018 suggested that many nephrologists do not feel confident about counseling patients regarding issues such as pregnancy outcomes in CKD. “We are not that comfortable with it, but we have to become more comfortable,” she said. “We need to be prepared to talk about contraception if they don’t want to have a child or the plan about how to have a child if they do.”
It’s especially important to understand that while women can fear birth defects and the exacerbation of their disease, they may also feel “they’re not fulfilling a societal norm to have a child like everyone else,” she said.
The risks of pregnancy in CKD can affect the mother (via worse kidney function) and/or the fetus (preeclampsia, poor fetal growth, preterm delivery).
In a 2015 study, Italian researchers compared 504 pregnancies in women with CKD to 836 low-risk pregnancies in women without CKD. They found that the risks of adverse outcomes increased in women at higher stages of CKD, compared with those at lower stages: “Renal function matters, and a stepwise increase in the risk of adverse maternal-fetal outcomes is observed from stage 1 to stages 4-5.”
In addition, the researchers noted that their research suggests “the presence of a baseline risk linked to CKD per se” (J Am Soc Nephrol. 2015 Aug; 26[8]:2011-22).
Dr. Hladunewich recommended focusing on “the safest window of opportunity.” Some patients will progress to end-stage renal disease, and an earlier pregnancy during CKD is a better option, she said. As a result, encouraging an earlier pregnancy can be a wise idea.
In some cases, though, a patient may be far into the stages of CKD. Dr. Hladunewich spoke about the case of a 31-year-old patient with a 29-year history of type 1 diabetes mellitus. She’d had one miscarriage, one preterm birth, and one twin pregnancy that was terminated because of safety concerns including rapid loss of kidney function.
The patient saw Dr. Hladunewich when she had a glomerular filtration rate of 25 mL/min, 3.5 g per 24 hour of proteinuria, and hypertension. The patient had a question: “Dr. Michelle, when can I try again?”
Dr. Hladunewich joked that “I had a small stroke.” But then, she said, “I got to the business of pregnancy counseling.”
She told the woman that her progression to end-stage renal disease was likely inevitable, and “adverse pregnancy outcomes were almost guaranteed.”
The woman responded: “Not now? When?” That, Dr. Hladunewich said, “was when I had my second stroke.”
But there is a possible solution: Pregnancy during dialysis. “Historically, we’ve said absolutely no pregnancy on dialysis,” she said, “but times are changing. We believe aggressive dialysis improves fetal maternal and fetal outcomes.”
Indeed, Dr. Hladunewich led a 2014 study that linked extensive dialysis during pregnancy (compared with less dialysis) to a better likelihood of outcomes such as live birth rate and normal birth weight (JASN May 2014;25[5]:1103-9).
As she noted, “we do offer it as a reproductive option” to patients like the one she mentioned – those who are in ESRD, approaching it, or are nearing the end of their child-bearing years with no transplant in sight. In transplant cases, she said, adequate graft function is linked to good pregnancy outcomes.
Dr. Hladunewich added that it’s important to monitor and adjust treatment of patients during the postpartum period. She said it’s especially important to understand the risks of drugs during breastfeeding. Both dialysis and transplant patients can breastfeed, she said.
Dr. Hladunewich reports no disclosures.
SOURCE: Kidney Week 2018, Abstract FR-OR078.
SAN DIEGO – Nephrologists are often uncomfortable with the idea of advising women with chronic kidney disease (CKD) about pregnancy, a physician told colleagues. They must do better, she said, with sensitivity and insight into once-extreme possibilities like pregnancy during dialysis.
“For many women, having a child is a life goal, and our women with chronic kidney disease are not different,” said Michelle Hladunewich, MD, of Toronto’s Sunnybrook Health Sciences Center. “When we don’t know what we should do, we tend to over-aggressively counsel our women, and that can traumatize them. It’s our role as nephrologists to help them find the safest window to have their pregnancy,” she said at the meeting sponsored by the American Society of Nephrology.
According to Dr. Hladunewich, there are tens of thousands of women of child-bearing age in the United States who have CKD, end-stage renal disease (ESRD), and kidney transplants. However, she said, research presented at Kidney Week 2018 suggested that many nephrologists do not feel confident about counseling patients regarding issues such as pregnancy outcomes in CKD. “We are not that comfortable with it, but we have to become more comfortable,” she said. “We need to be prepared to talk about contraception if they don’t want to have a child or the plan about how to have a child if they do.”
It’s especially important to understand that while women can fear birth defects and the exacerbation of their disease, they may also feel “they’re not fulfilling a societal norm to have a child like everyone else,” she said.
The risks of pregnancy in CKD can affect the mother (via worse kidney function) and/or the fetus (preeclampsia, poor fetal growth, preterm delivery).
In a 2015 study, Italian researchers compared 504 pregnancies in women with CKD to 836 low-risk pregnancies in women without CKD. They found that the risks of adverse outcomes increased in women at higher stages of CKD, compared with those at lower stages: “Renal function matters, and a stepwise increase in the risk of adverse maternal-fetal outcomes is observed from stage 1 to stages 4-5.”
In addition, the researchers noted that their research suggests “the presence of a baseline risk linked to CKD per se” (J Am Soc Nephrol. 2015 Aug; 26[8]:2011-22).
Dr. Hladunewich recommended focusing on “the safest window of opportunity.” Some patients will progress to end-stage renal disease, and an earlier pregnancy during CKD is a better option, she said. As a result, encouraging an earlier pregnancy can be a wise idea.
In some cases, though, a patient may be far into the stages of CKD. Dr. Hladunewich spoke about the case of a 31-year-old patient with a 29-year history of type 1 diabetes mellitus. She’d had one miscarriage, one preterm birth, and one twin pregnancy that was terminated because of safety concerns including rapid loss of kidney function.
The patient saw Dr. Hladunewich when she had a glomerular filtration rate of 25 mL/min, 3.5 g per 24 hour of proteinuria, and hypertension. The patient had a question: “Dr. Michelle, when can I try again?”
Dr. Hladunewich joked that “I had a small stroke.” But then, she said, “I got to the business of pregnancy counseling.”
She told the woman that her progression to end-stage renal disease was likely inevitable, and “adverse pregnancy outcomes were almost guaranteed.”
The woman responded: “Not now? When?” That, Dr. Hladunewich said, “was when I had my second stroke.”
But there is a possible solution: Pregnancy during dialysis. “Historically, we’ve said absolutely no pregnancy on dialysis,” she said, “but times are changing. We believe aggressive dialysis improves fetal maternal and fetal outcomes.”
Indeed, Dr. Hladunewich led a 2014 study that linked extensive dialysis during pregnancy (compared with less dialysis) to a better likelihood of outcomes such as live birth rate and normal birth weight (JASN May 2014;25[5]:1103-9).
As she noted, “we do offer it as a reproductive option” to patients like the one she mentioned – those who are in ESRD, approaching it, or are nearing the end of their child-bearing years with no transplant in sight. In transplant cases, she said, adequate graft function is linked to good pregnancy outcomes.
Dr. Hladunewich added that it’s important to monitor and adjust treatment of patients during the postpartum period. She said it’s especially important to understand the risks of drugs during breastfeeding. Both dialysis and transplant patients can breastfeed, she said.
Dr. Hladunewich reports no disclosures.
SOURCE: Kidney Week 2018, Abstract FR-OR078.
REPORTING FROM KIDNEY WEEK 2018
Monitoring limited in stage 3 chronic kidney disease
SAN DIEGO – Fewer than a quarter of patients with signs of stage 3 chronic kidney disease (CKD) underwent follow-up testing within 1 year, even though most of these patients underwent repeat cholesterol screening during the same time.
Considering that CKD can be asymptomatic until the late stages, “this is a lost opportunity to get a proper evaluation by a nephrologist,” study coauthor and nephrologist Barbara S. Gillespie, MD, MMS, of Covance, the drug development business of LabCorp, said in an interview. Dr. Gillespie and her colleagues presented their findings at Kidney Week 2018, sponsored by the American Society of Nephrology.
More than 90% of patients with stages 1-3 CKD didn’t know they had the condition, based on 2013-2016 data gathered by the United States Renal Data System . Just 57% of those with stage 4 CKD were aware of their disease.
For the retrospective study, the researchers identified 4.9 million patients (58% were women; mean age was 71) who had estimated glomerular filtration rate (eGFR) results below 60 mL/min per 1.73 m2 from 2011 to 2018, based on serum creatinine tests performed at least twice and at least 3 months apart by LabCorp. The researchers tracked the patients for a median 26 months.
Based on the initial results, 92% of the patients had stage 3 CKD, 6% had stage 4, and 2% had stage 5. However, at 1 year, the percentages of overall patients who underwent urine albumin/creatinine ratio, serum phosphorus, and plasma parathyroid hormone were 24%, 12%, and 17%, respectively, lead author Jennifer Ennis, MD, of LabCorp, said in an interview.
Kidney Disease Improving Global Outcomes guidelines from 2012 recommend “assessments of GFR and albuminuria at least annually ... and more often for individuals at higher risk of progression, and/or where measurement will impact therapeutic decisions” (Ann Intern Med. 2013 Jun 4;158[11]:825-30).
Yet 76% of these patients also underwent annual LDL cholesterol screening. “This suggests that the patients were receiving evaluation and treatment for other common conditions, but that CKD may not have been specifically addressed,” Dr. Ennis said.
“These results suggest that guideline recommendations for monitoring of CKD are not well implemented in the primary care setting, which is where the majority of this testing took place,” she added. “There are possibly many reasons for this, including lack of guideline awareness, familiarity, or agreement; inertia; or other external barriers such as time constraints and the burden of having to remember numerous guidelines for a single patient with multiple conditions.”
Dr. Gillespie said the findings may help to explain why so many patients with CKD are unaware of their condition and “crash into dialysis” within 24 hours of winding up in the emergency department with kidney failure. “Often they note they did not know they had kidney disease,” she said, “or did not know how bad it was.”
The authors disclosed employment by LabCorp, which funded the study.
SOURCE: Ennis JL et al. Kidney Week 2018, Abstract PUB111.
SAN DIEGO – Fewer than a quarter of patients with signs of stage 3 chronic kidney disease (CKD) underwent follow-up testing within 1 year, even though most of these patients underwent repeat cholesterol screening during the same time.
Considering that CKD can be asymptomatic until the late stages, “this is a lost opportunity to get a proper evaluation by a nephrologist,” study coauthor and nephrologist Barbara S. Gillespie, MD, MMS, of Covance, the drug development business of LabCorp, said in an interview. Dr. Gillespie and her colleagues presented their findings at Kidney Week 2018, sponsored by the American Society of Nephrology.
More than 90% of patients with stages 1-3 CKD didn’t know they had the condition, based on 2013-2016 data gathered by the United States Renal Data System . Just 57% of those with stage 4 CKD were aware of their disease.
For the retrospective study, the researchers identified 4.9 million patients (58% were women; mean age was 71) who had estimated glomerular filtration rate (eGFR) results below 60 mL/min per 1.73 m2 from 2011 to 2018, based on serum creatinine tests performed at least twice and at least 3 months apart by LabCorp. The researchers tracked the patients for a median 26 months.
Based on the initial results, 92% of the patients had stage 3 CKD, 6% had stage 4, and 2% had stage 5. However, at 1 year, the percentages of overall patients who underwent urine albumin/creatinine ratio, serum phosphorus, and plasma parathyroid hormone were 24%, 12%, and 17%, respectively, lead author Jennifer Ennis, MD, of LabCorp, said in an interview.
Kidney Disease Improving Global Outcomes guidelines from 2012 recommend “assessments of GFR and albuminuria at least annually ... and more often for individuals at higher risk of progression, and/or where measurement will impact therapeutic decisions” (Ann Intern Med. 2013 Jun 4;158[11]:825-30).
Yet 76% of these patients also underwent annual LDL cholesterol screening. “This suggests that the patients were receiving evaluation and treatment for other common conditions, but that CKD may not have been specifically addressed,” Dr. Ennis said.
“These results suggest that guideline recommendations for monitoring of CKD are not well implemented in the primary care setting, which is where the majority of this testing took place,” she added. “There are possibly many reasons for this, including lack of guideline awareness, familiarity, or agreement; inertia; or other external barriers such as time constraints and the burden of having to remember numerous guidelines for a single patient with multiple conditions.”
Dr. Gillespie said the findings may help to explain why so many patients with CKD are unaware of their condition and “crash into dialysis” within 24 hours of winding up in the emergency department with kidney failure. “Often they note they did not know they had kidney disease,” she said, “or did not know how bad it was.”
The authors disclosed employment by LabCorp, which funded the study.
SOURCE: Ennis JL et al. Kidney Week 2018, Abstract PUB111.
SAN DIEGO – Fewer than a quarter of patients with signs of stage 3 chronic kidney disease (CKD) underwent follow-up testing within 1 year, even though most of these patients underwent repeat cholesterol screening during the same time.
Considering that CKD can be asymptomatic until the late stages, “this is a lost opportunity to get a proper evaluation by a nephrologist,” study coauthor and nephrologist Barbara S. Gillespie, MD, MMS, of Covance, the drug development business of LabCorp, said in an interview. Dr. Gillespie and her colleagues presented their findings at Kidney Week 2018, sponsored by the American Society of Nephrology.
More than 90% of patients with stages 1-3 CKD didn’t know they had the condition, based on 2013-2016 data gathered by the United States Renal Data System . Just 57% of those with stage 4 CKD were aware of their disease.
For the retrospective study, the researchers identified 4.9 million patients (58% were women; mean age was 71) who had estimated glomerular filtration rate (eGFR) results below 60 mL/min per 1.73 m2 from 2011 to 2018, based on serum creatinine tests performed at least twice and at least 3 months apart by LabCorp. The researchers tracked the patients for a median 26 months.
Based on the initial results, 92% of the patients had stage 3 CKD, 6% had stage 4, and 2% had stage 5. However, at 1 year, the percentages of overall patients who underwent urine albumin/creatinine ratio, serum phosphorus, and plasma parathyroid hormone were 24%, 12%, and 17%, respectively, lead author Jennifer Ennis, MD, of LabCorp, said in an interview.
Kidney Disease Improving Global Outcomes guidelines from 2012 recommend “assessments of GFR and albuminuria at least annually ... and more often for individuals at higher risk of progression, and/or where measurement will impact therapeutic decisions” (Ann Intern Med. 2013 Jun 4;158[11]:825-30).
Yet 76% of these patients also underwent annual LDL cholesterol screening. “This suggests that the patients were receiving evaluation and treatment for other common conditions, but that CKD may not have been specifically addressed,” Dr. Ennis said.
“These results suggest that guideline recommendations for monitoring of CKD are not well implemented in the primary care setting, which is where the majority of this testing took place,” she added. “There are possibly many reasons for this, including lack of guideline awareness, familiarity, or agreement; inertia; or other external barriers such as time constraints and the burden of having to remember numerous guidelines for a single patient with multiple conditions.”
Dr. Gillespie said the findings may help to explain why so many patients with CKD are unaware of their condition and “crash into dialysis” within 24 hours of winding up in the emergency department with kidney failure. “Often they note they did not know they had kidney disease,” she said, “or did not know how bad it was.”
The authors disclosed employment by LabCorp, which funded the study.
SOURCE: Ennis JL et al. Kidney Week 2018, Abstract PUB111.
REPORTING FROM KIDNEY WEEK 2018
Key clinical point: Physicians often ignore blood test results that indicate chronic kidney disease.
Major finding: Over 1 year, 24% of patients with signs of CKD underwent a recommended follow-up test, even though about 76% had cholesterol screening.
Study details: Retrospective study of 4.9 million U.S. patients who had signs of CKD based on LabCorp blood tests during 2011-2018.
Disclosures: The authors disclosed employment by LabCorp, which funded the study.
Source: Ennis JL et al. Kidney Week 2018, Abstract PUB111.
Dialysis decision in elderly needs to factor in comorbidities
SAN DIEGO – The wider picture of the patient’s health and prognosis, not just chronologic age, should enter into the clinical decision to initiate dialysis, according to Bjorg Thorsteinsdottir, MD, a palliative care physician at the Mayo Clinic in Rochester, Minn.
“People perceive they have no choice [but treatment], and we perceive we have to do things to them until everything is lost, then we expect them to do a 180 [degree turn],” she said in a presentation at the meeting sponsored by the American Society of Nephrology.
“A 90-year-old fit individual, with minimal comorbidity living independently, would absolutely be a good candidate for dialysis, while a 75-year-old patient with bad peripheral vascular disease and dementia, living in a nursing home, would be unlikely to live longer on dialysis than off dialysis,” she said. “We need to weigh the risks and benefits for each individual patient against their goals and values. We need to be honest about the lack of benefit for certain subgroups of patients and the heavy treatment burdens of dialysis. Age, comorbidity, and frailty all factor into these deliberations and prognosis.”
More than 107,000 people over age 75 in the United States received dialysis in 2015, according to statistics gathered by the National Kidney Foundation. Yet the survival advantage of dialysis is more limited in elderly patients with multiple comorbidities, Dr. Thorsteinsdottir said. “It becomes important to think about the harms of treatment.”
A 2016 study from the Netherlands found no survival advantage to dialysis, compared with conservative management among kidney failure patients aged 80 and older. The survival advantage was limited with dialysis in patients aged 70 and older who also had multiple comorbidities. (Clin J Am Soc Nephrol. 2016 Apr;11(4):633-40)
In an interview, Dr. Thorsteinsdottir acknowledged that “determining who is unlikely to benefit from dialysis is complicated.” However, she said, “we know that the following comorbidities are the worst: dementia and peripheral vascular disease.”
“No one that I know of currently has an age cutoff for dialysis,” Dr. Thorsteinsdottir said in the interview, “and I do not believe the U.S. is ready for any kind of explicit limit setting by the government on dialysis treatment.”
“We must respond to legitimate concerns raised by recent studies that suggest that strong moral imperatives – to treat anyone we can treat – have created a situation where we are not pausing and asking hard questions about whether the patient in front of us is likely to benefit from dialysis,” she said in the interview. “Patients sense this and do not feel that they are given any alternatives to dialysis treatment. This needs to change.”
Dr. Thorsteinsdottir reported no relevant financial disclosures.
SAN DIEGO – The wider picture of the patient’s health and prognosis, not just chronologic age, should enter into the clinical decision to initiate dialysis, according to Bjorg Thorsteinsdottir, MD, a palliative care physician at the Mayo Clinic in Rochester, Minn.
“People perceive they have no choice [but treatment], and we perceive we have to do things to them until everything is lost, then we expect them to do a 180 [degree turn],” she said in a presentation at the meeting sponsored by the American Society of Nephrology.
“A 90-year-old fit individual, with minimal comorbidity living independently, would absolutely be a good candidate for dialysis, while a 75-year-old patient with bad peripheral vascular disease and dementia, living in a nursing home, would be unlikely to live longer on dialysis than off dialysis,” she said. “We need to weigh the risks and benefits for each individual patient against their goals and values. We need to be honest about the lack of benefit for certain subgroups of patients and the heavy treatment burdens of dialysis. Age, comorbidity, and frailty all factor into these deliberations and prognosis.”
More than 107,000 people over age 75 in the United States received dialysis in 2015, according to statistics gathered by the National Kidney Foundation. Yet the survival advantage of dialysis is more limited in elderly patients with multiple comorbidities, Dr. Thorsteinsdottir said. “It becomes important to think about the harms of treatment.”
A 2016 study from the Netherlands found no survival advantage to dialysis, compared with conservative management among kidney failure patients aged 80 and older. The survival advantage was limited with dialysis in patients aged 70 and older who also had multiple comorbidities. (Clin J Am Soc Nephrol. 2016 Apr;11(4):633-40)
In an interview, Dr. Thorsteinsdottir acknowledged that “determining who is unlikely to benefit from dialysis is complicated.” However, she said, “we know that the following comorbidities are the worst: dementia and peripheral vascular disease.”
“No one that I know of currently has an age cutoff for dialysis,” Dr. Thorsteinsdottir said in the interview, “and I do not believe the U.S. is ready for any kind of explicit limit setting by the government on dialysis treatment.”
“We must respond to legitimate concerns raised by recent studies that suggest that strong moral imperatives – to treat anyone we can treat – have created a situation where we are not pausing and asking hard questions about whether the patient in front of us is likely to benefit from dialysis,” she said in the interview. “Patients sense this and do not feel that they are given any alternatives to dialysis treatment. This needs to change.”
Dr. Thorsteinsdottir reported no relevant financial disclosures.
SAN DIEGO – The wider picture of the patient’s health and prognosis, not just chronologic age, should enter into the clinical decision to initiate dialysis, according to Bjorg Thorsteinsdottir, MD, a palliative care physician at the Mayo Clinic in Rochester, Minn.
“People perceive they have no choice [but treatment], and we perceive we have to do things to them until everything is lost, then we expect them to do a 180 [degree turn],” she said in a presentation at the meeting sponsored by the American Society of Nephrology.
“A 90-year-old fit individual, with minimal comorbidity living independently, would absolutely be a good candidate for dialysis, while a 75-year-old patient with bad peripheral vascular disease and dementia, living in a nursing home, would be unlikely to live longer on dialysis than off dialysis,” she said. “We need to weigh the risks and benefits for each individual patient against their goals and values. We need to be honest about the lack of benefit for certain subgroups of patients and the heavy treatment burdens of dialysis. Age, comorbidity, and frailty all factor into these deliberations and prognosis.”
More than 107,000 people over age 75 in the United States received dialysis in 2015, according to statistics gathered by the National Kidney Foundation. Yet the survival advantage of dialysis is more limited in elderly patients with multiple comorbidities, Dr. Thorsteinsdottir said. “It becomes important to think about the harms of treatment.”
A 2016 study from the Netherlands found no survival advantage to dialysis, compared with conservative management among kidney failure patients aged 80 and older. The survival advantage was limited with dialysis in patients aged 70 and older who also had multiple comorbidities. (Clin J Am Soc Nephrol. 2016 Apr;11(4):633-40)
In an interview, Dr. Thorsteinsdottir acknowledged that “determining who is unlikely to benefit from dialysis is complicated.” However, she said, “we know that the following comorbidities are the worst: dementia and peripheral vascular disease.”
“No one that I know of currently has an age cutoff for dialysis,” Dr. Thorsteinsdottir said in the interview, “and I do not believe the U.S. is ready for any kind of explicit limit setting by the government on dialysis treatment.”
“We must respond to legitimate concerns raised by recent studies that suggest that strong moral imperatives – to treat anyone we can treat – have created a situation where we are not pausing and asking hard questions about whether the patient in front of us is likely to benefit from dialysis,” she said in the interview. “Patients sense this and do not feel that they are given any alternatives to dialysis treatment. This needs to change.”
Dr. Thorsteinsdottir reported no relevant financial disclosures.
REPORTING FROM KIDNEY WEEK 2018