User login
Blunted cardiac reserve strongly predicts incident hepatorenal syndrome
VIENNA – Patients with cirrhosis and undergoing work-up for a possible liver transplant who had low cardiac reserve had a nearly fourfold increased rate of developing hepatorenal syndrome (HRS) during an average 17 months of follow-up, compared with patients with normal cardiac reserve, in a review of 560 Australian patients assessed for a possible liver transplant.
The findings suggest that patients with advanced liver disease should routinely undergo assessment for low cardiac reserve, Anoop N. Koshy, MBBS, said at the meeting sponsored by the European Association for the Study of the Liver.
said Dr. Koshy, a cardiologist with Austin Health in Melbourne. “We propose that it’s not low cardiac output that leads to HRS, but an inability of patients to increase their cardiac output” in response to usual stimuli.
The findings also add to the concerns about using nonselective beta-blocker drugs in patients with cirrhosis because of the potential of these drugs to further blunt increases in cardiac output; they also suggest that noninvasive measurement of cardiac reserve could identify patients with low cardiac reserve who could benefit from closer monitoring and new approaches to treatment, he suggested. About 10%-30% of patients with cirrhosis develop HRS, and the new finding suggests a noninvasive way to identify patients with the highest risk for this complication.
The study included 560 consecutive patients with cirrhosis and end-stage liver disease who were awaiting a liver transplant at the Victoria Liver Transplant Unit in Melbourne and underwent assessment by stress echocardiography using low-dose dobutamine (10 mcg/kg per min) during 2010-2017 as part of their standard pretransplant work-up. Exclusion of patients with known cardiac disease prior to their stress echo examination or incomplete measurement left 488 patients, of whom 424 were free from HRS at baseline. Patients with HRS at the time of their stress echo assessment had on average a cardiac output that was about 25% higher than patients without HRS, a statistically significant difference driven by both a significantly increased heart rate and stroke volume.
Among the 424 patients free from HRS at baseline, 85 developed HRS during an average 17-month follow-up. Patients with low cardiac reserve after dobutamine challenge, defined as an increase in cardiac output of less than 25%, had a 3.9-fold increased rate of incident HRS during follow-up, compared with patients who had a larger rise in their cardiac output after adjustment for several clinical and echocardiographic baseline variables, Dr. Koshy reported. In this analysis low cardiac reserve was the strongest predictor of subsequent HRS, he said.
Dr. Koshy had no disclosures.
SOURCE: Koshy AN et al. J Hepatol. 2019 April;70(1):e56.
Cardiovascular abnormalities develop in patients with advanced chronic liver disease to produce a hyperdynamic systemic circulation with splanchnic vasodilation, decreased systemic vascular resistance, and increased cardiac output (J Hepatol. 2018;69[4]:958-60). The term cirrhotic cardiomyopathy has also been used for the changes of systolic dysfunction with impaired cardiac contractile response to stress and altered diastolic relaxation that develops in patients with cirrhosis (J Hepatol. 2010;53[1]:179-90).
In this study by Dr. Koshy and colleagues, the inability to increase cardiac output during dobutamine stress echo (DSE) was associated with a greater subsequent risk for hepatorenal syndrome (J Hepatol. 2019;70:e56).
All patients in the study were undergoing pretransplant liver evaluation. Those who developed hepatorenal syndrome (HRS) in follow-up had a higher mean cardiac output with a reduction of the increase in cardiac output that follows dobutamine administration when compared with those who did not develop HRS. A multivariate analysis that adjusted for age, gender, MELD score, and Child-Pugh score found that “impaired contractile response was the strongest predictor of hepatorenal syndrome” as defined by a less than 22% increase in cardiac output following dobutamine. Overall, 40% of those with impaired contractile reserve developed hepatorenal syndrome, compared with 25% of those with normal contractile reserve following dobutamine (P = .006). It is of interest that cirrhotic patients with HRS at the time of initial dobutamine stress echo had a 25% higher average cardiac output than those without HRS. Patients who subsequently developed hepatorenal syndrome also had higher average cardiac output at initial evaluation than those who did not.
This study continues to raise important questions about the role of cardiovascular dysfunction and the risk of hepatorenal syndrome. Additional studies seem warranted to evaluate progression of cardiac changes and dobutamine response throughout follow-up of end-stage liver disease patients, including at the development of hepatorenal syndrome. Studies of HRS patients with specific associations such as sepsis, spontaneous bacterial peritonitis, and gastrointestinal bleeding may also provide information on the role of systolic and diastolic dysfunction during such events.
This article also draws attention to “concerns about using nonselective beta-blocker drugs in patients with cirrhosis.” Current data indicate that nonselective beta-blockers reduce all-cause mortality and the risk of first variceal hemorrhage in patients with advanced liver disease (Hepatology. 2019;69[4]:1657-75). Until we have studies that reveal a clear association between beta-blockers and development of hepatorenal syndrome, I will continue to recommend the use of beta-blockers in cirrhotic patients at risk for first variceal hemorrhage.
Rowen K. Zetterman, MD, is dean emeritus of the Creighton University School of Medicine in Omaha, Neb. He serves as the Associate Vice Chancellor for Academic Affairs and the Associate Vice Chancellor for Planning at the University of Nebraska Medical Center in Omaha. Dr. Zetterman, a gastroenterologist and hepatologist, is also a member of the editorial advisory board of Internal Medicine News.
Cardiovascular abnormalities develop in patients with advanced chronic liver disease to produce a hyperdynamic systemic circulation with splanchnic vasodilation, decreased systemic vascular resistance, and increased cardiac output (J Hepatol. 2018;69[4]:958-60). The term cirrhotic cardiomyopathy has also been used for the changes of systolic dysfunction with impaired cardiac contractile response to stress and altered diastolic relaxation that develops in patients with cirrhosis (J Hepatol. 2010;53[1]:179-90).
In this study by Dr. Koshy and colleagues, the inability to increase cardiac output during dobutamine stress echo (DSE) was associated with a greater subsequent risk for hepatorenal syndrome (J Hepatol. 2019;70:e56).
All patients in the study were undergoing pretransplant liver evaluation. Those who developed hepatorenal syndrome (HRS) in follow-up had a higher mean cardiac output with a reduction of the increase in cardiac output that follows dobutamine administration when compared with those who did not develop HRS. A multivariate analysis that adjusted for age, gender, MELD score, and Child-Pugh score found that “impaired contractile response was the strongest predictor of hepatorenal syndrome” as defined by a less than 22% increase in cardiac output following dobutamine. Overall, 40% of those with impaired contractile reserve developed hepatorenal syndrome, compared with 25% of those with normal contractile reserve following dobutamine (P = .006). It is of interest that cirrhotic patients with HRS at the time of initial dobutamine stress echo had a 25% higher average cardiac output than those without HRS. Patients who subsequently developed hepatorenal syndrome also had higher average cardiac output at initial evaluation than those who did not.
This study continues to raise important questions about the role of cardiovascular dysfunction and the risk of hepatorenal syndrome. Additional studies seem warranted to evaluate progression of cardiac changes and dobutamine response throughout follow-up of end-stage liver disease patients, including at the development of hepatorenal syndrome. Studies of HRS patients with specific associations such as sepsis, spontaneous bacterial peritonitis, and gastrointestinal bleeding may also provide information on the role of systolic and diastolic dysfunction during such events.
This article also draws attention to “concerns about using nonselective beta-blocker drugs in patients with cirrhosis.” Current data indicate that nonselective beta-blockers reduce all-cause mortality and the risk of first variceal hemorrhage in patients with advanced liver disease (Hepatology. 2019;69[4]:1657-75). Until we have studies that reveal a clear association between beta-blockers and development of hepatorenal syndrome, I will continue to recommend the use of beta-blockers in cirrhotic patients at risk for first variceal hemorrhage.
Rowen K. Zetterman, MD, is dean emeritus of the Creighton University School of Medicine in Omaha, Neb. He serves as the Associate Vice Chancellor for Academic Affairs and the Associate Vice Chancellor for Planning at the University of Nebraska Medical Center in Omaha. Dr. Zetterman, a gastroenterologist and hepatologist, is also a member of the editorial advisory board of Internal Medicine News.
Cardiovascular abnormalities develop in patients with advanced chronic liver disease to produce a hyperdynamic systemic circulation with splanchnic vasodilation, decreased systemic vascular resistance, and increased cardiac output (J Hepatol. 2018;69[4]:958-60). The term cirrhotic cardiomyopathy has also been used for the changes of systolic dysfunction with impaired cardiac contractile response to stress and altered diastolic relaxation that develops in patients with cirrhosis (J Hepatol. 2010;53[1]:179-90).
In this study by Dr. Koshy and colleagues, the inability to increase cardiac output during dobutamine stress echo (DSE) was associated with a greater subsequent risk for hepatorenal syndrome (J Hepatol. 2019;70:e56).
All patients in the study were undergoing pretransplant liver evaluation. Those who developed hepatorenal syndrome (HRS) in follow-up had a higher mean cardiac output with a reduction of the increase in cardiac output that follows dobutamine administration when compared with those who did not develop HRS. A multivariate analysis that adjusted for age, gender, MELD score, and Child-Pugh score found that “impaired contractile response was the strongest predictor of hepatorenal syndrome” as defined by a less than 22% increase in cardiac output following dobutamine. Overall, 40% of those with impaired contractile reserve developed hepatorenal syndrome, compared with 25% of those with normal contractile reserve following dobutamine (P = .006). It is of interest that cirrhotic patients with HRS at the time of initial dobutamine stress echo had a 25% higher average cardiac output than those without HRS. Patients who subsequently developed hepatorenal syndrome also had higher average cardiac output at initial evaluation than those who did not.
This study continues to raise important questions about the role of cardiovascular dysfunction and the risk of hepatorenal syndrome. Additional studies seem warranted to evaluate progression of cardiac changes and dobutamine response throughout follow-up of end-stage liver disease patients, including at the development of hepatorenal syndrome. Studies of HRS patients with specific associations such as sepsis, spontaneous bacterial peritonitis, and gastrointestinal bleeding may also provide information on the role of systolic and diastolic dysfunction during such events.
This article also draws attention to “concerns about using nonselective beta-blocker drugs in patients with cirrhosis.” Current data indicate that nonselective beta-blockers reduce all-cause mortality and the risk of first variceal hemorrhage in patients with advanced liver disease (Hepatology. 2019;69[4]:1657-75). Until we have studies that reveal a clear association between beta-blockers and development of hepatorenal syndrome, I will continue to recommend the use of beta-blockers in cirrhotic patients at risk for first variceal hemorrhage.
Rowen K. Zetterman, MD, is dean emeritus of the Creighton University School of Medicine in Omaha, Neb. He serves as the Associate Vice Chancellor for Academic Affairs and the Associate Vice Chancellor for Planning at the University of Nebraska Medical Center in Omaha. Dr. Zetterman, a gastroenterologist and hepatologist, is also a member of the editorial advisory board of Internal Medicine News.
VIENNA – Patients with cirrhosis and undergoing work-up for a possible liver transplant who had low cardiac reserve had a nearly fourfold increased rate of developing hepatorenal syndrome (HRS) during an average 17 months of follow-up, compared with patients with normal cardiac reserve, in a review of 560 Australian patients assessed for a possible liver transplant.
The findings suggest that patients with advanced liver disease should routinely undergo assessment for low cardiac reserve, Anoop N. Koshy, MBBS, said at the meeting sponsored by the European Association for the Study of the Liver.
said Dr. Koshy, a cardiologist with Austin Health in Melbourne. “We propose that it’s not low cardiac output that leads to HRS, but an inability of patients to increase their cardiac output” in response to usual stimuli.
The findings also add to the concerns about using nonselective beta-blocker drugs in patients with cirrhosis because of the potential of these drugs to further blunt increases in cardiac output; they also suggest that noninvasive measurement of cardiac reserve could identify patients with low cardiac reserve who could benefit from closer monitoring and new approaches to treatment, he suggested. About 10%-30% of patients with cirrhosis develop HRS, and the new finding suggests a noninvasive way to identify patients with the highest risk for this complication.
The study included 560 consecutive patients with cirrhosis and end-stage liver disease who were awaiting a liver transplant at the Victoria Liver Transplant Unit in Melbourne and underwent assessment by stress echocardiography using low-dose dobutamine (10 mcg/kg per min) during 2010-2017 as part of their standard pretransplant work-up. Exclusion of patients with known cardiac disease prior to their stress echo examination or incomplete measurement left 488 patients, of whom 424 were free from HRS at baseline. Patients with HRS at the time of their stress echo assessment had on average a cardiac output that was about 25% higher than patients without HRS, a statistically significant difference driven by both a significantly increased heart rate and stroke volume.
Among the 424 patients free from HRS at baseline, 85 developed HRS during an average 17-month follow-up. Patients with low cardiac reserve after dobutamine challenge, defined as an increase in cardiac output of less than 25%, had a 3.9-fold increased rate of incident HRS during follow-up, compared with patients who had a larger rise in their cardiac output after adjustment for several clinical and echocardiographic baseline variables, Dr. Koshy reported. In this analysis low cardiac reserve was the strongest predictor of subsequent HRS, he said.
Dr. Koshy had no disclosures.
SOURCE: Koshy AN et al. J Hepatol. 2019 April;70(1):e56.
VIENNA – Patients with cirrhosis and undergoing work-up for a possible liver transplant who had low cardiac reserve had a nearly fourfold increased rate of developing hepatorenal syndrome (HRS) during an average 17 months of follow-up, compared with patients with normal cardiac reserve, in a review of 560 Australian patients assessed for a possible liver transplant.
The findings suggest that patients with advanced liver disease should routinely undergo assessment for low cardiac reserve, Anoop N. Koshy, MBBS, said at the meeting sponsored by the European Association for the Study of the Liver.
said Dr. Koshy, a cardiologist with Austin Health in Melbourne. “We propose that it’s not low cardiac output that leads to HRS, but an inability of patients to increase their cardiac output” in response to usual stimuli.
The findings also add to the concerns about using nonselective beta-blocker drugs in patients with cirrhosis because of the potential of these drugs to further blunt increases in cardiac output; they also suggest that noninvasive measurement of cardiac reserve could identify patients with low cardiac reserve who could benefit from closer monitoring and new approaches to treatment, he suggested. About 10%-30% of patients with cirrhosis develop HRS, and the new finding suggests a noninvasive way to identify patients with the highest risk for this complication.
The study included 560 consecutive patients with cirrhosis and end-stage liver disease who were awaiting a liver transplant at the Victoria Liver Transplant Unit in Melbourne and underwent assessment by stress echocardiography using low-dose dobutamine (10 mcg/kg per min) during 2010-2017 as part of their standard pretransplant work-up. Exclusion of patients with known cardiac disease prior to their stress echo examination or incomplete measurement left 488 patients, of whom 424 were free from HRS at baseline. Patients with HRS at the time of their stress echo assessment had on average a cardiac output that was about 25% higher than patients without HRS, a statistically significant difference driven by both a significantly increased heart rate and stroke volume.
Among the 424 patients free from HRS at baseline, 85 developed HRS during an average 17-month follow-up. Patients with low cardiac reserve after dobutamine challenge, defined as an increase in cardiac output of less than 25%, had a 3.9-fold increased rate of incident HRS during follow-up, compared with patients who had a larger rise in their cardiac output after adjustment for several clinical and echocardiographic baseline variables, Dr. Koshy reported. In this analysis low cardiac reserve was the strongest predictor of subsequent HRS, he said.
Dr. Koshy had no disclosures.
SOURCE: Koshy AN et al. J Hepatol. 2019 April;70(1):e56.
REPORTING FROM ILC 2019
Combo B-cell depletion advances in SLE
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
REPORTING FROM LUPUS 2019
Low-dose IL-2 found effective in SLE
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.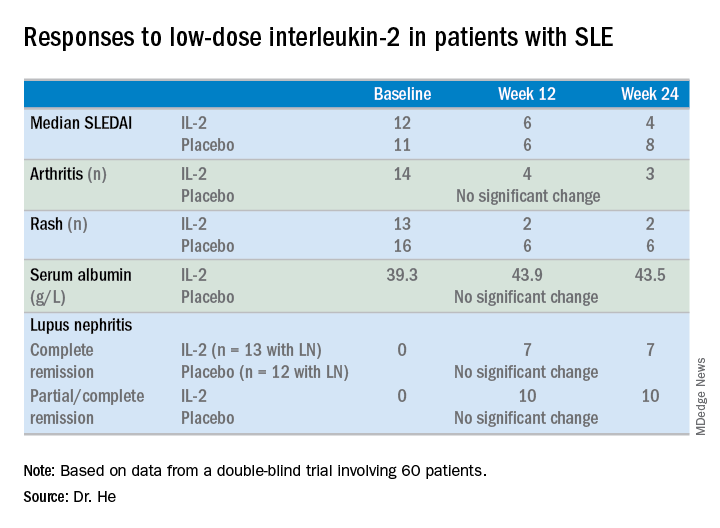
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
REPORTING FROM LUPUS 2019
Dr. Joseph Vassalotti: Cancer risk minimal with ARBs
PHILADELPHIA – according to a senior officer of the National Kidney Foundation.
“I’ve been telling everyone not to stop on their own,” said Joseph A. Vassalotti, MD, chief medical officer for the National Kidney Foundation and associate clinical professor of medicine at Icahn School of Medicine at Mount Sinai, New York.
“The risk of cardiovascular events acutely and the long-term risk of kidney disease progression is much more concerning to me, if they self-discontinue the ARB, than the small risk of cancer,” Dr. Vassalotti said in a meet-the-professor session at the annual meeting of the American College of Physicians.
Put in perspective, the absolute risk of cancer according to the Food and Drug Administration is one new malignancy per 8,000 patients treated with 320 mg of valsartan daily – the highest ARB dose that contained N-Nitrosodimethylamine (NDMA), one of several impurities that led to the recent recalls.
Dr. Vassalotti said that so far, he’s been able to avoid switching patients from one ARB to another by working with pharmacies to get the same medication in a different generic brand not affected by the FDA recalls.
He advised caution in switching ARBs, noting a paucity of head-to-head comparative data between ARBs.
“There may be variable effects,” he said.
If switching is thought to be warranted, he said, some extra tests or visits might be needed to ensure avoidance of hyperkalemia, undertreated hypertension, or hypotension.
Dr. Vassalotti encouraged attendees to review a perspective piece in the New England Journal of Medicine (2019 Mar 13. doi: 10.1056/NEJMp1901657) describing this hypertension “hot potato” resulting from the large-scale voluntary recalls of products containing valsartan, losartan, and irbesartan due to nitrosamine contamination.
Patients may hear about recalls of hypertension drugs, but may not know what products or manufacturers are involved, leaving the burden on clinicians, pharmacies, and health care systems to respond to their concerns, said authors of that perspective piece, led by J. Brian Byrd, MD, of the University of Michigan, Ann Arbor.
“Recalls may trigger unnecessary concern among many people receiving antihypertensive therapy – and may be ignored by people who take ARBs for heart failure or chronic kidney disease,” wrote Dr. Byrd and his colleagues.
The FDA, which said it has worked with manufacturers to “swiftly” remove ARB drug products with impurity levels above acceptable limits, is now maintaining a list of other currently marketed ARB products that are being tested for impurities.
As of the latest update on April 4, the FDA listed more than 40 products with an overall nitrosamine impurity determination of “not present” and more than 300 additional products for which assessments are not yet complete.
“Essentially, we have a safe list now of ARBs that is being developed,” Dr. Vassalotti said. “So if a patient really wanted to change, I would consult that list, and consider picking one that’s been tested already on that list, and the FDA hopefully will complete testing on all the ARB drugs in the near future.”
Dr. Vassalotti is a consultant with Merck, Janssen, and the U.S. Nephrology Advisory Board.
PHILADELPHIA – according to a senior officer of the National Kidney Foundation.
“I’ve been telling everyone not to stop on their own,” said Joseph A. Vassalotti, MD, chief medical officer for the National Kidney Foundation and associate clinical professor of medicine at Icahn School of Medicine at Mount Sinai, New York.
“The risk of cardiovascular events acutely and the long-term risk of kidney disease progression is much more concerning to me, if they self-discontinue the ARB, than the small risk of cancer,” Dr. Vassalotti said in a meet-the-professor session at the annual meeting of the American College of Physicians.
Put in perspective, the absolute risk of cancer according to the Food and Drug Administration is one new malignancy per 8,000 patients treated with 320 mg of valsartan daily – the highest ARB dose that contained N-Nitrosodimethylamine (NDMA), one of several impurities that led to the recent recalls.
Dr. Vassalotti said that so far, he’s been able to avoid switching patients from one ARB to another by working with pharmacies to get the same medication in a different generic brand not affected by the FDA recalls.
He advised caution in switching ARBs, noting a paucity of head-to-head comparative data between ARBs.
“There may be variable effects,” he said.
If switching is thought to be warranted, he said, some extra tests or visits might be needed to ensure avoidance of hyperkalemia, undertreated hypertension, or hypotension.
Dr. Vassalotti encouraged attendees to review a perspective piece in the New England Journal of Medicine (2019 Mar 13. doi: 10.1056/NEJMp1901657) describing this hypertension “hot potato” resulting from the large-scale voluntary recalls of products containing valsartan, losartan, and irbesartan due to nitrosamine contamination.
Patients may hear about recalls of hypertension drugs, but may not know what products or manufacturers are involved, leaving the burden on clinicians, pharmacies, and health care systems to respond to their concerns, said authors of that perspective piece, led by J. Brian Byrd, MD, of the University of Michigan, Ann Arbor.
“Recalls may trigger unnecessary concern among many people receiving antihypertensive therapy – and may be ignored by people who take ARBs for heart failure or chronic kidney disease,” wrote Dr. Byrd and his colleagues.
The FDA, which said it has worked with manufacturers to “swiftly” remove ARB drug products with impurity levels above acceptable limits, is now maintaining a list of other currently marketed ARB products that are being tested for impurities.
As of the latest update on April 4, the FDA listed more than 40 products with an overall nitrosamine impurity determination of “not present” and more than 300 additional products for which assessments are not yet complete.
“Essentially, we have a safe list now of ARBs that is being developed,” Dr. Vassalotti said. “So if a patient really wanted to change, I would consult that list, and consider picking one that’s been tested already on that list, and the FDA hopefully will complete testing on all the ARB drugs in the near future.”
Dr. Vassalotti is a consultant with Merck, Janssen, and the U.S. Nephrology Advisory Board.
PHILADELPHIA – according to a senior officer of the National Kidney Foundation.
“I’ve been telling everyone not to stop on their own,” said Joseph A. Vassalotti, MD, chief medical officer for the National Kidney Foundation and associate clinical professor of medicine at Icahn School of Medicine at Mount Sinai, New York.
“The risk of cardiovascular events acutely and the long-term risk of kidney disease progression is much more concerning to me, if they self-discontinue the ARB, than the small risk of cancer,” Dr. Vassalotti said in a meet-the-professor session at the annual meeting of the American College of Physicians.
Put in perspective, the absolute risk of cancer according to the Food and Drug Administration is one new malignancy per 8,000 patients treated with 320 mg of valsartan daily – the highest ARB dose that contained N-Nitrosodimethylamine (NDMA), one of several impurities that led to the recent recalls.
Dr. Vassalotti said that so far, he’s been able to avoid switching patients from one ARB to another by working with pharmacies to get the same medication in a different generic brand not affected by the FDA recalls.
He advised caution in switching ARBs, noting a paucity of head-to-head comparative data between ARBs.
“There may be variable effects,” he said.
If switching is thought to be warranted, he said, some extra tests or visits might be needed to ensure avoidance of hyperkalemia, undertreated hypertension, or hypotension.
Dr. Vassalotti encouraged attendees to review a perspective piece in the New England Journal of Medicine (2019 Mar 13. doi: 10.1056/NEJMp1901657) describing this hypertension “hot potato” resulting from the large-scale voluntary recalls of products containing valsartan, losartan, and irbesartan due to nitrosamine contamination.
Patients may hear about recalls of hypertension drugs, but may not know what products or manufacturers are involved, leaving the burden on clinicians, pharmacies, and health care systems to respond to their concerns, said authors of that perspective piece, led by J. Brian Byrd, MD, of the University of Michigan, Ann Arbor.
“Recalls may trigger unnecessary concern among many people receiving antihypertensive therapy – and may be ignored by people who take ARBs for heart failure or chronic kidney disease,” wrote Dr. Byrd and his colleagues.
The FDA, which said it has worked with manufacturers to “swiftly” remove ARB drug products with impurity levels above acceptable limits, is now maintaining a list of other currently marketed ARB products that are being tested for impurities.
As of the latest update on April 4, the FDA listed more than 40 products with an overall nitrosamine impurity determination of “not present” and more than 300 additional products for which assessments are not yet complete.
“Essentially, we have a safe list now of ARBs that is being developed,” Dr. Vassalotti said. “So if a patient really wanted to change, I would consult that list, and consider picking one that’s been tested already on that list, and the FDA hopefully will complete testing on all the ARB drugs in the near future.”
Dr. Vassalotti is a consultant with Merck, Janssen, and the U.S. Nephrology Advisory Board.
FROM INTERNAL MEDICINE 2019
Canagliflozin lowers kidney failure risk in T2D: CREDENCE
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Delay RRT for severe AKI in septic shock or ARDS
Clinical question: Does early renal replacement therapy (RRT) initiation affect clinical outcomes in patients with severe acute kidney injury (AKI) in the setting of septic shock or acute respiratory distress syndrome (ARDS)?
Background: Critically ill patients with AKI can benefit from RRT via improvement of electrolyte abnormalities, volume overload, and acid-base status. Potential harm from RRT includes complications of central venous access, intradialytic hypotension, and the bleeding risk of anticoagulation. The optimal timing of the elective initiation of RRT for AKI in septic shock or ARDS is unknown.
Study design: A post hoc subgroup study of a randomized, controlled trial.
Setting: Thirty-one ICUs in France.
Synopsis: Using data from the Artificial Kidney Initiation in Kidney Injury trial, the authors evaluated 619 patients with severe AKI and requirement for catecholamine infusion and/or invasive mechanical ventilation. Patients were randomly given RRT in an early or a delayed time frame. The early strategy involved RRT as soon as possible after randomization. In addition to the other parameters, the patients in the delayed group were given RRT for the following: anuria/oliguria 72 hours after randomization, blood urea nitrogen greater than 112 mg/dL, serum potassium greater than 6 mmol/L, metabolic acidosis with pH less than 7.15, or pulmonary edema from fluid overload causing severe hypoxia.
Early RRT did not show significant improvement in 60-day mortality, length of mechanical ventilation, or length of stay, compared with delayed RRT. The delayed RRT strategy was significantly associated with renal function recovery, with hazard ratios of 1.7 in ARDS (P = .009) and 1.9 in septic shock (P less than .001). Additionally, the likelihood of adequate urinary output was greater in the delayed RRT group.
Bottom line: A delayed RRT strategy in those with severe AKI and septic shock or ARDS may safely afford time for renal recovery in some patients.
Citation: Gaudry S et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome. A post hoc analysis of the AKIKI randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):58-66.
Dr. James is a hospitalist at Emory University Hospital Midtown and an assistant professor at Emory University, both in Atlanta.
Clinical question: Does early renal replacement therapy (RRT) initiation affect clinical outcomes in patients with severe acute kidney injury (AKI) in the setting of septic shock or acute respiratory distress syndrome (ARDS)?
Background: Critically ill patients with AKI can benefit from RRT via improvement of electrolyte abnormalities, volume overload, and acid-base status. Potential harm from RRT includes complications of central venous access, intradialytic hypotension, and the bleeding risk of anticoagulation. The optimal timing of the elective initiation of RRT for AKI in septic shock or ARDS is unknown.
Study design: A post hoc subgroup study of a randomized, controlled trial.
Setting: Thirty-one ICUs in France.
Synopsis: Using data from the Artificial Kidney Initiation in Kidney Injury trial, the authors evaluated 619 patients with severe AKI and requirement for catecholamine infusion and/or invasive mechanical ventilation. Patients were randomly given RRT in an early or a delayed time frame. The early strategy involved RRT as soon as possible after randomization. In addition to the other parameters, the patients in the delayed group were given RRT for the following: anuria/oliguria 72 hours after randomization, blood urea nitrogen greater than 112 mg/dL, serum potassium greater than 6 mmol/L, metabolic acidosis with pH less than 7.15, or pulmonary edema from fluid overload causing severe hypoxia.
Early RRT did not show significant improvement in 60-day mortality, length of mechanical ventilation, or length of stay, compared with delayed RRT. The delayed RRT strategy was significantly associated with renal function recovery, with hazard ratios of 1.7 in ARDS (P = .009) and 1.9 in septic shock (P less than .001). Additionally, the likelihood of adequate urinary output was greater in the delayed RRT group.
Bottom line: A delayed RRT strategy in those with severe AKI and septic shock or ARDS may safely afford time for renal recovery in some patients.
Citation: Gaudry S et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome. A post hoc analysis of the AKIKI randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):58-66.
Dr. James is a hospitalist at Emory University Hospital Midtown and an assistant professor at Emory University, both in Atlanta.
Clinical question: Does early renal replacement therapy (RRT) initiation affect clinical outcomes in patients with severe acute kidney injury (AKI) in the setting of septic shock or acute respiratory distress syndrome (ARDS)?
Background: Critically ill patients with AKI can benefit from RRT via improvement of electrolyte abnormalities, volume overload, and acid-base status. Potential harm from RRT includes complications of central venous access, intradialytic hypotension, and the bleeding risk of anticoagulation. The optimal timing of the elective initiation of RRT for AKI in septic shock or ARDS is unknown.
Study design: A post hoc subgroup study of a randomized, controlled trial.
Setting: Thirty-one ICUs in France.
Synopsis: Using data from the Artificial Kidney Initiation in Kidney Injury trial, the authors evaluated 619 patients with severe AKI and requirement for catecholamine infusion and/or invasive mechanical ventilation. Patients were randomly given RRT in an early or a delayed time frame. The early strategy involved RRT as soon as possible after randomization. In addition to the other parameters, the patients in the delayed group were given RRT for the following: anuria/oliguria 72 hours after randomization, blood urea nitrogen greater than 112 mg/dL, serum potassium greater than 6 mmol/L, metabolic acidosis with pH less than 7.15, or pulmonary edema from fluid overload causing severe hypoxia.
Early RRT did not show significant improvement in 60-day mortality, length of mechanical ventilation, or length of stay, compared with delayed RRT. The delayed RRT strategy was significantly associated with renal function recovery, with hazard ratios of 1.7 in ARDS (P = .009) and 1.9 in septic shock (P less than .001). Additionally, the likelihood of adequate urinary output was greater in the delayed RRT group.
Bottom line: A delayed RRT strategy in those with severe AKI and septic shock or ARDS may safely afford time for renal recovery in some patients.
Citation: Gaudry S et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome. A post hoc analysis of the AKIKI randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):58-66.
Dr. James is a hospitalist at Emory University Hospital Midtown and an assistant professor at Emory University, both in Atlanta.
Acute kidney injury after hip or knee replacement: Can we lower the risk?
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
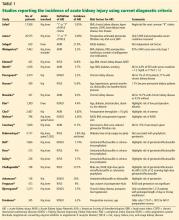
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
KEY POINTS
- Using current diagnostic criteria, the incidence of acute kidney injury complicating primary total joint arthroplasty may be nearly 10%, and 25% after placement of an antibiotic-loaded cement spacer to treat infection.
- In primary total joint arthroplasty, significant risk factors include older age, higher body mass index, chronic kidney disease, comorbidity, anemia, perioperative transfusion, aminoglycoside prophylaxis and treatment, preoperative heart murmur, and renin-angiotensin-aldosterone system blockade.
- Acute kidney injury may arise from infection, systemic administration of nephrotoxic antibiotics, and elution of antibiotics from antibiotic-loaded cement.
- No randomized controlled trial aimed at reducing acute kidney injury in these settings has been published; however, suggestions for practice modification are made based on the available data.
New renal, CV disease indication sought for canagliflozin
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
Janssen has announced that it has submitted a supplemental new drug application to the Food and Drug Administration to add an indication for canagliflozin (Invokana). The sodium-glucose cotransporter 2 is currently indicated, in addition to diet and exercise, for glycemic control in type 2 diabetes. However, in hopes of reducing the risks of end-stage kidney disease and of renal or cardiovascular death, according to a press release from the manufacturer.
If approved, canagliflozin will be the first diabetes medicine for the treatment of people living with type 2 diabetes and chronic kidney disease, according to the press release.
The application was based on the results of the phase 3 CREDENCE trial, a randomized, double-blind, placebo-controlled, multicenter study of 4,401 patients with type 2 diabetes, stage 2 or 3 chronic kidney disease, and macroalbuminuria. The patients received standard of care as well. The trial was stopped early, in July 2018, because it had met the prespecified criteria for efficacy. Data from the trial will be presented in mid-April at the annual meeting of the International Society of Nephrology World Congress of Nephrology in Melbourne.
Canagliflozin is contraindicated in patients with severe renal impairment (an estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), patients with end-stage renal disease, or patients on dialysis. Serious side effects associated with canagliflozin include ketoacidosis, kidney problems, hyperkalemia, serious urinary tract infections, and hypoglycemia. The most common side effects are yeast infections of the vagina or penis, and changes in urination.
The full prescribing information for canagliflozin is available on the FDA website.
When Can You Stop Dialysis?
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
When to Start Dialysis
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.