User login
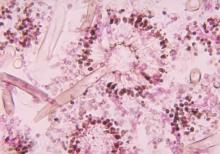
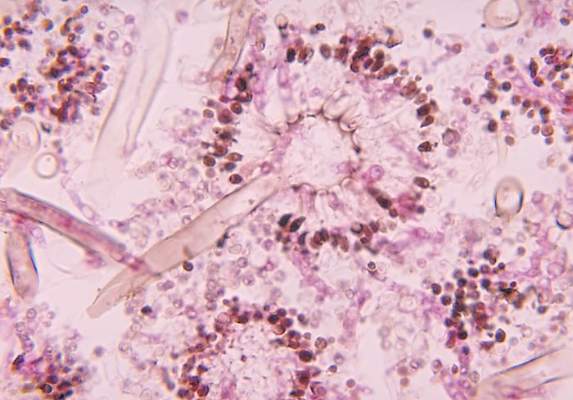
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.
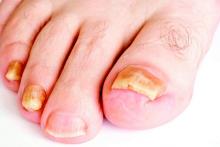
VIDEO: Which patients are best for new onychomycosis topicals?
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Dexamethasone harmful for patients with HIV-associated cryptococcal meningitis
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Dexamethasone failed to decrease mortality and induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis.
Major finding: The hazard ratio for death in the dexamethasone group relative to the placebo group was 0.77 at days 1-22 but rose to 1.94 at days 23-43, and to 2.50 at days 44-71.
Data source: An international, randomized, double-blind, placebo-controlled trial involving 451 patients treated for 6 weeks and followed for a further 4 weeks.
Disclosures: This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
VIDEO: New diagnostic, treatment methods for fungal infections
ORLANDO – New diagnostic and treatment options are at the forefront of what’s new and exciting in the area of superficial cutaneous fungal infections, according to Dr. Adam Friedman.
“Although superficial cutaneous mycoses [are] extremely common, they can be quite a challenge for several reasons,” explained Dr. Friedman of the George Washington University in Washington, at the Orlando Dermatology Aesthetic and Clinical annual meeting, adding that “many of the common skin diseases are often confused for tineum, and vice versa.”
In this video interview, Dr. Friedman discusses what dermatologists should look for in terms of diagnosing and treating dermatophytes and onychomycosis, two of most common and increasingly treatable fungal infections patients are likely to present with.
Dr. Friedman did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – New diagnostic and treatment options are at the forefront of what’s new and exciting in the area of superficial cutaneous fungal infections, according to Dr. Adam Friedman.
“Although superficial cutaneous mycoses [are] extremely common, they can be quite a challenge for several reasons,” explained Dr. Friedman of the George Washington University in Washington, at the Orlando Dermatology Aesthetic and Clinical annual meeting, adding that “many of the common skin diseases are often confused for tineum, and vice versa.”
In this video interview, Dr. Friedman discusses what dermatologists should look for in terms of diagnosing and treating dermatophytes and onychomycosis, two of most common and increasingly treatable fungal infections patients are likely to present with.
Dr. Friedman did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – New diagnostic and treatment options are at the forefront of what’s new and exciting in the area of superficial cutaneous fungal infections, according to Dr. Adam Friedman.
“Although superficial cutaneous mycoses [are] extremely common, they can be quite a challenge for several reasons,” explained Dr. Friedman of the George Washington University in Washington, at the Orlando Dermatology Aesthetic and Clinical annual meeting, adding that “many of the common skin diseases are often confused for tineum, and vice versa.”
In this video interview, Dr. Friedman discusses what dermatologists should look for in terms of diagnosing and treating dermatophytes and onychomycosis, two of most common and increasingly treatable fungal infections patients are likely to present with.
Dr. Friedman did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ODAC 2016
Hong Kong zygomycosis deaths pinned to dirty hospital laundry
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Clinicians need to maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,
Major finding: Of 195 environmental samples at the contaminated laundry, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples.
Data source: Epidemiological study in Hong Kong.
Disclosures: Hong Kong government services helped support the work. The authors do not have any financial conflicts of interest.
Oral fluconazole raises miscarriage risk
The use of oral fluconazole during pregnancy significantly raises the risk of spontaneous abortion, according to a report published online Jan. 5 in JAMA.
In a nationwide cohort study in Denmark involving more than 1.4 million pregnancies that occurred from 1997-2013, oral fluconazole increased the risk of spontaneous abortion from 7-22 weeks gestation, compared with no exposure to fluconazole and with exposure to a topical azole.
The drug did not raise the risk of stillbirth significantly in this study, “but this outcome was relatively rare and the results were therefore imprecise,” wrote Ditte Molgaard-Nielsen of the department of epidemiology research, Statens Serum Institut, Copenhagen, and associates.
The study findings indicate that “cautious prescribing of oral fluconazole in pregnancy may be advisable,” at least until more data regarding this association become available.
Pregnant women are at increased risk for candidiasis because of hormonal changes, and the prevalence of the infection is estimated to be 10% among pregnant women in the U.S. Intravaginal topical azoles are considered first-line treatment during pregnancy, but oral fluconazole can be used instead if the patient prefers it, in recurrent cases, or if symptoms are severe.
Long-term, high-dose oral fluconazole is associated with distinct craniofacial and skeletal birth defects, and most safety studies have focused on the possible teratogenic effects of the lower doses typically used during pregnancy. Only two epidemiologic studies to date have assessed a possible association with spontaneous abortion and stillbirth, and both “may not have had sufficient power to detect even a moderately increased risk,” the investigators wrote.
To examine a possible association between oral fluconazole use and spontaneous abortion (pregnancy loss at 7-22 gestational weeks) or stillbirth (pregnancy loss at 23 weeks or later), the investigators analyzed data in a national registry of all births, stillbirths, spontaneous abortions, induced abortions, ectopic pregnancies, cases of hydatidiform mole, and all other abnormal products of gestation. They correlated this with data in registries of all hospitalizations and all prescriptions filled in Denmark, focusing on the period from 1997-2013.
There were 3,315 pregnancies in which the mother received oral fluconazole during weeks 7-22, and these were matched for propensity score and maternal age with 13,246 control pregnancies. A total of 147 spontaneous abortions occurred in the exposed group and 563 in the control group. Exposure to oral fluconazole significantly increased the risk of spontaneous abortion, with a hazard ratio (HR) of 1.48, the investigators found (JAMA. 2016 Jan;315(1):58-67. doi: 10.1001/jama.2015.17844). In a further analysis that controlled for confounding by underlying disease (vaginal candidiasis), pregnancies exposed to oral fluconazole were at significantly higher risk of spontaneous abortion compared with both pregnancies exposed to topical azoles (HR, 1.62) and those exposed to pivmecillinam (HR, 1.44).
In addition, there were 5,382 pregnancies in which the mother received oral fluconazole during weeks 23 onward, and these were matched with 21,506 control pregnancies. A total of 21 stillbirths occurred in the exposed group and 77 in the control group. The hazard ratio for stillbirth in exposed pregnancies, compared with control pregnancies, was 1.32, which was not statistically significant. However, this result should be interpreted with caution because of the small numbers in these categories, the investigators wrote.
The use of oral fluconazole during pregnancy significantly raises the risk of spontaneous abortion, according to a report published online Jan. 5 in JAMA.
In a nationwide cohort study in Denmark involving more than 1.4 million pregnancies that occurred from 1997-2013, oral fluconazole increased the risk of spontaneous abortion from 7-22 weeks gestation, compared with no exposure to fluconazole and with exposure to a topical azole.
The drug did not raise the risk of stillbirth significantly in this study, “but this outcome was relatively rare and the results were therefore imprecise,” wrote Ditte Molgaard-Nielsen of the department of epidemiology research, Statens Serum Institut, Copenhagen, and associates.
The study findings indicate that “cautious prescribing of oral fluconazole in pregnancy may be advisable,” at least until more data regarding this association become available.
Pregnant women are at increased risk for candidiasis because of hormonal changes, and the prevalence of the infection is estimated to be 10% among pregnant women in the U.S. Intravaginal topical azoles are considered first-line treatment during pregnancy, but oral fluconazole can be used instead if the patient prefers it, in recurrent cases, or if symptoms are severe.
Long-term, high-dose oral fluconazole is associated with distinct craniofacial and skeletal birth defects, and most safety studies have focused on the possible teratogenic effects of the lower doses typically used during pregnancy. Only two epidemiologic studies to date have assessed a possible association with spontaneous abortion and stillbirth, and both “may not have had sufficient power to detect even a moderately increased risk,” the investigators wrote.
To examine a possible association between oral fluconazole use and spontaneous abortion (pregnancy loss at 7-22 gestational weeks) or stillbirth (pregnancy loss at 23 weeks or later), the investigators analyzed data in a national registry of all births, stillbirths, spontaneous abortions, induced abortions, ectopic pregnancies, cases of hydatidiform mole, and all other abnormal products of gestation. They correlated this with data in registries of all hospitalizations and all prescriptions filled in Denmark, focusing on the period from 1997-2013.
There were 3,315 pregnancies in which the mother received oral fluconazole during weeks 7-22, and these were matched for propensity score and maternal age with 13,246 control pregnancies. A total of 147 spontaneous abortions occurred in the exposed group and 563 in the control group. Exposure to oral fluconazole significantly increased the risk of spontaneous abortion, with a hazard ratio (HR) of 1.48, the investigators found (JAMA. 2016 Jan;315(1):58-67. doi: 10.1001/jama.2015.17844). In a further analysis that controlled for confounding by underlying disease (vaginal candidiasis), pregnancies exposed to oral fluconazole were at significantly higher risk of spontaneous abortion compared with both pregnancies exposed to topical azoles (HR, 1.62) and those exposed to pivmecillinam (HR, 1.44).
In addition, there were 5,382 pregnancies in which the mother received oral fluconazole during weeks 23 onward, and these were matched with 21,506 control pregnancies. A total of 21 stillbirths occurred in the exposed group and 77 in the control group. The hazard ratio for stillbirth in exposed pregnancies, compared with control pregnancies, was 1.32, which was not statistically significant. However, this result should be interpreted with caution because of the small numbers in these categories, the investigators wrote.
The use of oral fluconazole during pregnancy significantly raises the risk of spontaneous abortion, according to a report published online Jan. 5 in JAMA.
In a nationwide cohort study in Denmark involving more than 1.4 million pregnancies that occurred from 1997-2013, oral fluconazole increased the risk of spontaneous abortion from 7-22 weeks gestation, compared with no exposure to fluconazole and with exposure to a topical azole.
The drug did not raise the risk of stillbirth significantly in this study, “but this outcome was relatively rare and the results were therefore imprecise,” wrote Ditte Molgaard-Nielsen of the department of epidemiology research, Statens Serum Institut, Copenhagen, and associates.
The study findings indicate that “cautious prescribing of oral fluconazole in pregnancy may be advisable,” at least until more data regarding this association become available.
Pregnant women are at increased risk for candidiasis because of hormonal changes, and the prevalence of the infection is estimated to be 10% among pregnant women in the U.S. Intravaginal topical azoles are considered first-line treatment during pregnancy, but oral fluconazole can be used instead if the patient prefers it, in recurrent cases, or if symptoms are severe.
Long-term, high-dose oral fluconazole is associated with distinct craniofacial and skeletal birth defects, and most safety studies have focused on the possible teratogenic effects of the lower doses typically used during pregnancy. Only two epidemiologic studies to date have assessed a possible association with spontaneous abortion and stillbirth, and both “may not have had sufficient power to detect even a moderately increased risk,” the investigators wrote.
To examine a possible association between oral fluconazole use and spontaneous abortion (pregnancy loss at 7-22 gestational weeks) or stillbirth (pregnancy loss at 23 weeks or later), the investigators analyzed data in a national registry of all births, stillbirths, spontaneous abortions, induced abortions, ectopic pregnancies, cases of hydatidiform mole, and all other abnormal products of gestation. They correlated this with data in registries of all hospitalizations and all prescriptions filled in Denmark, focusing on the period from 1997-2013.
There were 3,315 pregnancies in which the mother received oral fluconazole during weeks 7-22, and these were matched for propensity score and maternal age with 13,246 control pregnancies. A total of 147 spontaneous abortions occurred in the exposed group and 563 in the control group. Exposure to oral fluconazole significantly increased the risk of spontaneous abortion, with a hazard ratio (HR) of 1.48, the investigators found (JAMA. 2016 Jan;315(1):58-67. doi: 10.1001/jama.2015.17844). In a further analysis that controlled for confounding by underlying disease (vaginal candidiasis), pregnancies exposed to oral fluconazole were at significantly higher risk of spontaneous abortion compared with both pregnancies exposed to topical azoles (HR, 1.62) and those exposed to pivmecillinam (HR, 1.44).
In addition, there were 5,382 pregnancies in which the mother received oral fluconazole during weeks 23 onward, and these were matched with 21,506 control pregnancies. A total of 21 stillbirths occurred in the exposed group and 77 in the control group. The hazard ratio for stillbirth in exposed pregnancies, compared with control pregnancies, was 1.32, which was not statistically significant. However, this result should be interpreted with caution because of the small numbers in these categories, the investigators wrote.
FROM JAMA
Key clinical point: The use of oral fluconazole during pregnancy significantly raises the risk of spontaneous abortion.
Major finding: A total of 147 spontaneous abortions occurred in the 3,315 pregnancies exposed to oral fluconazole, compared with 563 in the 13,246 control pregnancies.
Data source: A nationwide Danish cohort study involving 1,405,663 pregnancies from 1997-2013.
Disclosures: This study was supported by the Danish Medical Research Council. Ditte Molgaard-Nielsen and associates reported having no relevant financial disclosures.
European societies issue aspergillosis diagnosis, management guidelines
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Invasive candidiasis hospitalizations down overall
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: As of 2007, the incidence of hospital-associated invasive candidiasis appears to be decreasing.
Major finding: Between 2007 and 2012, the age-adjusted annual incidence of hospitalizations associated with invasive candidiasis in the United States decreased by 6.7% among men and by 7.4% among women.
Data source: A long-term analysis of data from the State Inpatient Database from the Agency for Healthcare Research and Quality.
Disclosures: The researchers reported having no financial disclosures.
New antifungals effective with shorter treatment course for tinea pedis
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
SDEF: Improved responses with newer topical onychomycosis treatments
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR