User login
Trends in Surveillance and Management of Dysplasia in IBD
Click to view more from Gastroenterology Data Trends 2022.
- Yeshi K, Ruscher R, Hunter L, et al. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med. 2020;9(5):1273. doi:10.3390/jcm9051273
- Xu F, Carlson SA, Liu Y, Greenlund KJ. Prevalence of inflammatory bowel disease among Medicare fee-for-service beneficiaries – United States, 2001-2018. MMWR Morb Mortal Wkly Rep. 2021;70(19):698-701. doi:10.15585/mmwr.mm7019a2
- Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11(5):1033. doi:10.3390/nu11051033
- Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31(3):168-178. doi:10.1055/s-0037-1602237
- Tariq H, Kamal MU, Sapkota B, et al. Evaluation of the combined effect of factors influencing bowel preparation and adenoma detection rates in patients undergoing colonoscopy. BMJ Open Gastroenterol. 2019;6(1):e000254. doi:10.1136/bmjgast-2018-000254
- May FP, Shaukat A. Time to add the "Q" (quality) factor to postpolypectomy surveillance? Gastroenterology. 2021;160(4):1007-1009. doi:10.1053/j.gastro.2020.12.067
- Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021;161(3):1043-1051.e4. doi:10.1053/j.gastro.2021.05.063
- van der Laan JJH, van der Waaij AM, Gabriëls RY, Festen EAM, Dijkstra G, Nagengast WB. Endoscopic imaging in inflammatory bowel disease: current developments and emerging strategies. Expert Rev Gastroenterol Hepatol. 2021;15(2):115-126. doi:10.1080/17474124.2021.1840352
- Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254-266. doi:10.1093/ecco-jcc/jjz131
- Atia O, Harel S, Ledderman N, et al. Risk of cancer in paediatric onset inflammatory bowel diseases: a nation-wide study from the epi-IIRN. J Crohns Colitis. 2022;16(5):786-795. doi:10.1093/ecco-jcc/jjab205
- Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375-381.e1. doi:10.1053/j.gastro.2012.04.016
- Di Palma JA, Bhandari R, Cleveland MV, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116(2):319-328. doi:10.14309/ajg.0000000000001020
- May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenterol. 2020;115(8):1183-1190. doi:10.14309/ajg.0000000000000622
- Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:651415. doi:10.3389/fphar.2021.651415
- Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32-40. doi:10.1093/ibd/izab011
Click to view more from Gastroenterology Data Trends 2022.
Click to view more from Gastroenterology Data Trends 2022.
- Yeshi K, Ruscher R, Hunter L, et al. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med. 2020;9(5):1273. doi:10.3390/jcm9051273
- Xu F, Carlson SA, Liu Y, Greenlund KJ. Prevalence of inflammatory bowel disease among Medicare fee-for-service beneficiaries – United States, 2001-2018. MMWR Morb Mortal Wkly Rep. 2021;70(19):698-701. doi:10.15585/mmwr.mm7019a2
- Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11(5):1033. doi:10.3390/nu11051033
- Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31(3):168-178. doi:10.1055/s-0037-1602237
- Tariq H, Kamal MU, Sapkota B, et al. Evaluation of the combined effect of factors influencing bowel preparation and adenoma detection rates in patients undergoing colonoscopy. BMJ Open Gastroenterol. 2019;6(1):e000254. doi:10.1136/bmjgast-2018-000254
- May FP, Shaukat A. Time to add the "Q" (quality) factor to postpolypectomy surveillance? Gastroenterology. 2021;160(4):1007-1009. doi:10.1053/j.gastro.2020.12.067
- Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021;161(3):1043-1051.e4. doi:10.1053/j.gastro.2021.05.063
- van der Laan JJH, van der Waaij AM, Gabriëls RY, Festen EAM, Dijkstra G, Nagengast WB. Endoscopic imaging in inflammatory bowel disease: current developments and emerging strategies. Expert Rev Gastroenterol Hepatol. 2021;15(2):115-126. doi:10.1080/17474124.2021.1840352
- Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254-266. doi:10.1093/ecco-jcc/jjz131
- Atia O, Harel S, Ledderman N, et al. Risk of cancer in paediatric onset inflammatory bowel diseases: a nation-wide study from the epi-IIRN. J Crohns Colitis. 2022;16(5):786-795. doi:10.1093/ecco-jcc/jjab205
- Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375-381.e1. doi:10.1053/j.gastro.2012.04.016
- Di Palma JA, Bhandari R, Cleveland MV, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116(2):319-328. doi:10.14309/ajg.0000000000001020
- May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenterol. 2020;115(8):1183-1190. doi:10.14309/ajg.0000000000000622
- Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:651415. doi:10.3389/fphar.2021.651415
- Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32-40. doi:10.1093/ibd/izab011
- Yeshi K, Ruscher R, Hunter L, et al. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med. 2020;9(5):1273. doi:10.3390/jcm9051273
- Xu F, Carlson SA, Liu Y, Greenlund KJ. Prevalence of inflammatory bowel disease among Medicare fee-for-service beneficiaries – United States, 2001-2018. MMWR Morb Mortal Wkly Rep. 2021;70(19):698-701. doi:10.15585/mmwr.mm7019a2
- Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11(5):1033. doi:10.3390/nu11051033
- Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31(3):168-178. doi:10.1055/s-0037-1602237
- Tariq H, Kamal MU, Sapkota B, et al. Evaluation of the combined effect of factors influencing bowel preparation and adenoma detection rates in patients undergoing colonoscopy. BMJ Open Gastroenterol. 2019;6(1):e000254. doi:10.1136/bmjgast-2018-000254
- May FP, Shaukat A. Time to add the "Q" (quality) factor to postpolypectomy surveillance? Gastroenterology. 2021;160(4):1007-1009. doi:10.1053/j.gastro.2020.12.067
- Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021;161(3):1043-1051.e4. doi:10.1053/j.gastro.2021.05.063
- van der Laan JJH, van der Waaij AM, Gabriëls RY, Festen EAM, Dijkstra G, Nagengast WB. Endoscopic imaging in inflammatory bowel disease: current developments and emerging strategies. Expert Rev Gastroenterol Hepatol. 2021;15(2):115-126. doi:10.1080/17474124.2021.1840352
- Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254-266. doi:10.1093/ecco-jcc/jjz131
- Atia O, Harel S, Ledderman N, et al. Risk of cancer in paediatric onset inflammatory bowel diseases: a nation-wide study from the epi-IIRN. J Crohns Colitis. 2022;16(5):786-795. doi:10.1093/ecco-jcc/jjab205
- Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375-381.e1. doi:10.1053/j.gastro.2012.04.016
- Di Palma JA, Bhandari R, Cleveland MV, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116(2):319-328. doi:10.14309/ajg.0000000000001020
- May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenterol. 2020;115(8):1183-1190. doi:10.14309/ajg.0000000000000622
- Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:651415. doi:10.3389/fphar.2021.651415
- Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32-40. doi:10.1093/ibd/izab011
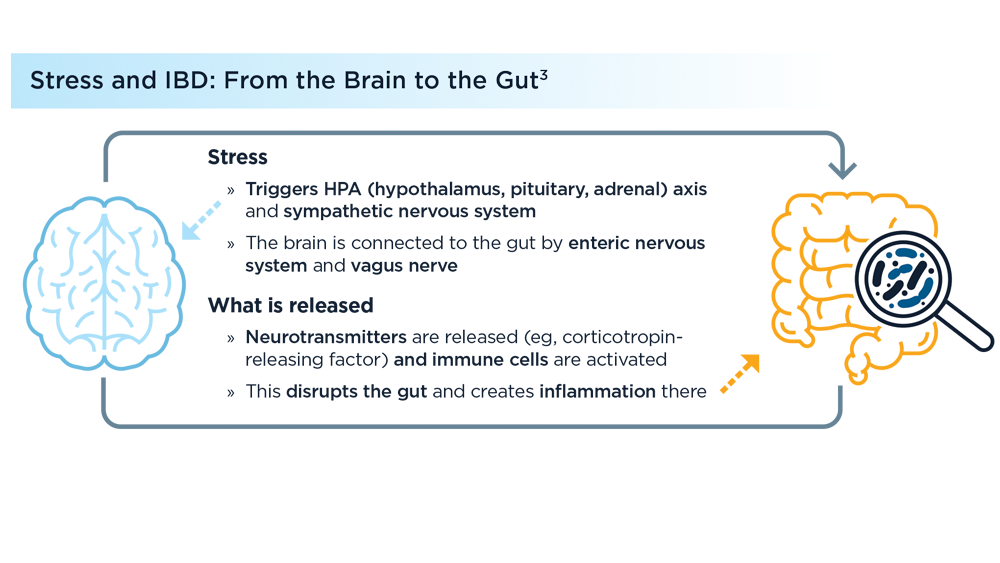
Environmental Factors in IBD: Diet and Stress
- Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7(7):666-678. doi:10.1016/S2468-1253(22)00021-8
- Byrne G, Rosenfeld G, Leung Y, et al. Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:6496727. doi:10.1155/2017/6496727
- Sun Y, Li L, Xie R, Wang B, Jiang K, Cao H. Stress triggers flare of inflammatory bowel disease in children and adults. Front Pediatr. 2019;7:432. doi:10.3389/fped.2019.00432
- Bernabeu P, van-der Hofstadt C, Rodríguez-Marín J, et al. Effectiveness of a multicomponent group psychological intervention program in patients with inflammatory bowel disease: a randomized trial. Int J Environ Res Public Health. 2021;18(10):5439. doi:10.3390/ijerph18105439
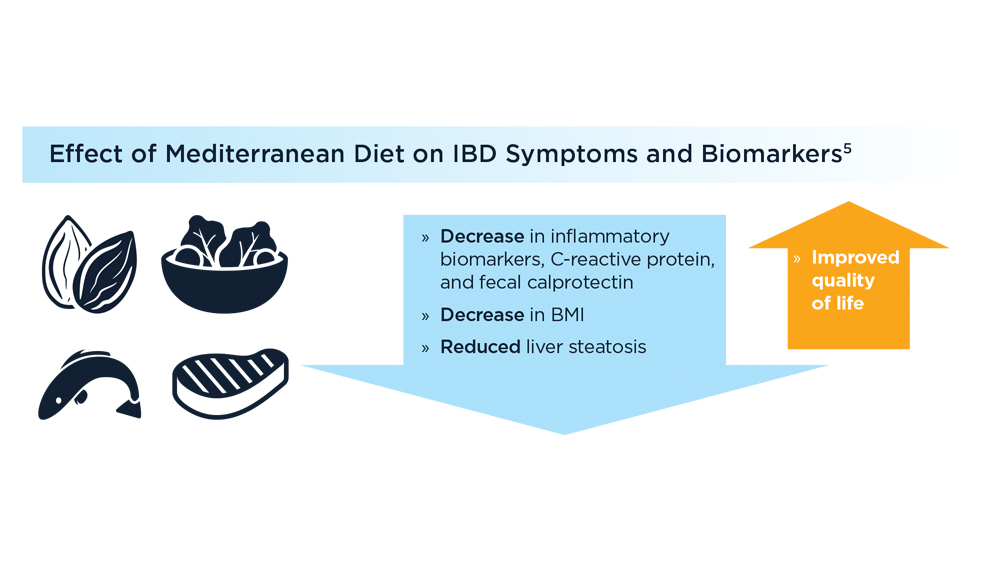
- Chicco F, Magrì S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27(1):1-9. doi:10.1093/ibd/izaa097
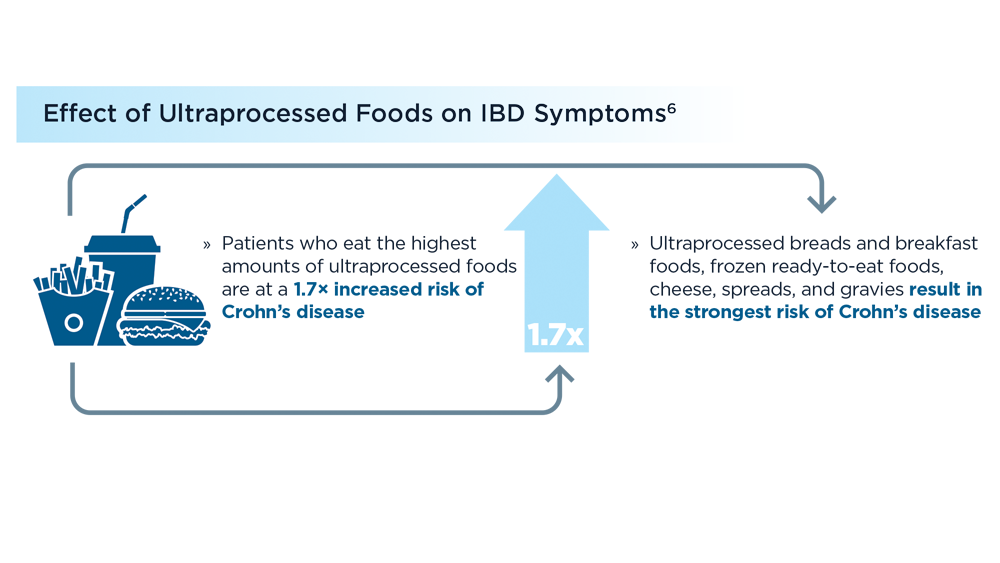
- Lo CH, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2022;20(6):e1323-e1337. doi:10.1016/j.cgh.2021.08.031
- Crooks B, McLaughlin J, Matsuoka K, Kobayashi T, Yamazaki H, Limdi JK. The dietary practices and beliefs of people living with inactive ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(3):372-379. doi:10.1097/MEG.0000000000001911
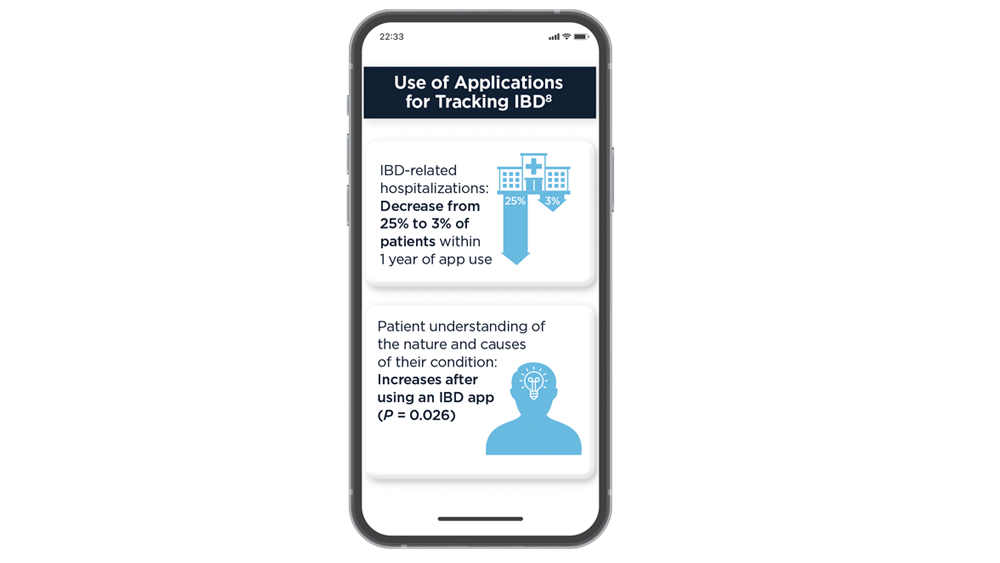
- Zhen J, Marshall JK, Nguyen GC, Atreja A, Narula N. Impact of digital health monitoring in the management of inflammatory bowel disease. J Med Syst. 2021;45(2):23. doi:10.1007/s10916-021-01706-x
- Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7(7):666-678. doi:10.1016/S2468-1253(22)00021-8
- Byrne G, Rosenfeld G, Leung Y, et al. Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:6496727. doi:10.1155/2017/6496727
- Sun Y, Li L, Xie R, Wang B, Jiang K, Cao H. Stress triggers flare of inflammatory bowel disease in children and adults. Front Pediatr. 2019;7:432. doi:10.3389/fped.2019.00432
- Bernabeu P, van-der Hofstadt C, Rodríguez-Marín J, et al. Effectiveness of a multicomponent group psychological intervention program in patients with inflammatory bowel disease: a randomized trial. Int J Environ Res Public Health. 2021;18(10):5439. doi:10.3390/ijerph18105439
- Chicco F, Magrì S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27(1):1-9. doi:10.1093/ibd/izaa097
- Lo CH, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2022;20(6):e1323-e1337. doi:10.1016/j.cgh.2021.08.031
- Crooks B, McLaughlin J, Matsuoka K, Kobayashi T, Yamazaki H, Limdi JK. The dietary practices and beliefs of people living with inactive ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(3):372-379. doi:10.1097/MEG.0000000000001911
- Zhen J, Marshall JK, Nguyen GC, Atreja A, Narula N. Impact of digital health monitoring in the management of inflammatory bowel disease. J Med Syst. 2021;45(2):23. doi:10.1007/s10916-021-01706-x
- Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7(7):666-678. doi:10.1016/S2468-1253(22)00021-8
- Byrne G, Rosenfeld G, Leung Y, et al. Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:6496727. doi:10.1155/2017/6496727
- Sun Y, Li L, Xie R, Wang B, Jiang K, Cao H. Stress triggers flare of inflammatory bowel disease in children and adults. Front Pediatr. 2019;7:432. doi:10.3389/fped.2019.00432
- Bernabeu P, van-der Hofstadt C, Rodríguez-Marín J, et al. Effectiveness of a multicomponent group psychological intervention program in patients with inflammatory bowel disease: a randomized trial. Int J Environ Res Public Health. 2021;18(10):5439. doi:10.3390/ijerph18105439
- Chicco F, Magrì S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27(1):1-9. doi:10.1093/ibd/izaa097
- Lo CH, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2022;20(6):e1323-e1337. doi:10.1016/j.cgh.2021.08.031
- Crooks B, McLaughlin J, Matsuoka K, Kobayashi T, Yamazaki H, Limdi JK. The dietary practices and beliefs of people living with inactive ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(3):372-379. doi:10.1097/MEG.0000000000001911
- Zhen J, Marshall JK, Nguyen GC, Atreja A, Narula N. Impact of digital health monitoring in the management of inflammatory bowel disease. J Med Syst. 2021;45(2):23. doi:10.1007/s10916-021-01706-x
Oral FMT on par with colonic FMT for recurrent C. difficile
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Then and now: Gut microbiome
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
The HMP, which was supported by “only” approximately $20 million of funding in its first year, served as a catalyst for the development of computational tools, clinical protocols, and reference datasets for an emerging field that now approaches nearly $2 billion per year in market value of diagnostics and therapeutics.
Over the past 15 years, many important discoveries about the microbiome have been made, particularly in the fields of gastroenterology, hepatology, and nutrition. The transplantation of gut microbiome from one person to another has been shown to be more than 90% effective in the treatment of recurrent C. difficile infection, disrupting our current therapeutic algorithms of repetitive antibiotics. Other exciting discoveries have included the relationship between the gut microbiome and enteric nervous system, and its roles in the regulation of metabolism and obesity and in the progression of liver fibrosis and cancer.
Looking ahead, several exciting areas related to digestive health and the microbiome are being prioritized, including the role of probiotics in nutrition, the complex relationship of the bidirectional “gut-brain” axis, and further development of analytics to define and deliver precision medicine across a wide range of digestive disorders. Without a doubt, emerging microbiome discoveries will be prominently featured in the pages of GI & Hepatology News over the coming years to keep our readers informed of these cutting-edge findings.
Dr. Rosenberg is medical director of the North Shore Endoscopy Center and director of clinical research at GI Alliance of Illinois in Gurnee, Ill. Dr. Rosenberg is a consultant for Aimmune Therapeutics and performs clinical research with Ferring Pharmaceuticals.
Commentary: Alternate considerations in treating IBS, November 2022
Acupuncture is a very popular treatment strategy in some areas of the world and is extensively applied in Chinese practice. Though this is a regularly applied treatment, a direct comparison with first-line antispasmodics has not been previously completed. The study by Shi and colleagues sought to compare the treatment of IBS using an adjusted indirect treatment comparison meta-analysis. This study proves that cimetropium was the most effective for relieving abdominal pain, whereas drotaverine, acupuncture, and pinaverium remained superior to the placebo. That being said, acupuncture was shown to be superior in relieving global IBS symptoms and caused fewer side effects than did antispasmodics. This shows that acupuncture may have a role in the treatment algorithm for IBS even outside of China, where it is used broadly.
The study by Formica and colleagues provides new insights on the etiology and pathogenesis of IBS. These insights include the positive correlation between disgust sensitivity and IBS quality of life (QOL) scores. The relationship between IBS and the emotional intensity of the experience of disgust is discussed throughout this study and more severe IBS-QOL scores were linked to those with high levels of disgust sensitivity. This study had gender limitations because of the low number of male participants, so these correlations were patterned mostly in female participants.
Acupuncture is a very popular treatment strategy in some areas of the world and is extensively applied in Chinese practice. Though this is a regularly applied treatment, a direct comparison with first-line antispasmodics has not been previously completed. The study by Shi and colleagues sought to compare the treatment of IBS using an adjusted indirect treatment comparison meta-analysis. This study proves that cimetropium was the most effective for relieving abdominal pain, whereas drotaverine, acupuncture, and pinaverium remained superior to the placebo. That being said, acupuncture was shown to be superior in relieving global IBS symptoms and caused fewer side effects than did antispasmodics. This shows that acupuncture may have a role in the treatment algorithm for IBS even outside of China, where it is used broadly.
The study by Formica and colleagues provides new insights on the etiology and pathogenesis of IBS. These insights include the positive correlation between disgust sensitivity and IBS quality of life (QOL) scores. The relationship between IBS and the emotional intensity of the experience of disgust is discussed throughout this study and more severe IBS-QOL scores were linked to those with high levels of disgust sensitivity. This study had gender limitations because of the low number of male participants, so these correlations were patterned mostly in female participants.
Acupuncture is a very popular treatment strategy in some areas of the world and is extensively applied in Chinese practice. Though this is a regularly applied treatment, a direct comparison with first-line antispasmodics has not been previously completed. The study by Shi and colleagues sought to compare the treatment of IBS using an adjusted indirect treatment comparison meta-analysis. This study proves that cimetropium was the most effective for relieving abdominal pain, whereas drotaverine, acupuncture, and pinaverium remained superior to the placebo. That being said, acupuncture was shown to be superior in relieving global IBS symptoms and caused fewer side effects than did antispasmodics. This shows that acupuncture may have a role in the treatment algorithm for IBS even outside of China, where it is used broadly.
The study by Formica and colleagues provides new insights on the etiology and pathogenesis of IBS. These insights include the positive correlation between disgust sensitivity and IBS quality of life (QOL) scores. The relationship between IBS and the emotional intensity of the experience of disgust is discussed throughout this study and more severe IBS-QOL scores were linked to those with high levels of disgust sensitivity. This study had gender limitations because of the low number of male participants, so these correlations were patterned mostly in female participants.
Endoscopic severity score helps guide treatment in immune-mediated colitis
, according to new research presented at the annual meeting of the American College of Gastroenterology.
An endoscopy score cutoff of 4 or higher had a specificity of 82.8% across all colitis grades, and a cutoff of 5 or higher had a specificity of 87.6%, said Yinghong Wang, MD, PhD, a gastroenterologist at the University of Texas MD Anderson Cancer Center, Houston.
Immune-mediated colitis (IMC) is a common immune-related adverse event associated with immune checkpoint inhibitors. Dr. Wang and colleagues previously reported on endoscopic presentations of IMC, including severe inflammation with deep ulcerated mucosa; moderate to severe inflammation with diffuse erythema, superficial ulcers, exudate, and loss of vasculature; and mild inflammation with patchy erythema, aphtha, edema, or normal mucosa associated with histological inflammation.
Endoscopic scoring systems haven’t been established for IMC, but previous studies have shown benefits from early endoscopic evaluation. The current Common Terminology Criteria for Adverse Events (CTCAE) grading system for clinical symptoms alone has been poorly correlated with endoscopic findings and unable to provide accurate assessments, Dr. Wang said.
“There is a critical and urgent need to develop a new scoring system that could provide accurate and comprehensive assessment for IMC severity to better predict the requirement of more aggressive selective immunosuppressive therapy (SIT), which includes infliximab and vedolizumab,” she said.
Dr. Wang and colleagues conducted a retrospective international study across 14 centers to develop a new comprehensive endoscopic scoring system to assess the severity of IMC and explore its utility in predicting the need for aggressive treatment with SIT. They included 674 adult cancer patients in the United States, United Kingdom, Germany, and Australia with IMC who underwent endoscopic evaluation between 2010 and 2020.
All patients had received immune checkpoint inhibitors, an IMC diagnosis, and endoscopy and histology evaluations for IMC. In addition, all patients had diarrhea, including 92% who had grade 2 diarrhea and higher and 80% who had grade 2 colitis and higher. About 85% were treated with corticosteroids, 31% were treated with infliximab, 10% were treated with vedolizumab, and 5% were treated with both treatment types, corticosteroids and SIT.
Based on endoscopic reports, the research team looked at 10 endoscopic features and assigned one point each for erythema, edema, loss of vasculature, friability, erosions, exudate, any ulcers, large ulcers, deep ulcers, and more than two ulcers. The median IMC endoscopic score was 2.
The scoring system was devised by measuring the specificity of a selected score cutoff in predicting the need for SIT based on clinical consensus from the study group.
The researchers divided the cohort into a training set and a validation set. In the training set, an IMC endoscopy score cutoff of 4 or more had a specificity of 82.8% across all colitis grades and 96.4% among grade 1 colitis to predict SIT use. A cutoff of 5 or more had a specificity of 87.6% across all colitis grades and 98.2% among grade 1. These specificities were comparable to those of the validation sets.
At the same time, the CTCAE score was poorly associated with prediction of future SIT use, with a specificity of 27.4% for clinical colitis grading and 12.3% for diarrhea grading.
In addition, an IMC endoscopic score of 4 or 5 plus ulcer factors had a numerically higher specificity than a Mayo Endoscopic Score of 3. The IMC endoscopic score had a specificity of 85% at a cutoff of 4 and 88.2% at a cutoff of 5, as compared with 74.6% for the Mayo score.
Early endoscopic evaluation in disease course was associated with early SIT use, with a P value of less than .001.
“Implementation of this novel endoscopic scoring system could guide future IMC treatment more precisely,” Dr. Wang said.
The study funding was not disclosed. The authors reported consultant roles, advisory roles, and research support from several pharmaceutical companies.
, according to new research presented at the annual meeting of the American College of Gastroenterology.
An endoscopy score cutoff of 4 or higher had a specificity of 82.8% across all colitis grades, and a cutoff of 5 or higher had a specificity of 87.6%, said Yinghong Wang, MD, PhD, a gastroenterologist at the University of Texas MD Anderson Cancer Center, Houston.
Immune-mediated colitis (IMC) is a common immune-related adverse event associated with immune checkpoint inhibitors. Dr. Wang and colleagues previously reported on endoscopic presentations of IMC, including severe inflammation with deep ulcerated mucosa; moderate to severe inflammation with diffuse erythema, superficial ulcers, exudate, and loss of vasculature; and mild inflammation with patchy erythema, aphtha, edema, or normal mucosa associated with histological inflammation.
Endoscopic scoring systems haven’t been established for IMC, but previous studies have shown benefits from early endoscopic evaluation. The current Common Terminology Criteria for Adverse Events (CTCAE) grading system for clinical symptoms alone has been poorly correlated with endoscopic findings and unable to provide accurate assessments, Dr. Wang said.
“There is a critical and urgent need to develop a new scoring system that could provide accurate and comprehensive assessment for IMC severity to better predict the requirement of more aggressive selective immunosuppressive therapy (SIT), which includes infliximab and vedolizumab,” she said.
Dr. Wang and colleagues conducted a retrospective international study across 14 centers to develop a new comprehensive endoscopic scoring system to assess the severity of IMC and explore its utility in predicting the need for aggressive treatment with SIT. They included 674 adult cancer patients in the United States, United Kingdom, Germany, and Australia with IMC who underwent endoscopic evaluation between 2010 and 2020.
All patients had received immune checkpoint inhibitors, an IMC diagnosis, and endoscopy and histology evaluations for IMC. In addition, all patients had diarrhea, including 92% who had grade 2 diarrhea and higher and 80% who had grade 2 colitis and higher. About 85% were treated with corticosteroids, 31% were treated with infliximab, 10% were treated with vedolizumab, and 5% were treated with both treatment types, corticosteroids and SIT.
Based on endoscopic reports, the research team looked at 10 endoscopic features and assigned one point each for erythema, edema, loss of vasculature, friability, erosions, exudate, any ulcers, large ulcers, deep ulcers, and more than two ulcers. The median IMC endoscopic score was 2.
The scoring system was devised by measuring the specificity of a selected score cutoff in predicting the need for SIT based on clinical consensus from the study group.
The researchers divided the cohort into a training set and a validation set. In the training set, an IMC endoscopy score cutoff of 4 or more had a specificity of 82.8% across all colitis grades and 96.4% among grade 1 colitis to predict SIT use. A cutoff of 5 or more had a specificity of 87.6% across all colitis grades and 98.2% among grade 1. These specificities were comparable to those of the validation sets.
At the same time, the CTCAE score was poorly associated with prediction of future SIT use, with a specificity of 27.4% for clinical colitis grading and 12.3% for diarrhea grading.
In addition, an IMC endoscopic score of 4 or 5 plus ulcer factors had a numerically higher specificity than a Mayo Endoscopic Score of 3. The IMC endoscopic score had a specificity of 85% at a cutoff of 4 and 88.2% at a cutoff of 5, as compared with 74.6% for the Mayo score.
Early endoscopic evaluation in disease course was associated with early SIT use, with a P value of less than .001.
“Implementation of this novel endoscopic scoring system could guide future IMC treatment more precisely,” Dr. Wang said.
The study funding was not disclosed. The authors reported consultant roles, advisory roles, and research support from several pharmaceutical companies.
, according to new research presented at the annual meeting of the American College of Gastroenterology.
An endoscopy score cutoff of 4 or higher had a specificity of 82.8% across all colitis grades, and a cutoff of 5 or higher had a specificity of 87.6%, said Yinghong Wang, MD, PhD, a gastroenterologist at the University of Texas MD Anderson Cancer Center, Houston.
Immune-mediated colitis (IMC) is a common immune-related adverse event associated with immune checkpoint inhibitors. Dr. Wang and colleagues previously reported on endoscopic presentations of IMC, including severe inflammation with deep ulcerated mucosa; moderate to severe inflammation with diffuse erythema, superficial ulcers, exudate, and loss of vasculature; and mild inflammation with patchy erythema, aphtha, edema, or normal mucosa associated with histological inflammation.
Endoscopic scoring systems haven’t been established for IMC, but previous studies have shown benefits from early endoscopic evaluation. The current Common Terminology Criteria for Adverse Events (CTCAE) grading system for clinical symptoms alone has been poorly correlated with endoscopic findings and unable to provide accurate assessments, Dr. Wang said.
“There is a critical and urgent need to develop a new scoring system that could provide accurate and comprehensive assessment for IMC severity to better predict the requirement of more aggressive selective immunosuppressive therapy (SIT), which includes infliximab and vedolizumab,” she said.
Dr. Wang and colleagues conducted a retrospective international study across 14 centers to develop a new comprehensive endoscopic scoring system to assess the severity of IMC and explore its utility in predicting the need for aggressive treatment with SIT. They included 674 adult cancer patients in the United States, United Kingdom, Germany, and Australia with IMC who underwent endoscopic evaluation between 2010 and 2020.
All patients had received immune checkpoint inhibitors, an IMC diagnosis, and endoscopy and histology evaluations for IMC. In addition, all patients had diarrhea, including 92% who had grade 2 diarrhea and higher and 80% who had grade 2 colitis and higher. About 85% were treated with corticosteroids, 31% were treated with infliximab, 10% were treated with vedolizumab, and 5% were treated with both treatment types, corticosteroids and SIT.
Based on endoscopic reports, the research team looked at 10 endoscopic features and assigned one point each for erythema, edema, loss of vasculature, friability, erosions, exudate, any ulcers, large ulcers, deep ulcers, and more than two ulcers. The median IMC endoscopic score was 2.
The scoring system was devised by measuring the specificity of a selected score cutoff in predicting the need for SIT based on clinical consensus from the study group.
The researchers divided the cohort into a training set and a validation set. In the training set, an IMC endoscopy score cutoff of 4 or more had a specificity of 82.8% across all colitis grades and 96.4% among grade 1 colitis to predict SIT use. A cutoff of 5 or more had a specificity of 87.6% across all colitis grades and 98.2% among grade 1. These specificities were comparable to those of the validation sets.
At the same time, the CTCAE score was poorly associated with prediction of future SIT use, with a specificity of 27.4% for clinical colitis grading and 12.3% for diarrhea grading.
In addition, an IMC endoscopic score of 4 or 5 plus ulcer factors had a numerically higher specificity than a Mayo Endoscopic Score of 3. The IMC endoscopic score had a specificity of 85% at a cutoff of 4 and 88.2% at a cutoff of 5, as compared with 74.6% for the Mayo score.
Early endoscopic evaluation in disease course was associated with early SIT use, with a P value of less than .001.
“Implementation of this novel endoscopic scoring system could guide future IMC treatment more precisely,” Dr. Wang said.
The study funding was not disclosed. The authors reported consultant roles, advisory roles, and research support from several pharmaceutical companies.
FROM ACG 2022
Guselkumab induction improves moderate to severe active UC at week 12
according to findings presented at the annual meeting of the American College of Gastroenterology.
The efficacy of the 200-mg dose and the 400-mg dose was comparable, said David Rubin, MD, a gastroenterologist at the University of Chicago Medicine Inflammatory Bowel Disease Center. Outcomes improved in all patients, with or without a history of inadequate response or intolerance to advanced therapy.
Guselkumab, an interleukin-12 p19 subunit antagonist, is currently being investigated in inflammatory bowel disease.
The QUASAR Induction Study 1 (NCT04033445) is a phase 2b, randomized, double-blind, placebo-controlled study that evaluates guselkumab as induction therapy in patients with moderately to severely active UC. Inclusion criteria specify a demonstrated inadequate response or intolerance to conventional therapy, such as thiopurines or corticosteroids, or to advanced therapy, such as tumor necrosis factor–alpha antagonists, vedolizumab, or tofacitinib. The study didn’t include patients exposed to ustekinumab.
Study participants were age 18 and older with moderately to severely active UC, defined as a modified Mayo score of 5-9 with a Mayo rectal bleeding subscore of 1 or greater and a Mayo endoscopy subscore of 2 or greater at baseline. The groups were randomized 1:1:1 to receive 400 mg of IV guselkumab, 200 mg of guselkumab, or placebo at weeks 0, 4, and 8.
At week 12, the research team looked for several key endpoints. Clinical response was defined as a modified Mayo score decrease of 30% or more and a drop in 2 or more points, with either a 1-point decrease or more in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
Clinical remission was defined as a stool frequency subscore of 0 or 1 that hadn’t increased from baseline, a rectal bleeding subscore of 0, and an endoscopy subscore of 0 or 1 with no friability present on the endoscopy.
In addition, symptomatic remission was defined as a stool subscore of 0 or 1 that hadn’t increased from baseline and rectal bleeding subscore of 0.
Endoscopic improvement was defined as an endoscopy subscore of 0 or 1 with no friability present on the endoscopy. Endoscopic normalization was an endoscopy subscore of 0.
Notably, the research team looked at histoendoscopic mucosal improvement, which includes a combination of endoscopic improvement and histologic improvement (neutrophil infiltration in less than 5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue, according to the Geboes grading system).
Among the 313 total patients, 47% had a history of inadequate response or intolerance to advanced therapy, and about half of these patients had prior inadequate response or intolerance to two or more advanced therapy classes.
At baseline, about 90% of patients had an endoscopic subscore of 3 (severe). More than half had extensive UC, and the average UC duration was 9 years. About 20% overall had extraintestinal manifestations present, which were noted in 33% of the 400 mg guselkumab treatment arm.
At week 12, clinical response was achieved by a higher proportion of patients treated with guselkumab versus placebo, at 50.5% versus 25.5% for patients with prior inadequate response or intolerance to advanced therapy and 70.3% versus 29.6% for those without prior inadequate response or intolerance to advanced therapy, the authors reported in the abstract.
Compared with placebo, higher proportions of patients treated with guselkumab achieved clinical, endoscopic, and histologic outcomes in both groups with or without inadequate response or intolerance to advanced therapy. Generally, those without a history of inadequate response had higher response rates across all endpoints.
Overall, both the 200-mg and 400-mg doses of guselkumab were statistically superior to the placebo across all endpoints for both groups (with or without inadequate response or intolerance). Although the efficacy was comparable for the two doses, the 400-mg dose was associated with greater histoendoscopic mucosal improvement in both groups.
“It’s of interest to think about how we position and sequence our therapies with this additional data,” Dr. Rubin said.
The study was sponsored by Janssen Research & Development. Several authors are employees for and have stock options with Johnson & Johnson and Janssen. The other authors reported consultant roles, advisory roles, and research support from numerous pharmaceutical companies, including Janssen.
according to findings presented at the annual meeting of the American College of Gastroenterology.
The efficacy of the 200-mg dose and the 400-mg dose was comparable, said David Rubin, MD, a gastroenterologist at the University of Chicago Medicine Inflammatory Bowel Disease Center. Outcomes improved in all patients, with or without a history of inadequate response or intolerance to advanced therapy.
Guselkumab, an interleukin-12 p19 subunit antagonist, is currently being investigated in inflammatory bowel disease.
The QUASAR Induction Study 1 (NCT04033445) is a phase 2b, randomized, double-blind, placebo-controlled study that evaluates guselkumab as induction therapy in patients with moderately to severely active UC. Inclusion criteria specify a demonstrated inadequate response or intolerance to conventional therapy, such as thiopurines or corticosteroids, or to advanced therapy, such as tumor necrosis factor–alpha antagonists, vedolizumab, or tofacitinib. The study didn’t include patients exposed to ustekinumab.
Study participants were age 18 and older with moderately to severely active UC, defined as a modified Mayo score of 5-9 with a Mayo rectal bleeding subscore of 1 or greater and a Mayo endoscopy subscore of 2 or greater at baseline. The groups were randomized 1:1:1 to receive 400 mg of IV guselkumab, 200 mg of guselkumab, or placebo at weeks 0, 4, and 8.
At week 12, the research team looked for several key endpoints. Clinical response was defined as a modified Mayo score decrease of 30% or more and a drop in 2 or more points, with either a 1-point decrease or more in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
Clinical remission was defined as a stool frequency subscore of 0 or 1 that hadn’t increased from baseline, a rectal bleeding subscore of 0, and an endoscopy subscore of 0 or 1 with no friability present on the endoscopy.
In addition, symptomatic remission was defined as a stool subscore of 0 or 1 that hadn’t increased from baseline and rectal bleeding subscore of 0.
Endoscopic improvement was defined as an endoscopy subscore of 0 or 1 with no friability present on the endoscopy. Endoscopic normalization was an endoscopy subscore of 0.
Notably, the research team looked at histoendoscopic mucosal improvement, which includes a combination of endoscopic improvement and histologic improvement (neutrophil infiltration in less than 5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue, according to the Geboes grading system).
Among the 313 total patients, 47% had a history of inadequate response or intolerance to advanced therapy, and about half of these patients had prior inadequate response or intolerance to two or more advanced therapy classes.
At baseline, about 90% of patients had an endoscopic subscore of 3 (severe). More than half had extensive UC, and the average UC duration was 9 years. About 20% overall had extraintestinal manifestations present, which were noted in 33% of the 400 mg guselkumab treatment arm.
At week 12, clinical response was achieved by a higher proportion of patients treated with guselkumab versus placebo, at 50.5% versus 25.5% for patients with prior inadequate response or intolerance to advanced therapy and 70.3% versus 29.6% for those without prior inadequate response or intolerance to advanced therapy, the authors reported in the abstract.
Compared with placebo, higher proportions of patients treated with guselkumab achieved clinical, endoscopic, and histologic outcomes in both groups with or without inadequate response or intolerance to advanced therapy. Generally, those without a history of inadequate response had higher response rates across all endpoints.
Overall, both the 200-mg and 400-mg doses of guselkumab were statistically superior to the placebo across all endpoints for both groups (with or without inadequate response or intolerance). Although the efficacy was comparable for the two doses, the 400-mg dose was associated with greater histoendoscopic mucosal improvement in both groups.
“It’s of interest to think about how we position and sequence our therapies with this additional data,” Dr. Rubin said.
The study was sponsored by Janssen Research & Development. Several authors are employees for and have stock options with Johnson & Johnson and Janssen. The other authors reported consultant roles, advisory roles, and research support from numerous pharmaceutical companies, including Janssen.
according to findings presented at the annual meeting of the American College of Gastroenterology.
The efficacy of the 200-mg dose and the 400-mg dose was comparable, said David Rubin, MD, a gastroenterologist at the University of Chicago Medicine Inflammatory Bowel Disease Center. Outcomes improved in all patients, with or without a history of inadequate response or intolerance to advanced therapy.
Guselkumab, an interleukin-12 p19 subunit antagonist, is currently being investigated in inflammatory bowel disease.
The QUASAR Induction Study 1 (NCT04033445) is a phase 2b, randomized, double-blind, placebo-controlled study that evaluates guselkumab as induction therapy in patients with moderately to severely active UC. Inclusion criteria specify a demonstrated inadequate response or intolerance to conventional therapy, such as thiopurines or corticosteroids, or to advanced therapy, such as tumor necrosis factor–alpha antagonists, vedolizumab, or tofacitinib. The study didn’t include patients exposed to ustekinumab.
Study participants were age 18 and older with moderately to severely active UC, defined as a modified Mayo score of 5-9 with a Mayo rectal bleeding subscore of 1 or greater and a Mayo endoscopy subscore of 2 or greater at baseline. The groups were randomized 1:1:1 to receive 400 mg of IV guselkumab, 200 mg of guselkumab, or placebo at weeks 0, 4, and 8.
At week 12, the research team looked for several key endpoints. Clinical response was defined as a modified Mayo score decrease of 30% or more and a drop in 2 or more points, with either a 1-point decrease or more in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
Clinical remission was defined as a stool frequency subscore of 0 or 1 that hadn’t increased from baseline, a rectal bleeding subscore of 0, and an endoscopy subscore of 0 or 1 with no friability present on the endoscopy.
In addition, symptomatic remission was defined as a stool subscore of 0 or 1 that hadn’t increased from baseline and rectal bleeding subscore of 0.
Endoscopic improvement was defined as an endoscopy subscore of 0 or 1 with no friability present on the endoscopy. Endoscopic normalization was an endoscopy subscore of 0.
Notably, the research team looked at histoendoscopic mucosal improvement, which includes a combination of endoscopic improvement and histologic improvement (neutrophil infiltration in less than 5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue, according to the Geboes grading system).
Among the 313 total patients, 47% had a history of inadequate response or intolerance to advanced therapy, and about half of these patients had prior inadequate response or intolerance to two or more advanced therapy classes.
At baseline, about 90% of patients had an endoscopic subscore of 3 (severe). More than half had extensive UC, and the average UC duration was 9 years. About 20% overall had extraintestinal manifestations present, which were noted in 33% of the 400 mg guselkumab treatment arm.
At week 12, clinical response was achieved by a higher proportion of patients treated with guselkumab versus placebo, at 50.5% versus 25.5% for patients with prior inadequate response or intolerance to advanced therapy and 70.3% versus 29.6% for those without prior inadequate response or intolerance to advanced therapy, the authors reported in the abstract.
Compared with placebo, higher proportions of patients treated with guselkumab achieved clinical, endoscopic, and histologic outcomes in both groups with or without inadequate response or intolerance to advanced therapy. Generally, those without a history of inadequate response had higher response rates across all endpoints.
Overall, both the 200-mg and 400-mg doses of guselkumab were statistically superior to the placebo across all endpoints for both groups (with or without inadequate response or intolerance). Although the efficacy was comparable for the two doses, the 400-mg dose was associated with greater histoendoscopic mucosal improvement in both groups.
“It’s of interest to think about how we position and sequence our therapies with this additional data,” Dr. Rubin said.
The study was sponsored by Janssen Research & Development. Several authors are employees for and have stock options with Johnson & Johnson and Janssen. The other authors reported consultant roles, advisory roles, and research support from numerous pharmaceutical companies, including Janssen.
FROM ACG 2022
Latiglutenase reduces symptoms in celiac patients exposed to gluten
according to a new study published in Gastroenterology.
Latiglutenase led to 95% gluten degradation in the stomach, as indicated by measurements of gluten-immunogenic peptides in urine, wrote Joseph A. Murray, MD, a gastroenterologist at the Mayo Clinic, Rochester, Minn., and Jack A. Syage, PhD, CEO and cofounder of ImmunogenX Inc., Newport Beach, Calif., and colleagues on behalf of the CeliacShield Study Group.
For patients with celiac disease, the only available treatment is a life-long gluten-free diet (GFD). Low levels of gluten exposure can lead to ongoing inflammation and the risk of complications, and about half of patients continue to experience moderate to severe symptoms.
“Although a GFD can reduce symptoms and intestinal damage, the diet is neither easy nor readily achievable by many patients and, furthermore, can be lacking in essential nutrients,” the authors wrote.
In a randomized, double-blind, placebo-controlled gluten challenge study, the research team assessed the efficacy and safety of a 1,200-mg dose of IMGX003, formerly known as ALV003. The dual-enzyme supplementation therapy was “designed to mitigate the impact of gluten exposure in patients who are attempting to adhere to a GFD.”
The phase 2 trial was conducted at the Mayo Clinic with adult patients (aged 18-80 years) who had physician-diagnosed and biopsy-confirmed celiac disease, followed a GFD for more than 1 year, and had histologically well-controlled disease. During the study, they were exposed to 2 g of gluten per day for 6 weeks.
The primary endpoint focused on the change in the ratio of villus height to crypt depth. The “secondary endpoints included density of intraepithelial lymphocytes and symptom severity. Additional endpoints included serology and gluten-immunogenic peptides in urine.”
Among the 50 patients randomized, 43 completed the study, with 21 assigned to the IMGX003 group. About 74% of the participants were women; the mean age of all participants was 43.8 years.
Overall, the mean change in the ratio of villus height to crypt depth was –0.04 for IMGX003, compared with –0.35 for placebo. In addition, the mean change in the density of intraepithelial lymphocytes for IMGX003 was 9.8, compared with 24.8 for placebo. Based on the ratio of the means for both groups, the researchers estimated an 88% reduction of change in villus height to crypt depth and a 60% reduction of change in intraepithelial lymphocytes.
The mean changes, or worsening from baseline, in symptom severity for IMGX003 vs. placebo were 0.22 vs. 1.63 for abdominal pain, 0.96 vs. 3.29 for bloating, 0.02 vs. 3.2 for tiredness, and 0.64 vs. 2.27 for nonstool composite. The calculated symptom reduction values were 93% for abdominal pain, 53% for bloating, 99% for tiredness, and 70% for nonstool composite.
The mean change from baseline for symptom severity was evaluated over three 2-week periods, and the percent changes showed consistent reduction of symptom worsening during that time. Based on the effect size and trend significance, the P values were .014 for abdominal pain, .030 for bloating, .002 for tiredness, and < . 001 for nonstool composite.
The mean change in gluten-immunogenic peptides in urine (GIP) relative to baseline was 0.59 for IMGX003, compared with 11.53 for placebo. The researchers estimated an efficacy of gluten degradation in vivo of 95%.
“Measurement of GIP in urine demonstrated the purported mechanism of action of IMGX003, namely, degradation of gluten in the stomach, thereby preventing the triggering of the immunogenic autoimmune response,” the authors wrote. “Targeting gluten by degrading the immunogenic peptides before absorption minimizes or abrogates the cascading innate and adaptive immune responses that characterize the inflammatory response to gluten in CeD [celiac disease].”
The study was sponsored by ImmunogenX Inc., and partially funded by a grant from the National Center for Complementary and Integrative Health. The project was further supported by grants from the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors reported receiving grants from numerous funders, including ImmunogenX, and some reported being a cofounder, stockholder, or board director of the company.
From the perspective of patients affected by other chronic GI diseases requiring constant treatment with drugs, the life of celiac patients must appear “a piece of cake” (pun intended). But this is not the case. In fact, the burden of following a gluten-free diet (GFD) can profoundly impact their quality of life. Furthermore, a substantial portion of patients trying their best on a GFD do not experience full clinical and histologic remission, mainly because of ongoing involuntary gluten ingestion. Therefore, it’s not surprising that several lines of research have been actively trying to address this unmet need.
In this phase 2 trial, the proprietary enzyme combination called IMGX003 (latiglutenase) was investigated for safety and efficacy. IMGX003 can digest gluten in the stomach, thus preventing its intact entry into the small intestine where it would trigger the immune reaction leading to the villi destruction. The study did indeed demonstrate that administering it to patients on GFD exposed to 2 grams of gluten daily (roughly the equivalent of half a slice of wheat bread) effectively reduced both the mucosal damage and symptom severity. Is this good news for patients with celiac? You bet it is! While not replacing the need for a GFD, having a safe drug that adds a substantial layer of protection to inadvertent gluten exposure or the so-called “cross-contamination” is surely to be welcome by them.
If and once approved for use, IMGX003 could be taken sporadically by patients on GFD: For instance, while eating out in “new” places, traveling, going to parties, or for younger patients, when having sleepovers, birthday celebrations, and so on. With the caveat, not to be forgotten, that this is not meant to be a wonder drug eliminating the need for vigilance; we still need to wait patiently for science to advance further for that.
Stefano Guandalini, MD, AGAF, is professor emeritus of pediatrics at the University of Chicago and director emeritus of the University of Chicago Celiac Disease Center. He declares no relevant conflicts of interest.
From the perspective of patients affected by other chronic GI diseases requiring constant treatment with drugs, the life of celiac patients must appear “a piece of cake” (pun intended). But this is not the case. In fact, the burden of following a gluten-free diet (GFD) can profoundly impact their quality of life. Furthermore, a substantial portion of patients trying their best on a GFD do not experience full clinical and histologic remission, mainly because of ongoing involuntary gluten ingestion. Therefore, it’s not surprising that several lines of research have been actively trying to address this unmet need.
In this phase 2 trial, the proprietary enzyme combination called IMGX003 (latiglutenase) was investigated for safety and efficacy. IMGX003 can digest gluten in the stomach, thus preventing its intact entry into the small intestine where it would trigger the immune reaction leading to the villi destruction. The study did indeed demonstrate that administering it to patients on GFD exposed to 2 grams of gluten daily (roughly the equivalent of half a slice of wheat bread) effectively reduced both the mucosal damage and symptom severity. Is this good news for patients with celiac? You bet it is! While not replacing the need for a GFD, having a safe drug that adds a substantial layer of protection to inadvertent gluten exposure or the so-called “cross-contamination” is surely to be welcome by them.
If and once approved for use, IMGX003 could be taken sporadically by patients on GFD: For instance, while eating out in “new” places, traveling, going to parties, or for younger patients, when having sleepovers, birthday celebrations, and so on. With the caveat, not to be forgotten, that this is not meant to be a wonder drug eliminating the need for vigilance; we still need to wait patiently for science to advance further for that.
Stefano Guandalini, MD, AGAF, is professor emeritus of pediatrics at the University of Chicago and director emeritus of the University of Chicago Celiac Disease Center. He declares no relevant conflicts of interest.
From the perspective of patients affected by other chronic GI diseases requiring constant treatment with drugs, the life of celiac patients must appear “a piece of cake” (pun intended). But this is not the case. In fact, the burden of following a gluten-free diet (GFD) can profoundly impact their quality of life. Furthermore, a substantial portion of patients trying their best on a GFD do not experience full clinical and histologic remission, mainly because of ongoing involuntary gluten ingestion. Therefore, it’s not surprising that several lines of research have been actively trying to address this unmet need.
In this phase 2 trial, the proprietary enzyme combination called IMGX003 (latiglutenase) was investigated for safety and efficacy. IMGX003 can digest gluten in the stomach, thus preventing its intact entry into the small intestine where it would trigger the immune reaction leading to the villi destruction. The study did indeed demonstrate that administering it to patients on GFD exposed to 2 grams of gluten daily (roughly the equivalent of half a slice of wheat bread) effectively reduced both the mucosal damage and symptom severity. Is this good news for patients with celiac? You bet it is! While not replacing the need for a GFD, having a safe drug that adds a substantial layer of protection to inadvertent gluten exposure or the so-called “cross-contamination” is surely to be welcome by them.
If and once approved for use, IMGX003 could be taken sporadically by patients on GFD: For instance, while eating out in “new” places, traveling, going to parties, or for younger patients, when having sleepovers, birthday celebrations, and so on. With the caveat, not to be forgotten, that this is not meant to be a wonder drug eliminating the need for vigilance; we still need to wait patiently for science to advance further for that.
Stefano Guandalini, MD, AGAF, is professor emeritus of pediatrics at the University of Chicago and director emeritus of the University of Chicago Celiac Disease Center. He declares no relevant conflicts of interest.
according to a new study published in Gastroenterology.
Latiglutenase led to 95% gluten degradation in the stomach, as indicated by measurements of gluten-immunogenic peptides in urine, wrote Joseph A. Murray, MD, a gastroenterologist at the Mayo Clinic, Rochester, Minn., and Jack A. Syage, PhD, CEO and cofounder of ImmunogenX Inc., Newport Beach, Calif., and colleagues on behalf of the CeliacShield Study Group.
For patients with celiac disease, the only available treatment is a life-long gluten-free diet (GFD). Low levels of gluten exposure can lead to ongoing inflammation and the risk of complications, and about half of patients continue to experience moderate to severe symptoms.
“Although a GFD can reduce symptoms and intestinal damage, the diet is neither easy nor readily achievable by many patients and, furthermore, can be lacking in essential nutrients,” the authors wrote.
In a randomized, double-blind, placebo-controlled gluten challenge study, the research team assessed the efficacy and safety of a 1,200-mg dose of IMGX003, formerly known as ALV003. The dual-enzyme supplementation therapy was “designed to mitigate the impact of gluten exposure in patients who are attempting to adhere to a GFD.”
The phase 2 trial was conducted at the Mayo Clinic with adult patients (aged 18-80 years) who had physician-diagnosed and biopsy-confirmed celiac disease, followed a GFD for more than 1 year, and had histologically well-controlled disease. During the study, they were exposed to 2 g of gluten per day for 6 weeks.
The primary endpoint focused on the change in the ratio of villus height to crypt depth. The “secondary endpoints included density of intraepithelial lymphocytes and symptom severity. Additional endpoints included serology and gluten-immunogenic peptides in urine.”
Among the 50 patients randomized, 43 completed the study, with 21 assigned to the IMGX003 group. About 74% of the participants were women; the mean age of all participants was 43.8 years.
Overall, the mean change in the ratio of villus height to crypt depth was –0.04 for IMGX003, compared with –0.35 for placebo. In addition, the mean change in the density of intraepithelial lymphocytes for IMGX003 was 9.8, compared with 24.8 for placebo. Based on the ratio of the means for both groups, the researchers estimated an 88% reduction of change in villus height to crypt depth and a 60% reduction of change in intraepithelial lymphocytes.
The mean changes, or worsening from baseline, in symptom severity for IMGX003 vs. placebo were 0.22 vs. 1.63 for abdominal pain, 0.96 vs. 3.29 for bloating, 0.02 vs. 3.2 for tiredness, and 0.64 vs. 2.27 for nonstool composite. The calculated symptom reduction values were 93% for abdominal pain, 53% for bloating, 99% for tiredness, and 70% for nonstool composite.
The mean change from baseline for symptom severity was evaluated over three 2-week periods, and the percent changes showed consistent reduction of symptom worsening during that time. Based on the effect size and trend significance, the P values were .014 for abdominal pain, .030 for bloating, .002 for tiredness, and < . 001 for nonstool composite.
The mean change in gluten-immunogenic peptides in urine (GIP) relative to baseline was 0.59 for IMGX003, compared with 11.53 for placebo. The researchers estimated an efficacy of gluten degradation in vivo of 95%.
“Measurement of GIP in urine demonstrated the purported mechanism of action of IMGX003, namely, degradation of gluten in the stomach, thereby preventing the triggering of the immunogenic autoimmune response,” the authors wrote. “Targeting gluten by degrading the immunogenic peptides before absorption minimizes or abrogates the cascading innate and adaptive immune responses that characterize the inflammatory response to gluten in CeD [celiac disease].”
The study was sponsored by ImmunogenX Inc., and partially funded by a grant from the National Center for Complementary and Integrative Health. The project was further supported by grants from the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors reported receiving grants from numerous funders, including ImmunogenX, and some reported being a cofounder, stockholder, or board director of the company.
according to a new study published in Gastroenterology.
Latiglutenase led to 95% gluten degradation in the stomach, as indicated by measurements of gluten-immunogenic peptides in urine, wrote Joseph A. Murray, MD, a gastroenterologist at the Mayo Clinic, Rochester, Minn., and Jack A. Syage, PhD, CEO and cofounder of ImmunogenX Inc., Newport Beach, Calif., and colleagues on behalf of the CeliacShield Study Group.
For patients with celiac disease, the only available treatment is a life-long gluten-free diet (GFD). Low levels of gluten exposure can lead to ongoing inflammation and the risk of complications, and about half of patients continue to experience moderate to severe symptoms.
“Although a GFD can reduce symptoms and intestinal damage, the diet is neither easy nor readily achievable by many patients and, furthermore, can be lacking in essential nutrients,” the authors wrote.
In a randomized, double-blind, placebo-controlled gluten challenge study, the research team assessed the efficacy and safety of a 1,200-mg dose of IMGX003, formerly known as ALV003. The dual-enzyme supplementation therapy was “designed to mitigate the impact of gluten exposure in patients who are attempting to adhere to a GFD.”
The phase 2 trial was conducted at the Mayo Clinic with adult patients (aged 18-80 years) who had physician-diagnosed and biopsy-confirmed celiac disease, followed a GFD for more than 1 year, and had histologically well-controlled disease. During the study, they were exposed to 2 g of gluten per day for 6 weeks.
The primary endpoint focused on the change in the ratio of villus height to crypt depth. The “secondary endpoints included density of intraepithelial lymphocytes and symptom severity. Additional endpoints included serology and gluten-immunogenic peptides in urine.”
Among the 50 patients randomized, 43 completed the study, with 21 assigned to the IMGX003 group. About 74% of the participants were women; the mean age of all participants was 43.8 years.
Overall, the mean change in the ratio of villus height to crypt depth was –0.04 for IMGX003, compared with –0.35 for placebo. In addition, the mean change in the density of intraepithelial lymphocytes for IMGX003 was 9.8, compared with 24.8 for placebo. Based on the ratio of the means for both groups, the researchers estimated an 88% reduction of change in villus height to crypt depth and a 60% reduction of change in intraepithelial lymphocytes.
The mean changes, or worsening from baseline, in symptom severity for IMGX003 vs. placebo were 0.22 vs. 1.63 for abdominal pain, 0.96 vs. 3.29 for bloating, 0.02 vs. 3.2 for tiredness, and 0.64 vs. 2.27 for nonstool composite. The calculated symptom reduction values were 93% for abdominal pain, 53% for bloating, 99% for tiredness, and 70% for nonstool composite.
The mean change from baseline for symptom severity was evaluated over three 2-week periods, and the percent changes showed consistent reduction of symptom worsening during that time. Based on the effect size and trend significance, the P values were .014 for abdominal pain, .030 for bloating, .002 for tiredness, and < . 001 for nonstool composite.
The mean change in gluten-immunogenic peptides in urine (GIP) relative to baseline was 0.59 for IMGX003, compared with 11.53 for placebo. The researchers estimated an efficacy of gluten degradation in vivo of 95%.
“Measurement of GIP in urine demonstrated the purported mechanism of action of IMGX003, namely, degradation of gluten in the stomach, thereby preventing the triggering of the immunogenic autoimmune response,” the authors wrote. “Targeting gluten by degrading the immunogenic peptides before absorption minimizes or abrogates the cascading innate and adaptive immune responses that characterize the inflammatory response to gluten in CeD [celiac disease].”
The study was sponsored by ImmunogenX Inc., and partially funded by a grant from the National Center for Complementary and Integrative Health. The project was further supported by grants from the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors reported receiving grants from numerous funders, including ImmunogenX, and some reported being a cofounder, stockholder, or board director of the company.
FROM GASTROENTEROLOGY
RBX2660 shows promise in breaking the cycle of recurrent C. difficile
CHARLOTTE, N.C. –
Following a standard course of antibiotics, a one-time treatment with RBX2660 was successful for three quarters of participants at 8 weeks, according to a new study. It also prevented additional bouts, with 84% of these initial responders remaining free of C. difficile infection at 6 months.
The ongoing phase 3, open-label PUNCH CD3-OLS study expands on clinical trial experience by treating more “real-world” patients. People who might have been excluded from previous research because of comorbidities, such as irritable bowel syndrome, inflammatory bowel disease, and immunosuppression, were included.
The study also placed no limit on the number of previous rounds of C. difficile infections.
“Even when you expand the patient population to make it more generalizable, we’re still seeing both a high cure rate and a high success rate,” Sahil Khanna, MBBS, a gastroenterologist and hepatologist at the Mayo Clinic in Rochester, Minn., said in an interview.
“We also are not seeing any kind of safety signals that can be attributed to this particular product,” he said.
Dr. Khanna presented the findings during the annual meeting of the American College of Gastroenterology, which were also published simultaneously in the journal Drugs. The research by Dr. Khanna and associates received an ACG Outstanding Research Award in the colon category.
Study design and results
RBX2660 (Rebyota) is a microbiota-based live biotherapeutic in development from Ferring Pharmaceuticals. The treatment contains human stool collected from prescreened, qualified donors and is prepared according to good manufacturing standards.
After standard-of-care antibiotics and a 72-hour washout period, participants received a single 150-mL dose rectally by enema. RBX2660 is administered by a health care professional.
The median age of study participants was 63 years, with 45% aged 65 years or older, and 70% were women. Overall, 37% of participants had Crohn’s disease and 4% had ulcerative colitis.
At the time of screening, about half of participants had a history of one or two infections with C. difficile, and the remaining half reported three or more episodes.
Of the 402 participants whose outcomes could be analyzed, 75% reported treatment success, meaning no further C. difficile infections at 8 weeks. This was consistent with the 75% of 60 participants free of C. difficile in the interim analysis reported in 2021. Efficacy results were based on a modified intent-to-treat analysis.
Of the 300 participants who responded to RBX2660 at 8 weeks, 262 were followed up to 6 months, with 84% of these reporting no C. difficile recurrence.
“If you succeeded to 8 weeks, there was a high likelihood that you would succeed up to 6 months,” Dr. Khanna said.
For the subset of participants with inflammatory bowel disease, Dr. Khanna noted that the success rates were in the 80% range, which is higher than what is seen in clinic fecal microbiota transplantation programs.
Adverse events
Of the participants, 63% reported treatment-emergent adverse events. Most events were mild to moderate in severity, the researchers reported, with diarrhea and abdominal pain being the most common.
“When you look at the treatment-emergent adverse events, it’s important to put them into context in terms of this patient population,” Dr. Khanna said. “This recurrent population has developed underlying gastrointestinal symptoms like abdominal pain, diarrhea, nausea, vomiting, and weight loss.”
Some of these adverse events persist beyond resolution of the C. difficile infection, and the adverse-event profile with RBX2660 is consistent with what is seen following fecal microbiota transplantation, he added.
The serious adverse events “were very, very few,” Dr. Khanna said.
Overall, 11% of participants reported a serious adverse event. The majority were related to the C. difficile infection or an underlying comorbidity, he noted.
“Excruciating for patients to deal with”
Traditionally, there could be “some hesitation on the patient’s part [to undergo therapy] just because it’s delivered rectally,” session comoderator Lisa Malter, MD, said in an interview.
However, C. difficile can be “excruciating for patients to deal with,” said Dr. Malter, a gastroenterologist and professor of medicine at New York University Langone Health. They “may be more than willing to take [this agent] because it gets them feeling better.”
“This is a positive adjunct to our current therapies for C. diff in terms of trying to knock it out once a standard course of antibiotics has been administered,” she added.
Currently, people with recurrent C. difficile seek fecal microbiota material from a biobank or from a close friend or loved one.
But Dr. Malter noted that asking someone you know to donate fecal matter for transplantation requires several steps. Donors are screened to make sure they are free of gastrointestinal illness, are not taking any contraindicated medications, and do not have active infection.
Fecal microbiota samples from a biobank are more standardized, but there have been intermittent shutdowns and availability has been limited during the pandemic, she said.
Dr. Malter added that one unanswered question is how much of the colon is covered by therapy delivery via enema compared with colonoscope delivery during fecal microbiota transplantation.
“If it’s delivered colonoscopically, you get the entire colon. In contrast with an enema, you really only hit the left side of the colon,” she said.
FDA advisory committee nod
On Sept. 26, the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee reviewed evidence for RBX2660. The committee voted 13 to 4 that data were adequate to support the effectiveness of RBX2660 to reduce the recurrence of C. difficile infection in adults following antibiotic treatment for recurrent infections.
Members also voted 12 to 4, with one abstention, that the data were adequate to support the product’s safety.
The FDA often follows its advisory committee recommendations but is not required to do so.
“The hope would be that this would get through the usual FDA pipeline of an approval in the near future,” Dr. Khanna said.
The study was funded by Ferring Pharmaceuticals. Dr. Khanna reported receiving grant and research funding from Ferring. Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. –
Following a standard course of antibiotics, a one-time treatment with RBX2660 was successful for three quarters of participants at 8 weeks, according to a new study. It also prevented additional bouts, with 84% of these initial responders remaining free of C. difficile infection at 6 months.
The ongoing phase 3, open-label PUNCH CD3-OLS study expands on clinical trial experience by treating more “real-world” patients. People who might have been excluded from previous research because of comorbidities, such as irritable bowel syndrome, inflammatory bowel disease, and immunosuppression, were included.
The study also placed no limit on the number of previous rounds of C. difficile infections.
“Even when you expand the patient population to make it more generalizable, we’re still seeing both a high cure rate and a high success rate,” Sahil Khanna, MBBS, a gastroenterologist and hepatologist at the Mayo Clinic in Rochester, Minn., said in an interview.
“We also are not seeing any kind of safety signals that can be attributed to this particular product,” he said.
Dr. Khanna presented the findings during the annual meeting of the American College of Gastroenterology, which were also published simultaneously in the journal Drugs. The research by Dr. Khanna and associates received an ACG Outstanding Research Award in the colon category.
Study design and results
RBX2660 (Rebyota) is a microbiota-based live biotherapeutic in development from Ferring Pharmaceuticals. The treatment contains human stool collected from prescreened, qualified donors and is prepared according to good manufacturing standards.
After standard-of-care antibiotics and a 72-hour washout period, participants received a single 150-mL dose rectally by enema. RBX2660 is administered by a health care professional.
The median age of study participants was 63 years, with 45% aged 65 years or older, and 70% were women. Overall, 37% of participants had Crohn’s disease and 4% had ulcerative colitis.
At the time of screening, about half of participants had a history of one or two infections with C. difficile, and the remaining half reported three or more episodes.
Of the 402 participants whose outcomes could be analyzed, 75% reported treatment success, meaning no further C. difficile infections at 8 weeks. This was consistent with the 75% of 60 participants free of C. difficile in the interim analysis reported in 2021. Efficacy results were based on a modified intent-to-treat analysis.
Of the 300 participants who responded to RBX2660 at 8 weeks, 262 were followed up to 6 months, with 84% of these reporting no C. difficile recurrence.
“If you succeeded to 8 weeks, there was a high likelihood that you would succeed up to 6 months,” Dr. Khanna said.
For the subset of participants with inflammatory bowel disease, Dr. Khanna noted that the success rates were in the 80% range, which is higher than what is seen in clinic fecal microbiota transplantation programs.
Adverse events
Of the participants, 63% reported treatment-emergent adverse events. Most events were mild to moderate in severity, the researchers reported, with diarrhea and abdominal pain being the most common.
“When you look at the treatment-emergent adverse events, it’s important to put them into context in terms of this patient population,” Dr. Khanna said. “This recurrent population has developed underlying gastrointestinal symptoms like abdominal pain, diarrhea, nausea, vomiting, and weight loss.”
Some of these adverse events persist beyond resolution of the C. difficile infection, and the adverse-event profile with RBX2660 is consistent with what is seen following fecal microbiota transplantation, he added.
The serious adverse events “were very, very few,” Dr. Khanna said.
Overall, 11% of participants reported a serious adverse event. The majority were related to the C. difficile infection or an underlying comorbidity, he noted.
“Excruciating for patients to deal with”
Traditionally, there could be “some hesitation on the patient’s part [to undergo therapy] just because it’s delivered rectally,” session comoderator Lisa Malter, MD, said in an interview.
However, C. difficile can be “excruciating for patients to deal with,” said Dr. Malter, a gastroenterologist and professor of medicine at New York University Langone Health. They “may be more than willing to take [this agent] because it gets them feeling better.”
“This is a positive adjunct to our current therapies for C. diff in terms of trying to knock it out once a standard course of antibiotics has been administered,” she added.
Currently, people with recurrent C. difficile seek fecal microbiota material from a biobank or from a close friend or loved one.
But Dr. Malter noted that asking someone you know to donate fecal matter for transplantation requires several steps. Donors are screened to make sure they are free of gastrointestinal illness, are not taking any contraindicated medications, and do not have active infection.
Fecal microbiota samples from a biobank are more standardized, but there have been intermittent shutdowns and availability has been limited during the pandemic, she said.
Dr. Malter added that one unanswered question is how much of the colon is covered by therapy delivery via enema compared with colonoscope delivery during fecal microbiota transplantation.
“If it’s delivered colonoscopically, you get the entire colon. In contrast with an enema, you really only hit the left side of the colon,” she said.
FDA advisory committee nod
On Sept. 26, the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee reviewed evidence for RBX2660. The committee voted 13 to 4 that data were adequate to support the effectiveness of RBX2660 to reduce the recurrence of C. difficile infection in adults following antibiotic treatment for recurrent infections.
Members also voted 12 to 4, with one abstention, that the data were adequate to support the product’s safety.
The FDA often follows its advisory committee recommendations but is not required to do so.
“The hope would be that this would get through the usual FDA pipeline of an approval in the near future,” Dr. Khanna said.
The study was funded by Ferring Pharmaceuticals. Dr. Khanna reported receiving grant and research funding from Ferring. Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. –
Following a standard course of antibiotics, a one-time treatment with RBX2660 was successful for three quarters of participants at 8 weeks, according to a new study. It also prevented additional bouts, with 84% of these initial responders remaining free of C. difficile infection at 6 months.
The ongoing phase 3, open-label PUNCH CD3-OLS study expands on clinical trial experience by treating more “real-world” patients. People who might have been excluded from previous research because of comorbidities, such as irritable bowel syndrome, inflammatory bowel disease, and immunosuppression, were included.
The study also placed no limit on the number of previous rounds of C. difficile infections.
“Even when you expand the patient population to make it more generalizable, we’re still seeing both a high cure rate and a high success rate,” Sahil Khanna, MBBS, a gastroenterologist and hepatologist at the Mayo Clinic in Rochester, Minn., said in an interview.
“We also are not seeing any kind of safety signals that can be attributed to this particular product,” he said.
Dr. Khanna presented the findings during the annual meeting of the American College of Gastroenterology, which were also published simultaneously in the journal Drugs. The research by Dr. Khanna and associates received an ACG Outstanding Research Award in the colon category.
Study design and results
RBX2660 (Rebyota) is a microbiota-based live biotherapeutic in development from Ferring Pharmaceuticals. The treatment contains human stool collected from prescreened, qualified donors and is prepared according to good manufacturing standards.
After standard-of-care antibiotics and a 72-hour washout period, participants received a single 150-mL dose rectally by enema. RBX2660 is administered by a health care professional.
The median age of study participants was 63 years, with 45% aged 65 years or older, and 70% were women. Overall, 37% of participants had Crohn’s disease and 4% had ulcerative colitis.
At the time of screening, about half of participants had a history of one or two infections with C. difficile, and the remaining half reported three or more episodes.
Of the 402 participants whose outcomes could be analyzed, 75% reported treatment success, meaning no further C. difficile infections at 8 weeks. This was consistent with the 75% of 60 participants free of C. difficile in the interim analysis reported in 2021. Efficacy results were based on a modified intent-to-treat analysis.
Of the 300 participants who responded to RBX2660 at 8 weeks, 262 were followed up to 6 months, with 84% of these reporting no C. difficile recurrence.
“If you succeeded to 8 weeks, there was a high likelihood that you would succeed up to 6 months,” Dr. Khanna said.
For the subset of participants with inflammatory bowel disease, Dr. Khanna noted that the success rates were in the 80% range, which is higher than what is seen in clinic fecal microbiota transplantation programs.
Adverse events
Of the participants, 63% reported treatment-emergent adverse events. Most events were mild to moderate in severity, the researchers reported, with diarrhea and abdominal pain being the most common.
“When you look at the treatment-emergent adverse events, it’s important to put them into context in terms of this patient population,” Dr. Khanna said. “This recurrent population has developed underlying gastrointestinal symptoms like abdominal pain, diarrhea, nausea, vomiting, and weight loss.”
Some of these adverse events persist beyond resolution of the C. difficile infection, and the adverse-event profile with RBX2660 is consistent with what is seen following fecal microbiota transplantation, he added.
The serious adverse events “were very, very few,” Dr. Khanna said.
Overall, 11% of participants reported a serious adverse event. The majority were related to the C. difficile infection or an underlying comorbidity, he noted.
“Excruciating for patients to deal with”
Traditionally, there could be “some hesitation on the patient’s part [to undergo therapy] just because it’s delivered rectally,” session comoderator Lisa Malter, MD, said in an interview.
However, C. difficile can be “excruciating for patients to deal with,” said Dr. Malter, a gastroenterologist and professor of medicine at New York University Langone Health. They “may be more than willing to take [this agent] because it gets them feeling better.”
“This is a positive adjunct to our current therapies for C. diff in terms of trying to knock it out once a standard course of antibiotics has been administered,” she added.
Currently, people with recurrent C. difficile seek fecal microbiota material from a biobank or from a close friend or loved one.
But Dr. Malter noted that asking someone you know to donate fecal matter for transplantation requires several steps. Donors are screened to make sure they are free of gastrointestinal illness, are not taking any contraindicated medications, and do not have active infection.
Fecal microbiota samples from a biobank are more standardized, but there have been intermittent shutdowns and availability has been limited during the pandemic, she said.
Dr. Malter added that one unanswered question is how much of the colon is covered by therapy delivery via enema compared with colonoscope delivery during fecal microbiota transplantation.
“If it’s delivered colonoscopically, you get the entire colon. In contrast with an enema, you really only hit the left side of the colon,” she said.
FDA advisory committee nod
On Sept. 26, the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee reviewed evidence for RBX2660. The committee voted 13 to 4 that data were adequate to support the effectiveness of RBX2660 to reduce the recurrence of C. difficile infection in adults following antibiotic treatment for recurrent infections.
Members also voted 12 to 4, with one abstention, that the data were adequate to support the product’s safety.
The FDA often follows its advisory committee recommendations but is not required to do so.
“The hope would be that this would get through the usual FDA pipeline of an approval in the near future,” Dr. Khanna said.
The study was funded by Ferring Pharmaceuticals. Dr. Khanna reported receiving grant and research funding from Ferring. Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2022
Guselkumab and golimumab: Better together for ulcerative colitis
CHARLOTTE, N.C. – , a phase 2a study demonstrates.
Researchers compared the combination therapy of guselkumab and golimumab (both from Janssen) for 12 weeks, followed by guselkumab monotherapy up to week 38, versus either agent as monotherapy for the full 38 weeks.
Guselkumab is an interleukin-23p19 subunit antagonist being studied to treat inflammatory bowel disease (IBD). Golimumab is a TNF-alpha antagonist being evaluated for ulcerative colitis.
The combination induction strategy “achieved higher rates of clinical remission, endoscopic improvement, composite endpoint of histologic remission, and endoscopic improvement,” said Brian G. Feagan, MD, senior scientific director at the contract research organization Alimentiv and a gastroenterologist at Western University in London, Ont.
The findings were presented at the annual meeting of the American College of Gastroenterology.
Study design
The current research builds on previous week 12 VEGA study results. The earlier findings indicated that blocking interleukin-23p19 by guselkumab and TNF-alpha with golimumab was superior on multiple measures, compared with monotherapy.
The new findings are from a randomized, double-blind, proof-of-concept study that included 214 adults with moderately to severely active ulcerative colitis. Participants were naive to TNF-alpha antagonists and refractory or intolerant to conventional therapy.
Of the participants, 71 were randomly assigned to receive guselkumab, 200 mg intravenous (IV) at baseline and at weeks 4 and 8, plus 100 mg subcutaneous (SC) every 8 weeks.
Another 72 participants received golimumab, 200 mg SC at baseline, and 100 mg SC at weeks 2, 6, and 10, and every 4 weeks thereafter.
The combination group of 71 participants received guselkumab 200 mg IV and golimumab 200 mg SC at baseline, followed by golimumab 100 mg SC at weeks 2, 6, and 10, and guselkumab 200 mg IV at weeks 4 and 8. At week 12, this group switched to monotherapy with guselkumab, 100 mg SC every 8 weeks.
Overall, 13% of patients discontinued treatment prior to week 34, the time of final dose of study intervention.
Dr. Feagan noted that they did not see differences between any adverse event, serious adverse event, or adverse event leading to discontinuation among the treatment groups.
Key findings through week 38
The rate of clinical remission in the combination group was 44%. The rate was lower with guselkumab monotherapy at 31% and golimumab monotherapy at 22% at week 38. These percentages were based on a full Mayo Score of 2 or less and no individual subscore greater than 1.
At the same time, the rates of clinical remission by modified Mayo score also favored the combination group at 48%, followed by 31% in the guselkumab group and 21% in the golimumab cohort.
Endoscopic improvement, endoscopic normalization, histologic remission, and composite histologic-endoscopic endpoints were also greater in the combination group than in the monotherapy groups.
“Quite striking differences were maintained up to week 38,” Dr. Feagan said. “This combination treatment warrants further investigation, and phase 3 trials are underway.”
He added that, while they were concerned about serious infection, they did not see any differences, with only two serious infections in each of the three groups.
Opportunistic infections were reported for two patients in the combination group: extrapulmonary tuberculosis and cytomegalovirus colitis. No opportunistic infections occurred in the monotherapy groups.
Valuable data
“The early study results, such as the VEGA study, appear promising for combination biologics with a good safety profile,” Jean-Paul Achkar, MD, staff physician in the Center for Inflammatory Bowel Disease at Cleveland Clinic and the Kenneth Rainin Endowed Chair for IBD Research, said when asked to comment.
“These data are particularly valuable as we have seemingly reached a therapeutic response ceiling for single-biologic therapy, and we need to determine the added benefit and safety profile of a combination of two biologics or the combination of a biologic and a small molecule,” added Dr. Achkar, who served as the session comoderator.
A meeting attendee asked about the likelihood of regulatory approval for this combination based on evidence like this study.
“I think they have to,” Dr. Feagan said. “We’ve probably seen our best results yet in Crohn’s disease, and we’re still at 50% [response rate for monotherapy]. If we’re ever going to come to terms with IBD, I don’t think it’s monotherapy.”
Dr. Feagan added that with combination therapy, “physicians will often worry about economics, but I think that’s a surrogate for their concerns about infection.”
However, he noted that “the better the agents we have, the better the incremental cost effectiveness. So, I don’t think economics is the issue; the issue is safety.”
Another meeting attendee asked if the results might apply to other biologic combinations.
“This model was picked to show the additive effect of the anti-p19 and the TNF antagonist,” Dr. Feagan said.
Similar results could be expected with a combination of treatments from the same classes, he said, but the treatment potential of other drug-class combination is unclear.
The study was funded by Janssen Research and Development. Dr. Feagan reports being a consultant for Janssen. Dr. Achkar reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. – , a phase 2a study demonstrates.
Researchers compared the combination therapy of guselkumab and golimumab (both from Janssen) for 12 weeks, followed by guselkumab monotherapy up to week 38, versus either agent as monotherapy for the full 38 weeks.
Guselkumab is an interleukin-23p19 subunit antagonist being studied to treat inflammatory bowel disease (IBD). Golimumab is a TNF-alpha antagonist being evaluated for ulcerative colitis.
The combination induction strategy “achieved higher rates of clinical remission, endoscopic improvement, composite endpoint of histologic remission, and endoscopic improvement,” said Brian G. Feagan, MD, senior scientific director at the contract research organization Alimentiv and a gastroenterologist at Western University in London, Ont.
The findings were presented at the annual meeting of the American College of Gastroenterology.
Study design
The current research builds on previous week 12 VEGA study results. The earlier findings indicated that blocking interleukin-23p19 by guselkumab and TNF-alpha with golimumab was superior on multiple measures, compared with monotherapy.
The new findings are from a randomized, double-blind, proof-of-concept study that included 214 adults with moderately to severely active ulcerative colitis. Participants were naive to TNF-alpha antagonists and refractory or intolerant to conventional therapy.
Of the participants, 71 were randomly assigned to receive guselkumab, 200 mg intravenous (IV) at baseline and at weeks 4 and 8, plus 100 mg subcutaneous (SC) every 8 weeks.
Another 72 participants received golimumab, 200 mg SC at baseline, and 100 mg SC at weeks 2, 6, and 10, and every 4 weeks thereafter.
The combination group of 71 participants received guselkumab 200 mg IV and golimumab 200 mg SC at baseline, followed by golimumab 100 mg SC at weeks 2, 6, and 10, and guselkumab 200 mg IV at weeks 4 and 8. At week 12, this group switched to monotherapy with guselkumab, 100 mg SC every 8 weeks.
Overall, 13% of patients discontinued treatment prior to week 34, the time of final dose of study intervention.
Dr. Feagan noted that they did not see differences between any adverse event, serious adverse event, or adverse event leading to discontinuation among the treatment groups.
Key findings through week 38
The rate of clinical remission in the combination group was 44%. The rate was lower with guselkumab monotherapy at 31% and golimumab monotherapy at 22% at week 38. These percentages were based on a full Mayo Score of 2 or less and no individual subscore greater than 1.
At the same time, the rates of clinical remission by modified Mayo score also favored the combination group at 48%, followed by 31% in the guselkumab group and 21% in the golimumab cohort.
Endoscopic improvement, endoscopic normalization, histologic remission, and composite histologic-endoscopic endpoints were also greater in the combination group than in the monotherapy groups.
“Quite striking differences were maintained up to week 38,” Dr. Feagan said. “This combination treatment warrants further investigation, and phase 3 trials are underway.”
He added that, while they were concerned about serious infection, they did not see any differences, with only two serious infections in each of the three groups.
Opportunistic infections were reported for two patients in the combination group: extrapulmonary tuberculosis and cytomegalovirus colitis. No opportunistic infections occurred in the monotherapy groups.
Valuable data
“The early study results, such as the VEGA study, appear promising for combination biologics with a good safety profile,” Jean-Paul Achkar, MD, staff physician in the Center for Inflammatory Bowel Disease at Cleveland Clinic and the Kenneth Rainin Endowed Chair for IBD Research, said when asked to comment.
“These data are particularly valuable as we have seemingly reached a therapeutic response ceiling for single-biologic therapy, and we need to determine the added benefit and safety profile of a combination of two biologics or the combination of a biologic and a small molecule,” added Dr. Achkar, who served as the session comoderator.
A meeting attendee asked about the likelihood of regulatory approval for this combination based on evidence like this study.
“I think they have to,” Dr. Feagan said. “We’ve probably seen our best results yet in Crohn’s disease, and we’re still at 50% [response rate for monotherapy]. If we’re ever going to come to terms with IBD, I don’t think it’s monotherapy.”
Dr. Feagan added that with combination therapy, “physicians will often worry about economics, but I think that’s a surrogate for their concerns about infection.”
However, he noted that “the better the agents we have, the better the incremental cost effectiveness. So, I don’t think economics is the issue; the issue is safety.”
Another meeting attendee asked if the results might apply to other biologic combinations.
“This model was picked to show the additive effect of the anti-p19 and the TNF antagonist,” Dr. Feagan said.
Similar results could be expected with a combination of treatments from the same classes, he said, but the treatment potential of other drug-class combination is unclear.
The study was funded by Janssen Research and Development. Dr. Feagan reports being a consultant for Janssen. Dr. Achkar reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. – , a phase 2a study demonstrates.
Researchers compared the combination therapy of guselkumab and golimumab (both from Janssen) for 12 weeks, followed by guselkumab monotherapy up to week 38, versus either agent as monotherapy for the full 38 weeks.
Guselkumab is an interleukin-23p19 subunit antagonist being studied to treat inflammatory bowel disease (IBD). Golimumab is a TNF-alpha antagonist being evaluated for ulcerative colitis.
The combination induction strategy “achieved higher rates of clinical remission, endoscopic improvement, composite endpoint of histologic remission, and endoscopic improvement,” said Brian G. Feagan, MD, senior scientific director at the contract research organization Alimentiv and a gastroenterologist at Western University in London, Ont.
The findings were presented at the annual meeting of the American College of Gastroenterology.
Study design
The current research builds on previous week 12 VEGA study results. The earlier findings indicated that blocking interleukin-23p19 by guselkumab and TNF-alpha with golimumab was superior on multiple measures, compared with monotherapy.
The new findings are from a randomized, double-blind, proof-of-concept study that included 214 adults with moderately to severely active ulcerative colitis. Participants were naive to TNF-alpha antagonists and refractory or intolerant to conventional therapy.
Of the participants, 71 were randomly assigned to receive guselkumab, 200 mg intravenous (IV) at baseline and at weeks 4 and 8, plus 100 mg subcutaneous (SC) every 8 weeks.
Another 72 participants received golimumab, 200 mg SC at baseline, and 100 mg SC at weeks 2, 6, and 10, and every 4 weeks thereafter.
The combination group of 71 participants received guselkumab 200 mg IV and golimumab 200 mg SC at baseline, followed by golimumab 100 mg SC at weeks 2, 6, and 10, and guselkumab 200 mg IV at weeks 4 and 8. At week 12, this group switched to monotherapy with guselkumab, 100 mg SC every 8 weeks.
Overall, 13% of patients discontinued treatment prior to week 34, the time of final dose of study intervention.
Dr. Feagan noted that they did not see differences between any adverse event, serious adverse event, or adverse event leading to discontinuation among the treatment groups.
Key findings through week 38
The rate of clinical remission in the combination group was 44%. The rate was lower with guselkumab monotherapy at 31% and golimumab monotherapy at 22% at week 38. These percentages were based on a full Mayo Score of 2 or less and no individual subscore greater than 1.
At the same time, the rates of clinical remission by modified Mayo score also favored the combination group at 48%, followed by 31% in the guselkumab group and 21% in the golimumab cohort.
Endoscopic improvement, endoscopic normalization, histologic remission, and composite histologic-endoscopic endpoints were also greater in the combination group than in the monotherapy groups.
“Quite striking differences were maintained up to week 38,” Dr. Feagan said. “This combination treatment warrants further investigation, and phase 3 trials are underway.”
He added that, while they were concerned about serious infection, they did not see any differences, with only two serious infections in each of the three groups.
Opportunistic infections were reported for two patients in the combination group: extrapulmonary tuberculosis and cytomegalovirus colitis. No opportunistic infections occurred in the monotherapy groups.
Valuable data
“The early study results, such as the VEGA study, appear promising for combination biologics with a good safety profile,” Jean-Paul Achkar, MD, staff physician in the Center for Inflammatory Bowel Disease at Cleveland Clinic and the Kenneth Rainin Endowed Chair for IBD Research, said when asked to comment.
“These data are particularly valuable as we have seemingly reached a therapeutic response ceiling for single-biologic therapy, and we need to determine the added benefit and safety profile of a combination of two biologics or the combination of a biologic and a small molecule,” added Dr. Achkar, who served as the session comoderator.
A meeting attendee asked about the likelihood of regulatory approval for this combination based on evidence like this study.
“I think they have to,” Dr. Feagan said. “We’ve probably seen our best results yet in Crohn’s disease, and we’re still at 50% [response rate for monotherapy]. If we’re ever going to come to terms with IBD, I don’t think it’s monotherapy.”
Dr. Feagan added that with combination therapy, “physicians will often worry about economics, but I think that’s a surrogate for their concerns about infection.”
However, he noted that “the better the agents we have, the better the incremental cost effectiveness. So, I don’t think economics is the issue; the issue is safety.”
Another meeting attendee asked if the results might apply to other biologic combinations.
“This model was picked to show the additive effect of the anti-p19 and the TNF antagonist,” Dr. Feagan said.
Similar results could be expected with a combination of treatments from the same classes, he said, but the treatment potential of other drug-class combination is unclear.
The study was funded by Janssen Research and Development. Dr. Feagan reports being a consultant for Janssen. Dr. Achkar reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2022