User login
Volumetric Considerations for Valving Long-Arm Casts: The Utility of the Cast Spacer
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
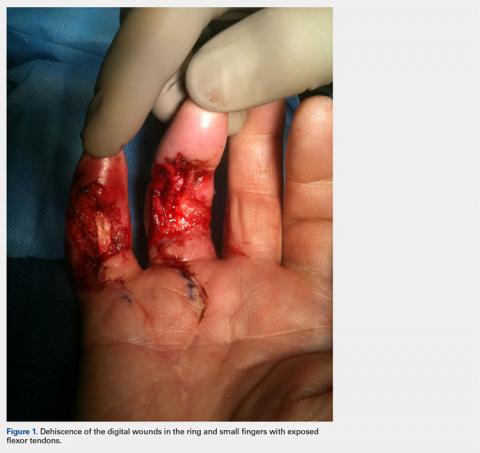
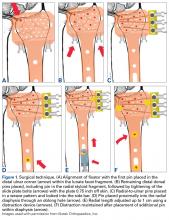
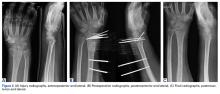
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.
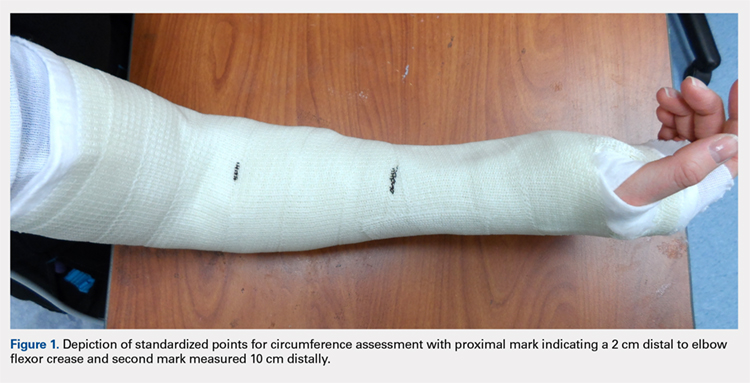
Continue to: Following valving, casts were secured via...
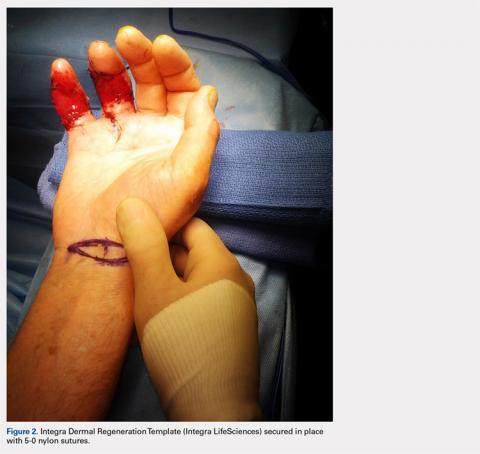
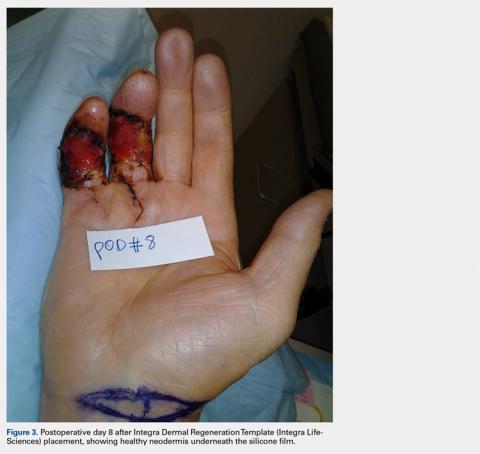
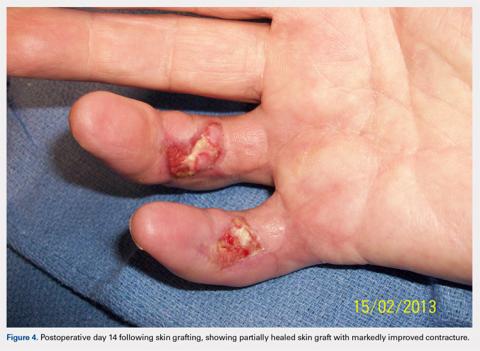
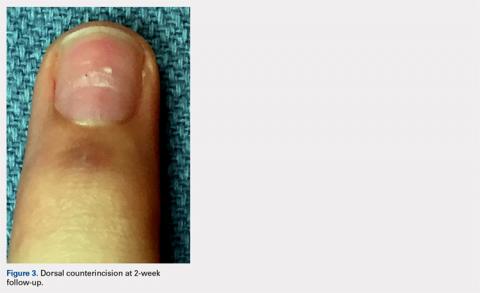
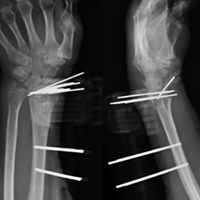
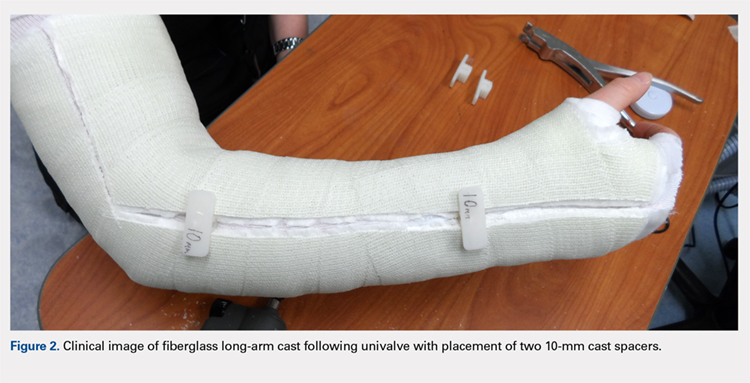
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).
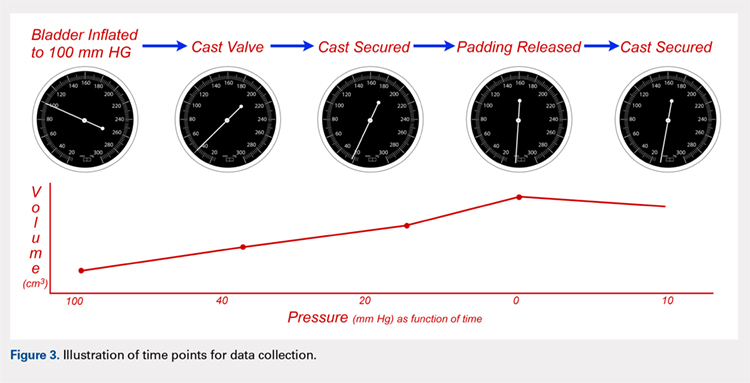
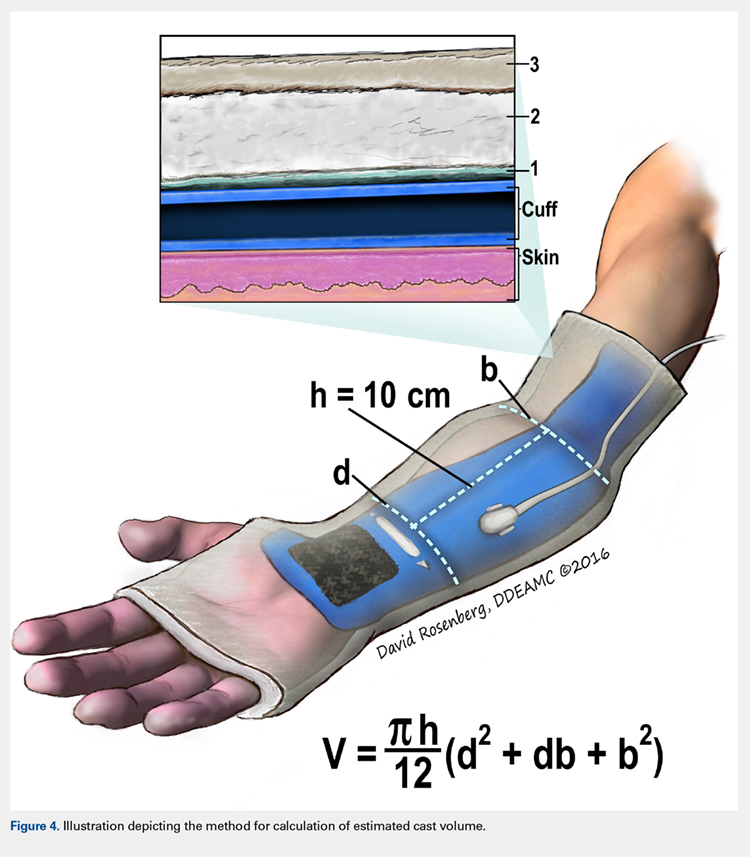
We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
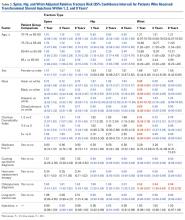
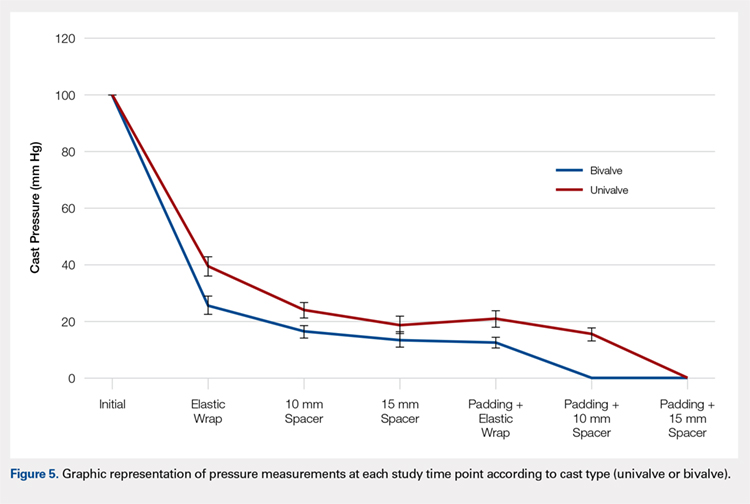
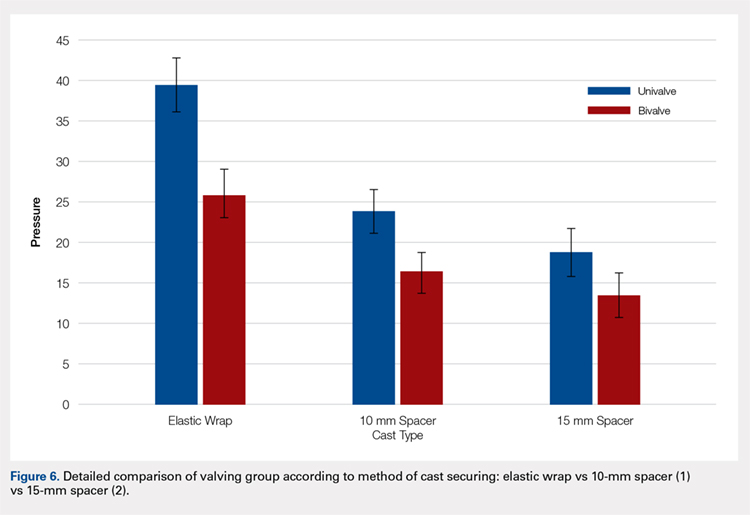
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |
Continue to: Table 2...
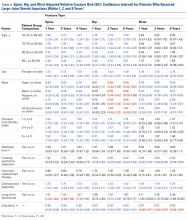
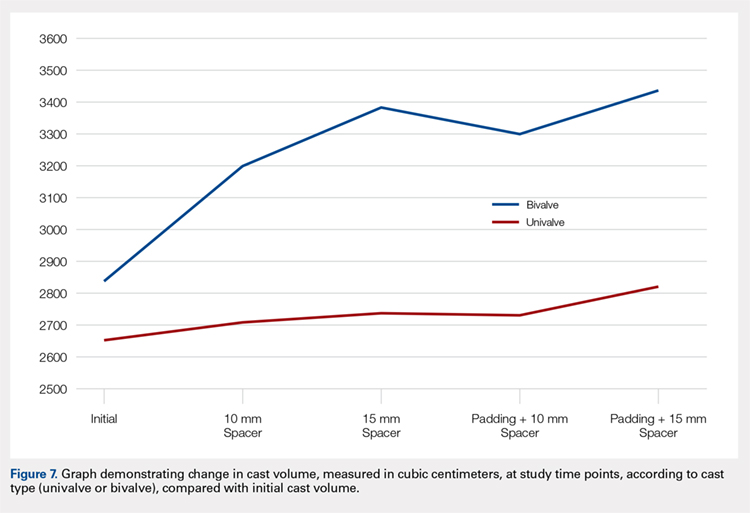
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |
Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
TAKE-HOME POINTS
- Valving a long-arm cast results in decreased cast pressures.
- Univalving can produce a 60% reduction in cast pressure.
- Bivalving produces a 75% reduction in cast pressure.
- Release of the underlying cast padding produces an additional pressure reduction.
- Adding a cast spacer to a univalved cast obtains similar pressure reduction to a bivalved cast.
Multi-Modal Pain Control in Ambulatory Hand Surgery
ABSTRACT
We evaluated postoperative pain control and narcotic usage after thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of the distal radius in patients given opiates with or without other non-opiate medication using a specific dosing regimen. A prospective, randomized study of 79 patients undergoing elective CMC arthroplasty or ORIF of the distal radius evaluated postoperative pain in the first 5 postoperative days. Patients were divided into 4 groups: Group 1, oxycodone and acetaminophen PRN; Group 2, oxycodone and acetaminophen with specific dosing; Group 3, oxycodone, acetaminophen, and OxyContin with specific dosing; and Group 4, oxycodone, acetaminophen, and ketorolac with specific dosing. During the first 5 postoperative days, we recorded pain levels according to a numeric pain scale, opioid usage, and complications. Although differences in our data did not reach statistical significance, overall pain scores, opioid usage, and complication rates were less prevalent in the oxycodone, acetaminophen, and ketorolac group. Postoperative pain following ambulatory hand and wrist surgery under regional anesthesia was more effectively controlled with fewer complications using a combination of oxycodone, acetaminophen, and ketorolac with a specific dosing regimen.
Continue to: Regional anesthesia...
Regional anesthesia is a safe and effective modality of perioperative pain control in patients undergoing ambulatory hand procedures.1-10 Often, as the regional block wears off, patients experience a rebound pain effect that can be challenging to manage.
We sought to determine if an organized, multimodal approach in patients undergoing thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of distal radius fractures would provide better postoperative pain control. We hypothesized that this approach would significantly reduce postoperative pain and the need for narcotic pain medication compared with PRN dosing of oxycodone/acetaminophen alone.11-14
MATERIALS AND METHODS
Our study was approved by our Institutional Review Board. Informed consent was obtained from each patient. Patients presenting for elective thumb CMC arthroplasty or ORIF of the distal radius were screened for inclusion in a prospective, randomized study. Inclusion criteria included patients aged 18 to 65 years who could provide informed consent. Patients with chronic pain syndromes, long-term narcotic usage, chronic medical conditions precluding the use of opiates or nonsteroidal anti-inflammatory drugs (NSAIDs), and those who did not have a complete sensory and motor block postoperatively were excluded.
Patients were randomly divided into 1 of 4 study arms. Randomization was performed via sealed envelopes, which were opened in the recovery area when postoperative prescriptions were written. The group distribution was as follows: Group 1, Percocet 5 mg/325 mg alone (control); Group 2, oxycodone 5 mg, acetaminophen 325 mg administered separately; Group 3, oxycodone 5 mg, acetaminophen 325 mg, and oxycodone SR (OxyContin) 10 mg; and Group 4, oxycodone 5 mg, acetaminophen 325 mg, and ketorolac (Toradol) 10 mg (Table 1). Patients in the control group were instructed to take 1 or 2 tablets every 4 to 6 hours as needed for pain. Patients in the 3 experimental groups were given detailed instructions regarding when and how to take their medications. All patients were instructed to take 650 mg of acetaminophen every 6 hours. Patients were provided a sliding scale to assist in dosing their opioid medications according to their numeric pain score (NPS) (Table 2). Group 2 patients were given oxycodone 10 mg in the postanesthesia care unit (PACU) and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Table 1. Patient Groups
Group | Anesthesia | Pain Medications |
1 (standard treatment) | Brachial plexus block | Percocet (oxycodone and acetaminophen) 5-10 mg every 4-6 hours as needed for pain. |
2 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. |
3 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on numeric pain scale. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. OxyContin (oxycodone sustained release) 10 mg twice a day, scheduled. |
4 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. Toradol (Ketorolac) 10mg every 6 hours, scheduled. |
Table 2. Sliding Scale for Pain Control in the Experimental Groups
Pain Score | Oxycodone Dose |
0-3 | 5 mg (1 tablet) |
4-7 | 10 mg (2 tablets) |
8-10 | 15 mg (3 tablets) |
Group 3 patients were given oxycodone 10 mg in the PACU and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours and OxyContin 10 mg every 12 hours on a scheduled basis until their block wore off, then dose themselves using NPS. Group 4 patients were given oxycodone 10 mg postoperatively and ketorolac 30 mg intravenously in the PACU and instructed to take oxycodone 10 mg, acetaminophen 650 mg, and ketorolac 10 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Patients were provided with a journal and asked to record their medication usage, NPS, and any adverse effects (nausea, vomiting, and uncontrolled pain were specifically mentioned) or complications for 5 days after their procedure. We also attempted to contact patients by telephone on each of the 5 days after their procedure to remind them to complete their logs. They were asked specifically if they were having difficulty with their medications. They were also asked specifically about nausea, vomiting, and over-sedation. If patients requested additional medication to help treat their pain, they were instructed to add an over-the-counter NSAID of their choice based on the label’s suggested dosing.
Continue to: All patients received a supraclavicular...
All patients received a supraclavicular brachial plexus block using 0.75% ropivacaine under the supervision of an attending anesthesiologist experienced in regional anesthesia. Patients underwent thumb CMC arthroplasty utilizing complete resection of the trapezium followed by abductor pollicis longus suspensionplasty under the supervision of 1 of 3 fellowship-trained hand surgeons. ORIF of the distal radius was completed utilizing a volar approach and distal radius locking plate under the supervision of 1 of 3 fellowship-trained hand surgeons.
Primary outcome measures were the total number of oxycodone tablets taken daily and the average daily NPS. Secondary outcomes measured included adverse effects as noted above and the need for a trip to the emergency department for unrelieved pain.
A power analysis was completed prior to the beginning of the study. To detect a difference of at least 1 on the NPS, we determined that 18 patients per group would provide 80% power. This was based on literature utilizing the visual analog scale (VAS), a 100-mm line on which patients can place a mark to describe the intensity of their pain. The standard deviation on the VAS is approximately 15 mm. To account for potential dropout, we elected to recruit 20 patients per group. Non-paired t tests were used to compare groups.
RESULTS
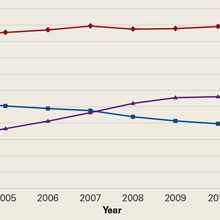
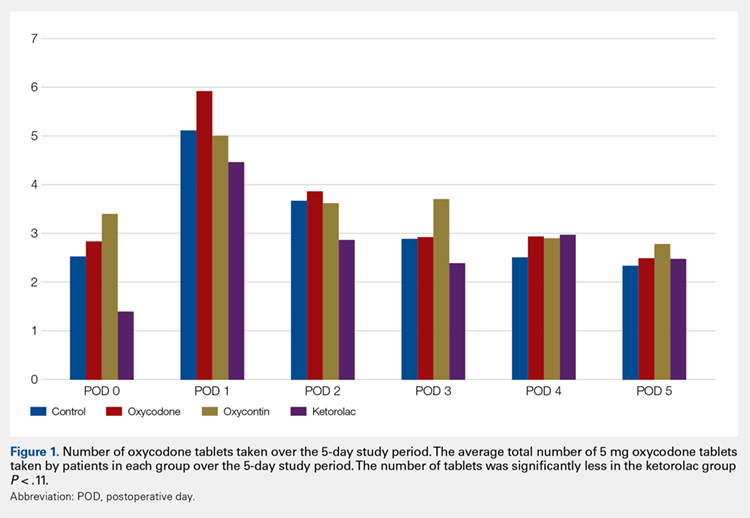
One hundred and eighteen patients enrolled in the study. Of those, 79 patients completed and returned their summary logs (by group: 18 control, 20 oxycodone, 17 OxyContin, and 24 ketorolac). The remaining patients were excluded from the final analysis because they did not return their summary logs. Only 1 patient was excluded from the analysis because he did not have adequate regional anesthesia. Demographic data were analyzed and showed no significant differences between groups at the P < .05 level of significance. Surgical procedures were completed by 3 fellowship-trained hand surgeons. Distal radius fractures were performed using a volar approach. CMC arthroplasty was performed using a procedure that was standardized across surgeons. There were no between-surgeon differences in outcomes.
Average daily NPS (Figure 1) and the total number of oxycodone tablets taken (Figure 2) over the 5-day study period were recorded. Patients in the ketorolac group used fewer oxycodone tablets (19.3) than patients in the other 3 groups (24.4), P =.11, but the difference was not statistically significant. The maximum number of oxycodone tablets used was 71 in the Percocet group, 57 in the oxycodone and ketorolac groups, and 73 in the OxyContin group. The average daily NPS was lower in the ketorolac group during the period of medication use. This value only reached statistical significance on postoperative day 0 when the ketorolac group was compared with the OxyContin group (P = .01) and on postoperative day 1 when the ketorolac group was compared with the oxycodone group (P = .04). Complications (Figure 3) were greater in the non-ketorolac groups. One patient each in the oxycodone and OxyContin groups required a trip to the emergency department for pain control after their block wore off. Nausea and vomiting were present in each of the 4 groups but to a much greater degree in the Percocet and OxyContin groups; however, these results did not reach statistical significance (P = .129). Eleven of the 18 patients in the Percocet group required an additional NSAID (naproxen) and still did not achieve pain control similar to the other groups. This may explain why the average daily pain score in the Percocet group was lower than that in the oxycodone group, in which only 4 of the 20 patients supplemented with naproxen. Patients did, however, require many more oxycodone tablets to achieve pain control in the Percocet group. Over-sedation was reported in 3 patients in the oxycodone group and in 1 patient in the OxyContin group. No patients were found to have bleeding, renal, or other systemic complications.
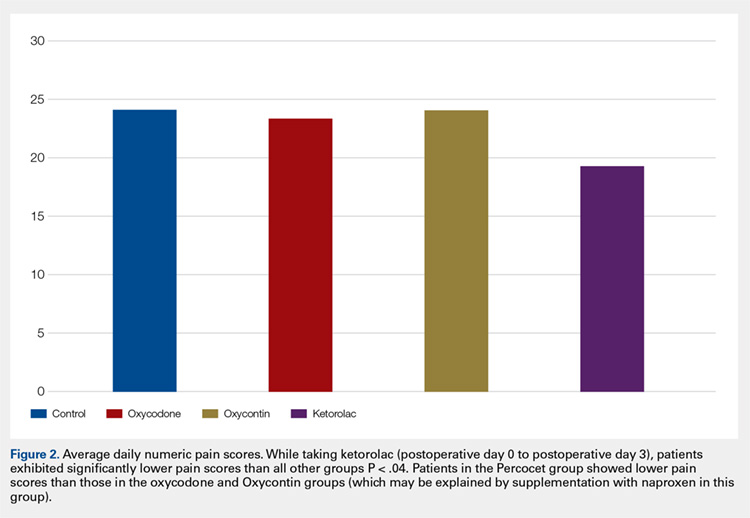
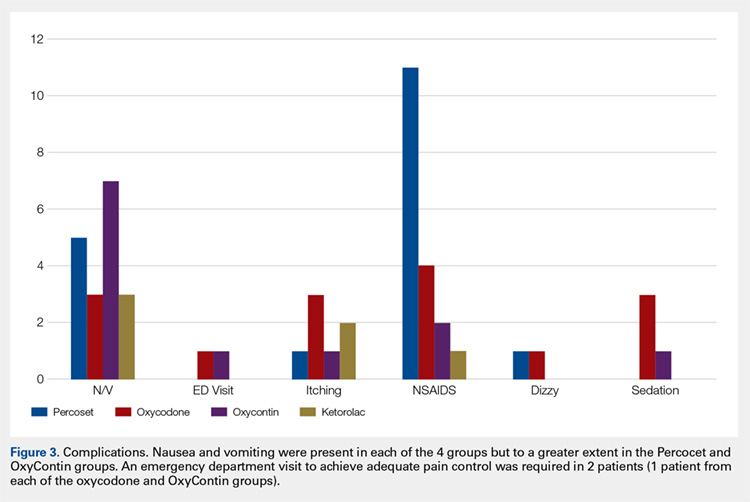
Continue to: Discussion...
DISCUSSION
In this prospective, randomized study, we sought to determine whether a more organized approach to treating postoperative pain using a specific dosing regimen or opiates in conjunction with non-opiate medications would lead to improved pain control and a decreased need for opiates. We found that adding ketorolac to the postoperative pain regimen and outlining a more detailed set of instructions could lower narcotic usage in the first 4 postoperative days. In addition, adding ketorolac decreased other complications commonly seen with narcotic usage and was shown to be safe in our patient population.
Ketorolac has been shown to decrease narcotic pain medication usage in several surgical settings and across different surgical specialties. It is hypothesized that ketorolac potentiates the effects of narcotics.11 Ketorolac given alone has a potent analgesic effect by acting as a strong non-selective cyclooxygenase inhibitor. The major drawback to ketorolac use has been its well-known side-effect profile. Ketorolac is renally excreted, and as such, should not be used in patients with renal insufficiency. In addition, ketorolac has been shown to cause increased gastrointestinal bleeding when used for >5 days.15 Caution should be taken when combining ketorolac with thromboprophylactic medications, especially in older patients.
Many surgeons use NSAIDs along with narcotics as part of a postoperative pain regimen. While this is often adequate for some procedures, when the surgery involves manipulating fractures, internal fixation, or resection arthroplasty, the variation in individual patient pain may call for a more robust protocol. Additionally, as surgeons expand to more complex procedures performed in the outpatient setting, evaluating different combinations of analgesics taken in a more structured manner may provide for improved pain control.
A major component of patient satisfaction is postoperative pain control.3-8,12,16,17 Regional anesthesia is an important tool that allows patients to undergo a surgical procedure with a greatly reduced amount of opioid pain medications. In addition, regional anesthesia can provide significant pain control after the patient has left the ambulatory surgery center, but this relief is short-lived because the medication is designed to lose effectiveness over time. As the effects of regional anesthesia wear off, patients can experience “rebound pain” with severe levels of pain that, on occasion, cannot be controlled with oral analgesics alone. The addition of ketorolac provided improved pain control when compared with the other regimens during this transition period when the regional anesthesia was becoming ineffective. In addition, because patients taking ketorolac used less narcotic medication, they experienced less nausea, vomiting, and over-sedation.
Additionally, patients were instructed to record their medication usage and pain scores on a prospective basis, with the hope of eliminating recall bias. A potential weakness is the inability to show significance for pain relief and reduced narcotic usage with the addition of ketorolac, although there was a trend toward significance. Many of the patients who enrolled in the trial did not return their medication logs. While these patients had to be excluded from data analysis, we continued enrollment until we obtained an adequate number of patients in each group. In addition, in the OxyContin group (Group 3), we could only recruit 17 participants, instead of the 18 needed based on our power study. Although this has a potential to alter the significance of our results, we do not feel this had a substantial impact on our results.
Many patients in the non-ketorolac groups supplemented their medication regimens with NSAIDs, which may have falsely lowered pain scores and narcotic usage. While this confounds our study results, we do not believe that it invalidates the conclusion that ketorolac can be an effective adjunct pain medication for use in patients undergoing ambulatory hand surgery.
The study examined postoperative pain control for only 2 procedures, thumb basal joint arthroplasty and distal radius fracture fixation, both commonly performed in the outpatient setting under regional anesthesia and both typically requiring narcotic pain medication. Perhaps the utilization of these medication regimens with different surgical procedures would have differing results.
We conclude that ketorolac potentially provides patients with improved pain control over the use of narcotic pain medications alone in the setting of ambulatory hand surgery.
This paper will be judged for the Resident Writer’s Award.
- Boezaart AP, Davis G, Le-Wendling L. Recovery after orthopedic surgery: techniques to increase duration of pain control. Curr Opin Anaesthesiol. 2012;25(6):665-672. doi:10.1097/ACO.0b013e328359ab5a.
- Buvanendran A, Kroin JS. Useful adjuvants for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2007;21(1):31-49. doi:10.1016/j.bpa.2006.12.003.
- Coluzzi F, Bragazzi L, Di Bussolo E, Pizza G, Mattia C. Determinants of patient satisfaction in postoperative pain management following hand ambulatory day-surgery. Minerva Med. 2011;102(3):177-186.
- Elvir-Lazo OL, White PF. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28(2):217-224. doi: 10.1016/j.anclin.2010.02.011.
- Kopp SL, Horlocker TT. Regional anaesthesia in day-stay and short-stay surgery. Anaesthesia. 2010;65(Suppl 1):84-96. doi:10.1111/j.1365-2044.2009.06204.x.
- Rawal N. Postoperative pain treatment for ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2007;21(1):129-148. doi:10.1016/j.bpa.2006.11.005.
- Schug SA, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2009;22(6):738-743. doi:10.1097/ACO.0b013e32833020f4.
- Sripada R, Bowens C Jr. Regional anesthesia procedures for shoulder and upper arm surgery upper extremity update--2005 to present. Int Anesthesiol Clin. 2012;50(1):26-46. doi:10.1097/AIA.0b013e31821a0284.
- Trompeter A, Camilleri G, Narang K, Hauf W, Venn R. Analgesia requirements after interscalene block for shoulder arthroscopy: the 5 days following surgery. Arch Orthop Trauma Surg. 2010;130(3):417-421. doi:10.1007/s00402-009-0959-9.
- Dufeu N, Marchand-Maillet F, Atchabahian A, et al. Efficacy and safety of ultrasound-guided distal blocks for analgesia without motor blockade after ambulatory hand surgery. J Hand Surg Am. 2014;39(4):737-743. doi:10.1016/j.jhsa.2014.01.011.
- Gutta R, Koehn CR, James LE. Does ketorolac have a preemptive analgesic effect? A randomized, double-blind, control study. J Oral Maxillofac Surg. 2013;71(12):2029-2034. doi:10.1016/j.joms.2013.06.220.
- Nossaman VE, Ramadhyani U, Kadowitz PJ, Nossaman BD. Advances in perioperative pain management: use of medications with dual analgesic mechanisms, tramadol & tapentadol. Anesthesiol Clin. 2010;28(4):647-666. doi:10.1016/j.anclin.2010.08.009.
- Warren-Stomberg M, Brattwall M, Jakobsson JG. Non-opioid analgesics for pain management following ambulatory surgery: a review. Minerva Anestesiol. 2013;79(9):1077-1087.
- Wickerts L, Warrén Stomberg M, Brattwall M, Jakobsson JJ. Coxibs: is there a benefit when compared to traditional non-selective NSAIDs in postoperative pain management? Minerva Anestesiol. 2011;77(11):1084-1098.
- Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA. 1996;275(5):376-382. doi:10.1001/jama.275.5.376.
- Hegarty M, Calder A, Davies K, et al. Does take-home analgesia improve postoperative pain after elective day case surgery? A comparison of hospital vs parent-supplied analgesia. Paediatr Anaesth. 2013;23(5):385-389. doi:10.1111/pan.12077.
- Weber SC, Jain R, Parise C. Pain scores in the management of postoperative pain in shoulder surgery. Arthroscopy. 2007;23(1):65-72. doi:10.1016/j.arthro.2006.11.002.
ABSTRACT
We evaluated postoperative pain control and narcotic usage after thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of the distal radius in patients given opiates with or without other non-opiate medication using a specific dosing regimen. A prospective, randomized study of 79 patients undergoing elective CMC arthroplasty or ORIF of the distal radius evaluated postoperative pain in the first 5 postoperative days. Patients were divided into 4 groups: Group 1, oxycodone and acetaminophen PRN; Group 2, oxycodone and acetaminophen with specific dosing; Group 3, oxycodone, acetaminophen, and OxyContin with specific dosing; and Group 4, oxycodone, acetaminophen, and ketorolac with specific dosing. During the first 5 postoperative days, we recorded pain levels according to a numeric pain scale, opioid usage, and complications. Although differences in our data did not reach statistical significance, overall pain scores, opioid usage, and complication rates were less prevalent in the oxycodone, acetaminophen, and ketorolac group. Postoperative pain following ambulatory hand and wrist surgery under regional anesthesia was more effectively controlled with fewer complications using a combination of oxycodone, acetaminophen, and ketorolac with a specific dosing regimen.
Continue to: Regional anesthesia...
Regional anesthesia is a safe and effective modality of perioperative pain control in patients undergoing ambulatory hand procedures.1-10 Often, as the regional block wears off, patients experience a rebound pain effect that can be challenging to manage.
We sought to determine if an organized, multimodal approach in patients undergoing thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of distal radius fractures would provide better postoperative pain control. We hypothesized that this approach would significantly reduce postoperative pain and the need for narcotic pain medication compared with PRN dosing of oxycodone/acetaminophen alone.11-14
MATERIALS AND METHODS
Our study was approved by our Institutional Review Board. Informed consent was obtained from each patient. Patients presenting for elective thumb CMC arthroplasty or ORIF of the distal radius were screened for inclusion in a prospective, randomized study. Inclusion criteria included patients aged 18 to 65 years who could provide informed consent. Patients with chronic pain syndromes, long-term narcotic usage, chronic medical conditions precluding the use of opiates or nonsteroidal anti-inflammatory drugs (NSAIDs), and those who did not have a complete sensory and motor block postoperatively were excluded.
Patients were randomly divided into 1 of 4 study arms. Randomization was performed via sealed envelopes, which were opened in the recovery area when postoperative prescriptions were written. The group distribution was as follows: Group 1, Percocet 5 mg/325 mg alone (control); Group 2, oxycodone 5 mg, acetaminophen 325 mg administered separately; Group 3, oxycodone 5 mg, acetaminophen 325 mg, and oxycodone SR (OxyContin) 10 mg; and Group 4, oxycodone 5 mg, acetaminophen 325 mg, and ketorolac (Toradol) 10 mg (Table 1). Patients in the control group were instructed to take 1 or 2 tablets every 4 to 6 hours as needed for pain. Patients in the 3 experimental groups were given detailed instructions regarding when and how to take their medications. All patients were instructed to take 650 mg of acetaminophen every 6 hours. Patients were provided a sliding scale to assist in dosing their opioid medications according to their numeric pain score (NPS) (Table 2). Group 2 patients were given oxycodone 10 mg in the postanesthesia care unit (PACU) and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Table 1. Patient Groups
Group | Anesthesia | Pain Medications |
1 (standard treatment) | Brachial plexus block | Percocet (oxycodone and acetaminophen) 5-10 mg every 4-6 hours as needed for pain. |
2 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. |
3 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on numeric pain scale. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. OxyContin (oxycodone sustained release) 10 mg twice a day, scheduled. |
4 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. Toradol (Ketorolac) 10mg every 6 hours, scheduled. |
Table 2. Sliding Scale for Pain Control in the Experimental Groups
Pain Score | Oxycodone Dose |
0-3 | 5 mg (1 tablet) |
4-7 | 10 mg (2 tablets) |
8-10 | 15 mg (3 tablets) |
Group 3 patients were given oxycodone 10 mg in the PACU and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours and OxyContin 10 mg every 12 hours on a scheduled basis until their block wore off, then dose themselves using NPS. Group 4 patients were given oxycodone 10 mg postoperatively and ketorolac 30 mg intravenously in the PACU and instructed to take oxycodone 10 mg, acetaminophen 650 mg, and ketorolac 10 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Patients were provided with a journal and asked to record their medication usage, NPS, and any adverse effects (nausea, vomiting, and uncontrolled pain were specifically mentioned) or complications for 5 days after their procedure. We also attempted to contact patients by telephone on each of the 5 days after their procedure to remind them to complete their logs. They were asked specifically if they were having difficulty with their medications. They were also asked specifically about nausea, vomiting, and over-sedation. If patients requested additional medication to help treat their pain, they were instructed to add an over-the-counter NSAID of their choice based on the label’s suggested dosing.
Continue to: All patients received a supraclavicular...
All patients received a supraclavicular brachial plexus block using 0.75% ropivacaine under the supervision of an attending anesthesiologist experienced in regional anesthesia. Patients underwent thumb CMC arthroplasty utilizing complete resection of the trapezium followed by abductor pollicis longus suspensionplasty under the supervision of 1 of 3 fellowship-trained hand surgeons. ORIF of the distal radius was completed utilizing a volar approach and distal radius locking plate under the supervision of 1 of 3 fellowship-trained hand surgeons.
Primary outcome measures were the total number of oxycodone tablets taken daily and the average daily NPS. Secondary outcomes measured included adverse effects as noted above and the need for a trip to the emergency department for unrelieved pain.
A power analysis was completed prior to the beginning of the study. To detect a difference of at least 1 on the NPS, we determined that 18 patients per group would provide 80% power. This was based on literature utilizing the visual analog scale (VAS), a 100-mm line on which patients can place a mark to describe the intensity of their pain. The standard deviation on the VAS is approximately 15 mm. To account for potential dropout, we elected to recruit 20 patients per group. Non-paired t tests were used to compare groups.
RESULTS
One hundred and eighteen patients enrolled in the study. Of those, 79 patients completed and returned their summary logs (by group: 18 control, 20 oxycodone, 17 OxyContin, and 24 ketorolac). The remaining patients were excluded from the final analysis because they did not return their summary logs. Only 1 patient was excluded from the analysis because he did not have adequate regional anesthesia. Demographic data were analyzed and showed no significant differences between groups at the P < .05 level of significance. Surgical procedures were completed by 3 fellowship-trained hand surgeons. Distal radius fractures were performed using a volar approach. CMC arthroplasty was performed using a procedure that was standardized across surgeons. There were no between-surgeon differences in outcomes.
Average daily NPS (Figure 1) and the total number of oxycodone tablets taken (Figure 2) over the 5-day study period were recorded. Patients in the ketorolac group used fewer oxycodone tablets (19.3) than patients in the other 3 groups (24.4), P =.11, but the difference was not statistically significant. The maximum number of oxycodone tablets used was 71 in the Percocet group, 57 in the oxycodone and ketorolac groups, and 73 in the OxyContin group. The average daily NPS was lower in the ketorolac group during the period of medication use. This value only reached statistical significance on postoperative day 0 when the ketorolac group was compared with the OxyContin group (P = .01) and on postoperative day 1 when the ketorolac group was compared with the oxycodone group (P = .04). Complications (Figure 3) were greater in the non-ketorolac groups. One patient each in the oxycodone and OxyContin groups required a trip to the emergency department for pain control after their block wore off. Nausea and vomiting were present in each of the 4 groups but to a much greater degree in the Percocet and OxyContin groups; however, these results did not reach statistical significance (P = .129). Eleven of the 18 patients in the Percocet group required an additional NSAID (naproxen) and still did not achieve pain control similar to the other groups. This may explain why the average daily pain score in the Percocet group was lower than that in the oxycodone group, in which only 4 of the 20 patients supplemented with naproxen. Patients did, however, require many more oxycodone tablets to achieve pain control in the Percocet group. Over-sedation was reported in 3 patients in the oxycodone group and in 1 patient in the OxyContin group. No patients were found to have bleeding, renal, or other systemic complications.



Continue to: Discussion...
DISCUSSION
In this prospective, randomized study, we sought to determine whether a more organized approach to treating postoperative pain using a specific dosing regimen or opiates in conjunction with non-opiate medications would lead to improved pain control and a decreased need for opiates. We found that adding ketorolac to the postoperative pain regimen and outlining a more detailed set of instructions could lower narcotic usage in the first 4 postoperative days. In addition, adding ketorolac decreased other complications commonly seen with narcotic usage and was shown to be safe in our patient population.
Ketorolac has been shown to decrease narcotic pain medication usage in several surgical settings and across different surgical specialties. It is hypothesized that ketorolac potentiates the effects of narcotics.11 Ketorolac given alone has a potent analgesic effect by acting as a strong non-selective cyclooxygenase inhibitor. The major drawback to ketorolac use has been its well-known side-effect profile. Ketorolac is renally excreted, and as such, should not be used in patients with renal insufficiency. In addition, ketorolac has been shown to cause increased gastrointestinal bleeding when used for >5 days.15 Caution should be taken when combining ketorolac with thromboprophylactic medications, especially in older patients.
Many surgeons use NSAIDs along with narcotics as part of a postoperative pain regimen. While this is often adequate for some procedures, when the surgery involves manipulating fractures, internal fixation, or resection arthroplasty, the variation in individual patient pain may call for a more robust protocol. Additionally, as surgeons expand to more complex procedures performed in the outpatient setting, evaluating different combinations of analgesics taken in a more structured manner may provide for improved pain control.
A major component of patient satisfaction is postoperative pain control.3-8,12,16,17 Regional anesthesia is an important tool that allows patients to undergo a surgical procedure with a greatly reduced amount of opioid pain medications. In addition, regional anesthesia can provide significant pain control after the patient has left the ambulatory surgery center, but this relief is short-lived because the medication is designed to lose effectiveness over time. As the effects of regional anesthesia wear off, patients can experience “rebound pain” with severe levels of pain that, on occasion, cannot be controlled with oral analgesics alone. The addition of ketorolac provided improved pain control when compared with the other regimens during this transition period when the regional anesthesia was becoming ineffective. In addition, because patients taking ketorolac used less narcotic medication, they experienced less nausea, vomiting, and over-sedation.
Additionally, patients were instructed to record their medication usage and pain scores on a prospective basis, with the hope of eliminating recall bias. A potential weakness is the inability to show significance for pain relief and reduced narcotic usage with the addition of ketorolac, although there was a trend toward significance. Many of the patients who enrolled in the trial did not return their medication logs. While these patients had to be excluded from data analysis, we continued enrollment until we obtained an adequate number of patients in each group. In addition, in the OxyContin group (Group 3), we could only recruit 17 participants, instead of the 18 needed based on our power study. Although this has a potential to alter the significance of our results, we do not feel this had a substantial impact on our results.
Many patients in the non-ketorolac groups supplemented their medication regimens with NSAIDs, which may have falsely lowered pain scores and narcotic usage. While this confounds our study results, we do not believe that it invalidates the conclusion that ketorolac can be an effective adjunct pain medication for use in patients undergoing ambulatory hand surgery.
The study examined postoperative pain control for only 2 procedures, thumb basal joint arthroplasty and distal radius fracture fixation, both commonly performed in the outpatient setting under regional anesthesia and both typically requiring narcotic pain medication. Perhaps the utilization of these medication regimens with different surgical procedures would have differing results.
We conclude that ketorolac potentially provides patients with improved pain control over the use of narcotic pain medications alone in the setting of ambulatory hand surgery.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
We evaluated postoperative pain control and narcotic usage after thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of the distal radius in patients given opiates with or without other non-opiate medication using a specific dosing regimen. A prospective, randomized study of 79 patients undergoing elective CMC arthroplasty or ORIF of the distal radius evaluated postoperative pain in the first 5 postoperative days. Patients were divided into 4 groups: Group 1, oxycodone and acetaminophen PRN; Group 2, oxycodone and acetaminophen with specific dosing; Group 3, oxycodone, acetaminophen, and OxyContin with specific dosing; and Group 4, oxycodone, acetaminophen, and ketorolac with specific dosing. During the first 5 postoperative days, we recorded pain levels according to a numeric pain scale, opioid usage, and complications. Although differences in our data did not reach statistical significance, overall pain scores, opioid usage, and complication rates were less prevalent in the oxycodone, acetaminophen, and ketorolac group. Postoperative pain following ambulatory hand and wrist surgery under regional anesthesia was more effectively controlled with fewer complications using a combination of oxycodone, acetaminophen, and ketorolac with a specific dosing regimen.
Continue to: Regional anesthesia...
Regional anesthesia is a safe and effective modality of perioperative pain control in patients undergoing ambulatory hand procedures.1-10 Often, as the regional block wears off, patients experience a rebound pain effect that can be challenging to manage.
We sought to determine if an organized, multimodal approach in patients undergoing thumb carpometacarpal (CMC) arthroplasty or open reduction and internal fixation (ORIF) of distal radius fractures would provide better postoperative pain control. We hypothesized that this approach would significantly reduce postoperative pain and the need for narcotic pain medication compared with PRN dosing of oxycodone/acetaminophen alone.11-14
MATERIALS AND METHODS
Our study was approved by our Institutional Review Board. Informed consent was obtained from each patient. Patients presenting for elective thumb CMC arthroplasty or ORIF of the distal radius were screened for inclusion in a prospective, randomized study. Inclusion criteria included patients aged 18 to 65 years who could provide informed consent. Patients with chronic pain syndromes, long-term narcotic usage, chronic medical conditions precluding the use of opiates or nonsteroidal anti-inflammatory drugs (NSAIDs), and those who did not have a complete sensory and motor block postoperatively were excluded.
Patients were randomly divided into 1 of 4 study arms. Randomization was performed via sealed envelopes, which were opened in the recovery area when postoperative prescriptions were written. The group distribution was as follows: Group 1, Percocet 5 mg/325 mg alone (control); Group 2, oxycodone 5 mg, acetaminophen 325 mg administered separately; Group 3, oxycodone 5 mg, acetaminophen 325 mg, and oxycodone SR (OxyContin) 10 mg; and Group 4, oxycodone 5 mg, acetaminophen 325 mg, and ketorolac (Toradol) 10 mg (Table 1). Patients in the control group were instructed to take 1 or 2 tablets every 4 to 6 hours as needed for pain. Patients in the 3 experimental groups were given detailed instructions regarding when and how to take their medications. All patients were instructed to take 650 mg of acetaminophen every 6 hours. Patients were provided a sliding scale to assist in dosing their opioid medications according to their numeric pain score (NPS) (Table 2). Group 2 patients were given oxycodone 10 mg in the postanesthesia care unit (PACU) and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Table 1. Patient Groups
Group | Anesthesia | Pain Medications |
1 (standard treatment) | Brachial plexus block | Percocet (oxycodone and acetaminophen) 5-10 mg every 4-6 hours as needed for pain. |
2 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. |
3 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on numeric pain scale. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. OxyContin (oxycodone sustained release) 10 mg twice a day, scheduled. |
4 | Brachial plexus block | 1. Oxycodone 0-15 mg every 4-6 hours as needed for pain based on pain scale score. 2. Tylenol (Acetaminophen) 650 mg every 6 hours, scheduled. 3. Toradol (Ketorolac) 10mg every 6 hours, scheduled. |
Table 2. Sliding Scale for Pain Control in the Experimental Groups
Pain Score | Oxycodone Dose |
0-3 | 5 mg (1 tablet) |
4-7 | 10 mg (2 tablets) |
8-10 | 15 mg (3 tablets) |
Group 3 patients were given oxycodone 10 mg in the PACU and instructed to take oxycodone 10 mg with acetaminophen 650 mg every 6 hours and OxyContin 10 mg every 12 hours on a scheduled basis until their block wore off, then dose themselves using NPS. Group 4 patients were given oxycodone 10 mg postoperatively and ketorolac 30 mg intravenously in the PACU and instructed to take oxycodone 10 mg, acetaminophen 650 mg, and ketorolac 10 mg every 6 hours on a scheduled basis until their block wore off, then dose themselves using the NPS.
Patients were provided with a journal and asked to record their medication usage, NPS, and any adverse effects (nausea, vomiting, and uncontrolled pain were specifically mentioned) or complications for 5 days after their procedure. We also attempted to contact patients by telephone on each of the 5 days after their procedure to remind them to complete their logs. They were asked specifically if they were having difficulty with their medications. They were also asked specifically about nausea, vomiting, and over-sedation. If patients requested additional medication to help treat their pain, they were instructed to add an over-the-counter NSAID of their choice based on the label’s suggested dosing.
Continue to: All patients received a supraclavicular...
All patients received a supraclavicular brachial plexus block using 0.75% ropivacaine under the supervision of an attending anesthesiologist experienced in regional anesthesia. Patients underwent thumb CMC arthroplasty utilizing complete resection of the trapezium followed by abductor pollicis longus suspensionplasty under the supervision of 1 of 3 fellowship-trained hand surgeons. ORIF of the distal radius was completed utilizing a volar approach and distal radius locking plate under the supervision of 1 of 3 fellowship-trained hand surgeons.
Primary outcome measures were the total number of oxycodone tablets taken daily and the average daily NPS. Secondary outcomes measured included adverse effects as noted above and the need for a trip to the emergency department for unrelieved pain.
A power analysis was completed prior to the beginning of the study. To detect a difference of at least 1 on the NPS, we determined that 18 patients per group would provide 80% power. This was based on literature utilizing the visual analog scale (VAS), a 100-mm line on which patients can place a mark to describe the intensity of their pain. The standard deviation on the VAS is approximately 15 mm. To account for potential dropout, we elected to recruit 20 patients per group. Non-paired t tests were used to compare groups.
RESULTS
One hundred and eighteen patients enrolled in the study. Of those, 79 patients completed and returned their summary logs (by group: 18 control, 20 oxycodone, 17 OxyContin, and 24 ketorolac). The remaining patients were excluded from the final analysis because they did not return their summary logs. Only 1 patient was excluded from the analysis because he did not have adequate regional anesthesia. Demographic data were analyzed and showed no significant differences between groups at the P < .05 level of significance. Surgical procedures were completed by 3 fellowship-trained hand surgeons. Distal radius fractures were performed using a volar approach. CMC arthroplasty was performed using a procedure that was standardized across surgeons. There were no between-surgeon differences in outcomes.
Average daily NPS (Figure 1) and the total number of oxycodone tablets taken (Figure 2) over the 5-day study period were recorded. Patients in the ketorolac group used fewer oxycodone tablets (19.3) than patients in the other 3 groups (24.4), P =.11, but the difference was not statistically significant. The maximum number of oxycodone tablets used was 71 in the Percocet group, 57 in the oxycodone and ketorolac groups, and 73 in the OxyContin group. The average daily NPS was lower in the ketorolac group during the period of medication use. This value only reached statistical significance on postoperative day 0 when the ketorolac group was compared with the OxyContin group (P = .01) and on postoperative day 1 when the ketorolac group was compared with the oxycodone group (P = .04). Complications (Figure 3) were greater in the non-ketorolac groups. One patient each in the oxycodone and OxyContin groups required a trip to the emergency department for pain control after their block wore off. Nausea and vomiting were present in each of the 4 groups but to a much greater degree in the Percocet and OxyContin groups; however, these results did not reach statistical significance (P = .129). Eleven of the 18 patients in the Percocet group required an additional NSAID (naproxen) and still did not achieve pain control similar to the other groups. This may explain why the average daily pain score in the Percocet group was lower than that in the oxycodone group, in which only 4 of the 20 patients supplemented with naproxen. Patients did, however, require many more oxycodone tablets to achieve pain control in the Percocet group. Over-sedation was reported in 3 patients in the oxycodone group and in 1 patient in the OxyContin group. No patients were found to have bleeding, renal, or other systemic complications.



Continue to: Discussion...
DISCUSSION
In this prospective, randomized study, we sought to determine whether a more organized approach to treating postoperative pain using a specific dosing regimen or opiates in conjunction with non-opiate medications would lead to improved pain control and a decreased need for opiates. We found that adding ketorolac to the postoperative pain regimen and outlining a more detailed set of instructions could lower narcotic usage in the first 4 postoperative days. In addition, adding ketorolac decreased other complications commonly seen with narcotic usage and was shown to be safe in our patient population.
Ketorolac has been shown to decrease narcotic pain medication usage in several surgical settings and across different surgical specialties. It is hypothesized that ketorolac potentiates the effects of narcotics.11 Ketorolac given alone has a potent analgesic effect by acting as a strong non-selective cyclooxygenase inhibitor. The major drawback to ketorolac use has been its well-known side-effect profile. Ketorolac is renally excreted, and as such, should not be used in patients with renal insufficiency. In addition, ketorolac has been shown to cause increased gastrointestinal bleeding when used for >5 days.15 Caution should be taken when combining ketorolac with thromboprophylactic medications, especially in older patients.
Many surgeons use NSAIDs along with narcotics as part of a postoperative pain regimen. While this is often adequate for some procedures, when the surgery involves manipulating fractures, internal fixation, or resection arthroplasty, the variation in individual patient pain may call for a more robust protocol. Additionally, as surgeons expand to more complex procedures performed in the outpatient setting, evaluating different combinations of analgesics taken in a more structured manner may provide for improved pain control.
A major component of patient satisfaction is postoperative pain control.3-8,12,16,17 Regional anesthesia is an important tool that allows patients to undergo a surgical procedure with a greatly reduced amount of opioid pain medications. In addition, regional anesthesia can provide significant pain control after the patient has left the ambulatory surgery center, but this relief is short-lived because the medication is designed to lose effectiveness over time. As the effects of regional anesthesia wear off, patients can experience “rebound pain” with severe levels of pain that, on occasion, cannot be controlled with oral analgesics alone. The addition of ketorolac provided improved pain control when compared with the other regimens during this transition period when the regional anesthesia was becoming ineffective. In addition, because patients taking ketorolac used less narcotic medication, they experienced less nausea, vomiting, and over-sedation.
Additionally, patients were instructed to record their medication usage and pain scores on a prospective basis, with the hope of eliminating recall bias. A potential weakness is the inability to show significance for pain relief and reduced narcotic usage with the addition of ketorolac, although there was a trend toward significance. Many of the patients who enrolled in the trial did not return their medication logs. While these patients had to be excluded from data analysis, we continued enrollment until we obtained an adequate number of patients in each group. In addition, in the OxyContin group (Group 3), we could only recruit 17 participants, instead of the 18 needed based on our power study. Although this has a potential to alter the significance of our results, we do not feel this had a substantial impact on our results.
Many patients in the non-ketorolac groups supplemented their medication regimens with NSAIDs, which may have falsely lowered pain scores and narcotic usage. While this confounds our study results, we do not believe that it invalidates the conclusion that ketorolac can be an effective adjunct pain medication for use in patients undergoing ambulatory hand surgery.
The study examined postoperative pain control for only 2 procedures, thumb basal joint arthroplasty and distal radius fracture fixation, both commonly performed in the outpatient setting under regional anesthesia and both typically requiring narcotic pain medication. Perhaps the utilization of these medication regimens with different surgical procedures would have differing results.
We conclude that ketorolac potentially provides patients with improved pain control over the use of narcotic pain medications alone in the setting of ambulatory hand surgery.
This paper will be judged for the Resident Writer’s Award.
- Boezaart AP, Davis G, Le-Wendling L. Recovery after orthopedic surgery: techniques to increase duration of pain control. Curr Opin Anaesthesiol. 2012;25(6):665-672. doi:10.1097/ACO.0b013e328359ab5a.
- Buvanendran A, Kroin JS. Useful adjuvants for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2007;21(1):31-49. doi:10.1016/j.bpa.2006.12.003.
- Coluzzi F, Bragazzi L, Di Bussolo E, Pizza G, Mattia C. Determinants of patient satisfaction in postoperative pain management following hand ambulatory day-surgery. Minerva Med. 2011;102(3):177-186.
- Elvir-Lazo OL, White PF. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28(2):217-224. doi: 10.1016/j.anclin.2010.02.011.
- Kopp SL, Horlocker TT. Regional anaesthesia in day-stay and short-stay surgery. Anaesthesia. 2010;65(Suppl 1):84-96. doi:10.1111/j.1365-2044.2009.06204.x.
- Rawal N. Postoperative pain treatment for ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2007;21(1):129-148. doi:10.1016/j.bpa.2006.11.005.
- Schug SA, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2009;22(6):738-743. doi:10.1097/ACO.0b013e32833020f4.
- Sripada R, Bowens C Jr. Regional anesthesia procedures for shoulder and upper arm surgery upper extremity update--2005 to present. Int Anesthesiol Clin. 2012;50(1):26-46. doi:10.1097/AIA.0b013e31821a0284.
- Trompeter A, Camilleri G, Narang K, Hauf W, Venn R. Analgesia requirements after interscalene block for shoulder arthroscopy: the 5 days following surgery. Arch Orthop Trauma Surg. 2010;130(3):417-421. doi:10.1007/s00402-009-0959-9.
- Dufeu N, Marchand-Maillet F, Atchabahian A, et al. Efficacy and safety of ultrasound-guided distal blocks for analgesia without motor blockade after ambulatory hand surgery. J Hand Surg Am. 2014;39(4):737-743. doi:10.1016/j.jhsa.2014.01.011.
- Gutta R, Koehn CR, James LE. Does ketorolac have a preemptive analgesic effect? A randomized, double-blind, control study. J Oral Maxillofac Surg. 2013;71(12):2029-2034. doi:10.1016/j.joms.2013.06.220.
- Nossaman VE, Ramadhyani U, Kadowitz PJ, Nossaman BD. Advances in perioperative pain management: use of medications with dual analgesic mechanisms, tramadol & tapentadol. Anesthesiol Clin. 2010;28(4):647-666. doi:10.1016/j.anclin.2010.08.009.
- Warren-Stomberg M, Brattwall M, Jakobsson JG. Non-opioid analgesics for pain management following ambulatory surgery: a review. Minerva Anestesiol. 2013;79(9):1077-1087.
- Wickerts L, Warrén Stomberg M, Brattwall M, Jakobsson JJ. Coxibs: is there a benefit when compared to traditional non-selective NSAIDs in postoperative pain management? Minerva Anestesiol. 2011;77(11):1084-1098.
- Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA. 1996;275(5):376-382. doi:10.1001/jama.275.5.376.
- Hegarty M, Calder A, Davies K, et al. Does take-home analgesia improve postoperative pain after elective day case surgery? A comparison of hospital vs parent-supplied analgesia. Paediatr Anaesth. 2013;23(5):385-389. doi:10.1111/pan.12077.
- Weber SC, Jain R, Parise C. Pain scores in the management of postoperative pain in shoulder surgery. Arthroscopy. 2007;23(1):65-72. doi:10.1016/j.arthro.2006.11.002.
- Boezaart AP, Davis G, Le-Wendling L. Recovery after orthopedic surgery: techniques to increase duration of pain control. Curr Opin Anaesthesiol. 2012;25(6):665-672. doi:10.1097/ACO.0b013e328359ab5a.
- Buvanendran A, Kroin JS. Useful adjuvants for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2007;21(1):31-49. doi:10.1016/j.bpa.2006.12.003.
- Coluzzi F, Bragazzi L, Di Bussolo E, Pizza G, Mattia C. Determinants of patient satisfaction in postoperative pain management following hand ambulatory day-surgery. Minerva Med. 2011;102(3):177-186.
- Elvir-Lazo OL, White PF. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28(2):217-224. doi: 10.1016/j.anclin.2010.02.011.
- Kopp SL, Horlocker TT. Regional anaesthesia in day-stay and short-stay surgery. Anaesthesia. 2010;65(Suppl 1):84-96. doi:10.1111/j.1365-2044.2009.06204.x.
- Rawal N. Postoperative pain treatment for ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2007;21(1):129-148. doi:10.1016/j.bpa.2006.11.005.
- Schug SA, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2009;22(6):738-743. doi:10.1097/ACO.0b013e32833020f4.
- Sripada R, Bowens C Jr. Regional anesthesia procedures for shoulder and upper arm surgery upper extremity update--2005 to present. Int Anesthesiol Clin. 2012;50(1):26-46. doi:10.1097/AIA.0b013e31821a0284.
- Trompeter A, Camilleri G, Narang K, Hauf W, Venn R. Analgesia requirements after interscalene block for shoulder arthroscopy: the 5 days following surgery. Arch Orthop Trauma Surg. 2010;130(3):417-421. doi:10.1007/s00402-009-0959-9.
- Dufeu N, Marchand-Maillet F, Atchabahian A, et al. Efficacy and safety of ultrasound-guided distal blocks for analgesia without motor blockade after ambulatory hand surgery. J Hand Surg Am. 2014;39(4):737-743. doi:10.1016/j.jhsa.2014.01.011.
- Gutta R, Koehn CR, James LE. Does ketorolac have a preemptive analgesic effect? A randomized, double-blind, control study. J Oral Maxillofac Surg. 2013;71(12):2029-2034. doi:10.1016/j.joms.2013.06.220.
- Nossaman VE, Ramadhyani U, Kadowitz PJ, Nossaman BD. Advances in perioperative pain management: use of medications with dual analgesic mechanisms, tramadol & tapentadol. Anesthesiol Clin. 2010;28(4):647-666. doi:10.1016/j.anclin.2010.08.009.
- Warren-Stomberg M, Brattwall M, Jakobsson JG. Non-opioid analgesics for pain management following ambulatory surgery: a review. Minerva Anestesiol. 2013;79(9):1077-1087.
- Wickerts L, Warrén Stomberg M, Brattwall M, Jakobsson JJ. Coxibs: is there a benefit when compared to traditional non-selective NSAIDs in postoperative pain management? Minerva Anestesiol. 2011;77(11):1084-1098.
- Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA. 1996;275(5):376-382. doi:10.1001/jama.275.5.376.
- Hegarty M, Calder A, Davies K, et al. Does take-home analgesia improve postoperative pain after elective day case surgery? A comparison of hospital vs parent-supplied analgesia. Paediatr Anaesth. 2013;23(5):385-389. doi:10.1111/pan.12077.
- Weber SC, Jain R, Parise C. Pain scores in the management of postoperative pain in shoulder surgery. Arthroscopy. 2007;23(1):65-72. doi:10.1016/j.arthro.2006.11.002.
TAKE-HOME POINTS
- While regional anesthesia is safe and effective for patients who undergo ambulatory hand surgery, patients often experience rebound pain as it wears off.
- We tested a multimodal approach for patients who underwent thumb CMC arthroplasty or ORIF of distal radius fracture.
- Patients were provided with a journal and asked to record medication usage, a NPS, and adverse effects. Seventy-nine patients completed the study.
- We found that adding ketorolac to the postoperative pain protocol, with detailed instructions, lowered narcotic usage in the first 4 postoperative days.
- Ketorolac potentially provides patients with improved pain control over the use of narcotic pain medication alone in an ambulatory hand surgery setting.
Coverage of Hand Defects with Exposed Tendons: The Use of Dermal Regeneration Template
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
TAKE-HOME POINTS
- Full thickness skin grafts are generally considered unreliable for coverage of 3-dimensional defects of the hand with tendon exposure.
- Integra (Integra LifeSciences) is a bilayer skin substitute. The “dermal” (lower) layer is a bovine collagen base with glycosaminoglycan chondroitin-6-sulfate while the upper layer is a silicone sheet that acts as a temporary epidermis.
- Despite its popularity of Integra in burn reconstruction, little has been published regarding its utility in complex hand wounds with exposed tendons.
- Small areas of exposed tendons without remaining paratenon can be successfully grafted with Integra.
- In the presence of a healthy wound bed and no necrotic tissue or infection, Integra offers a reconstructive option that allows immediate coverage of complex hand wounds.
A Novel Technique for the Treatment of Jersey Fingers
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
TAKE-HOME POINTS
- Transosseous repair of FDP has been long utilized, tying the sutures over a polyethylene button at the nail plate, which is associated with significant complications.
- Avoiding use of a button decreases these complications, eliminating damage to the nailbed and eliminating external sutures, thus decreasing infection risk.
- Keith needles can be utilized to pass the sutures from volar to dorsal, and can be inserted using a wire drive; their position can be checked fluoroscopically prior to suture passage.
- This technique can be used in conjunction with skeletal fixation of associated fractures.
- This technique can be utilized in pediatric patients, placing the sutures distal to the physis.
Dual Radial Styloid and Volar Plating for Unstable Fractures of the Distal Radius
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).
A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.
At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.
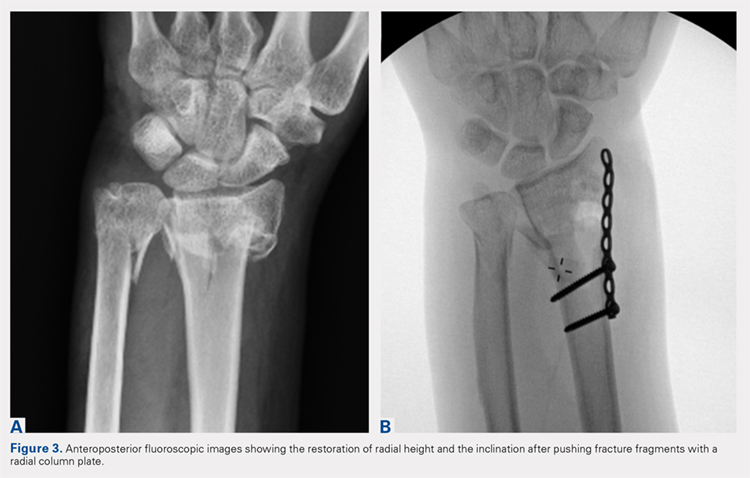
At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).

A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.

At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.

At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.

MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).

A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.

At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.

At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.

MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
TAKE-HOME POINTS
- Radial column fixation can be used as a reduction tool in unstable distal radius fractures.
- Radial column fixation can help maintain reduction until union in unstable distal radius fractures when combined with volar plating.
- When operating without an assistant, radial column plating can assist in reduction maintenance when other techniques are not successful and holding a reduction manually is not possible.
- Acceptable clinical and radiographic outcomes can be achieved with the use of dual radial styloid and volar plating for unstable distal radius fractures.
- The effects of increased dissection during radial column fixation in distal radius fractures with regard to scar formation and wrist ROM is currently not known.
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
Treating Unstable Distal Radius Fractures With a Nonspanning External Fixation Device: Comparison With Volar Locking Plates in Historical Control Group
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Carpal tunnel syndrome may flag cardiac amyloidosis in elderly
DALLAS – Older patients with carpal tunnel syndrome that requires release surgery appear to have a relatively high prevalence of amyloidosis that, in some, involves their heart, suggesting that routine screening for amyloidosis is warranted in elderly patients undergoing the surgery.
Routine Congo red staining of a tenosynovial biopsy taken at the time of carpal tunnel release surgery in a single-center experience with 96 patients showed that 10 (10%) were positive for amyloidosis, Mazen Hanna, MD, said at the annual scientific meeting of the Heart Failure Society of America.
Clinicians “should be aware of the association between carpal tunnel syndrome [CTS] and amyloidosis.” When a 60-year old shows up with bilateral CTS without a clear cause, it’s reasonable to suspect amyloidosis, he suggested.
The prospective study run by Dr. Hanna and his associates included men at least 50 years old and women at least 60 years old who underwent CTS release surgery at the Cleveland Clinic during May 2016–June 2017. Enrollment excluded patients with known amyloidosis or rheumatoid arthritis. The patients averaged 68 years of age, 51% were men, and 85% had bilateral CTS that required surgery. The surgeons removed a tenosynovial biopsy at the time of surgery from each of the 96 patients, a “low-risk procedure,” Dr. Hanna said.
The 10 patients with positive staining for amyloid underwent a work-up that included a comprehensive physical examination, a series of blood tests for cardiac biomarkers, an ECG, echocardiography including assessment of cardiac strain, and a technetium-99m pyrophosphate scan. This identified two patients with cardiac involvement. The examinations identified one case by the echocardiographic strain findings and the second case by the technetium pyrophosphate scan. Seven of the 10 patients with amyloid had a history of prior carpal tunnel release surgery.
The researchers also used mass spectroscopy to identify the amyloid type. Seven patients had the transthyretin subtype, including one patient with cardiac involvement; two patients had light chain amyloidosis, including the second patient with cardiac involvement. The tenth patient had inconclusive results but the researchers presumed the amyloid was of the transthyretin type, Dr. Hanna said.
The eight patients identified with amyloid but no cardiac involvement at baseline will continue to receive annual work ups to see whether their hearts become affected over time. The protocol delays a repeat technetium pyrophosphate scan until the 4th year following study entry.
The potential usefulness of early identification and treatment of cardiac amyloidosis received support in results from another study reported at the meeting. Researchers from Columbia University Medical Center, New York, and New York Presbyterian Hospital reported their retrospective, nonrandomized experience with 126 patients who had been diagnosed with transthyretin cardiac amyloidosis. Thirty of these patients had received treatment with a transthyretin-stabilizing drug, either the investigational agent tafamidis or diflunisal, while the other 96 patients received no stabilizing treatment. During a median follow-up of 2 years, patients treated with a stabilizing agent had a statistically significant 68% reduced rate of either death or orthotopic heart transplant, compared with the untreated patients in a multivariate analysis that controlled for various baseline differences between the treated and untreated patients.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
DALLAS – Older patients with carpal tunnel syndrome that requires release surgery appear to have a relatively high prevalence of amyloidosis that, in some, involves their heart, suggesting that routine screening for amyloidosis is warranted in elderly patients undergoing the surgery.
Routine Congo red staining of a tenosynovial biopsy taken at the time of carpal tunnel release surgery in a single-center experience with 96 patients showed that 10 (10%) were positive for amyloidosis, Mazen Hanna, MD, said at the annual scientific meeting of the Heart Failure Society of America.
Clinicians “should be aware of the association between carpal tunnel syndrome [CTS] and amyloidosis.” When a 60-year old shows up with bilateral CTS without a clear cause, it’s reasonable to suspect amyloidosis, he suggested.
The prospective study run by Dr. Hanna and his associates included men at least 50 years old and women at least 60 years old who underwent CTS release surgery at the Cleveland Clinic during May 2016–June 2017. Enrollment excluded patients with known amyloidosis or rheumatoid arthritis. The patients averaged 68 years of age, 51% were men, and 85% had bilateral CTS that required surgery. The surgeons removed a tenosynovial biopsy at the time of surgery from each of the 96 patients, a “low-risk procedure,” Dr. Hanna said.
The 10 patients with positive staining for amyloid underwent a work-up that included a comprehensive physical examination, a series of blood tests for cardiac biomarkers, an ECG, echocardiography including assessment of cardiac strain, and a technetium-99m pyrophosphate scan. This identified two patients with cardiac involvement. The examinations identified one case by the echocardiographic strain findings and the second case by the technetium pyrophosphate scan. Seven of the 10 patients with amyloid had a history of prior carpal tunnel release surgery.
The researchers also used mass spectroscopy to identify the amyloid type. Seven patients had the transthyretin subtype, including one patient with cardiac involvement; two patients had light chain amyloidosis, including the second patient with cardiac involvement. The tenth patient had inconclusive results but the researchers presumed the amyloid was of the transthyretin type, Dr. Hanna said.
The eight patients identified with amyloid but no cardiac involvement at baseline will continue to receive annual work ups to see whether their hearts become affected over time. The protocol delays a repeat technetium pyrophosphate scan until the 4th year following study entry.
The potential usefulness of early identification and treatment of cardiac amyloidosis received support in results from another study reported at the meeting. Researchers from Columbia University Medical Center, New York, and New York Presbyterian Hospital reported their retrospective, nonrandomized experience with 126 patients who had been diagnosed with transthyretin cardiac amyloidosis. Thirty of these patients had received treatment with a transthyretin-stabilizing drug, either the investigational agent tafamidis or diflunisal, while the other 96 patients received no stabilizing treatment. During a median follow-up of 2 years, patients treated with a stabilizing agent had a statistically significant 68% reduced rate of either death or orthotopic heart transplant, compared with the untreated patients in a multivariate analysis that controlled for various baseline differences between the treated and untreated patients.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
DALLAS – Older patients with carpal tunnel syndrome that requires release surgery appear to have a relatively high prevalence of amyloidosis that, in some, involves their heart, suggesting that routine screening for amyloidosis is warranted in elderly patients undergoing the surgery.
Routine Congo red staining of a tenosynovial biopsy taken at the time of carpal tunnel release surgery in a single-center experience with 96 patients showed that 10 (10%) were positive for amyloidosis, Mazen Hanna, MD, said at the annual scientific meeting of the Heart Failure Society of America.
Clinicians “should be aware of the association between carpal tunnel syndrome [CTS] and amyloidosis.” When a 60-year old shows up with bilateral CTS without a clear cause, it’s reasonable to suspect amyloidosis, he suggested.
The prospective study run by Dr. Hanna and his associates included men at least 50 years old and women at least 60 years old who underwent CTS release surgery at the Cleveland Clinic during May 2016–June 2017. Enrollment excluded patients with known amyloidosis or rheumatoid arthritis. The patients averaged 68 years of age, 51% were men, and 85% had bilateral CTS that required surgery. The surgeons removed a tenosynovial biopsy at the time of surgery from each of the 96 patients, a “low-risk procedure,” Dr. Hanna said.
The 10 patients with positive staining for amyloid underwent a work-up that included a comprehensive physical examination, a series of blood tests for cardiac biomarkers, an ECG, echocardiography including assessment of cardiac strain, and a technetium-99m pyrophosphate scan. This identified two patients with cardiac involvement. The examinations identified one case by the echocardiographic strain findings and the second case by the technetium pyrophosphate scan. Seven of the 10 patients with amyloid had a history of prior carpal tunnel release surgery.
The researchers also used mass spectroscopy to identify the amyloid type. Seven patients had the transthyretin subtype, including one patient with cardiac involvement; two patients had light chain amyloidosis, including the second patient with cardiac involvement. The tenth patient had inconclusive results but the researchers presumed the amyloid was of the transthyretin type, Dr. Hanna said.
The eight patients identified with amyloid but no cardiac involvement at baseline will continue to receive annual work ups to see whether their hearts become affected over time. The protocol delays a repeat technetium pyrophosphate scan until the 4th year following study entry.
The potential usefulness of early identification and treatment of cardiac amyloidosis received support in results from another study reported at the meeting. Researchers from Columbia University Medical Center, New York, and New York Presbyterian Hospital reported their retrospective, nonrandomized experience with 126 patients who had been diagnosed with transthyretin cardiac amyloidosis. Thirty of these patients had received treatment with a transthyretin-stabilizing drug, either the investigational agent tafamidis or diflunisal, while the other 96 patients received no stabilizing treatment. During a median follow-up of 2 years, patients treated with a stabilizing agent had a statistically significant 68% reduced rate of either death or orthotopic heart transplant, compared with the untreated patients in a multivariate analysis that controlled for various baseline differences between the treated and untreated patients.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Ten of 96 patients undergoing carpal tunnel release surgery had amyloidosis, and two had cardiac involvement.
Data source: Prospective, single-center series of 96 patients undergoing carpal tunnel release surgery.
Disclosures: Dr. Hanna had no disclosures.
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Distal Radius Fractures: Reconstruction Approaches, Planning, and Principles
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84