User login
Unicondylar Knee Arthroplasty in the U.S. Patient Population: Prevalence and Epidemiology
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
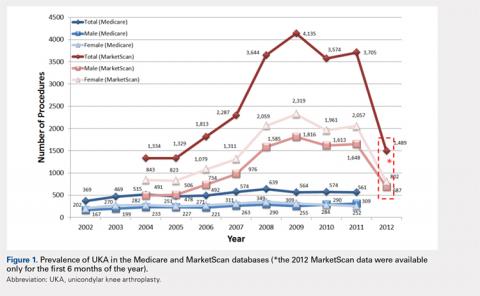
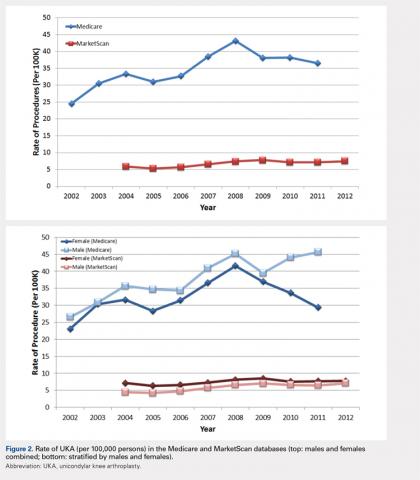
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
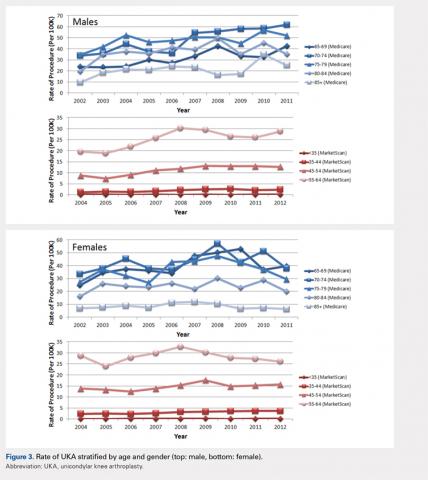
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
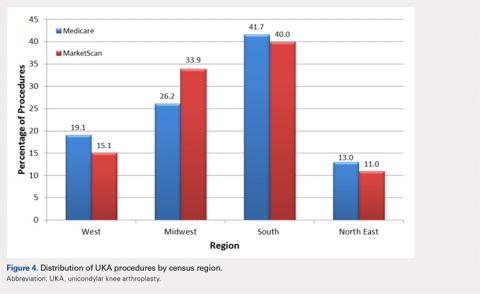
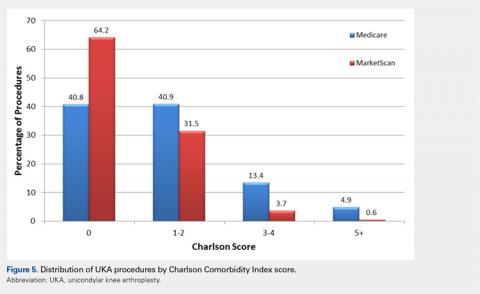
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
ABSTRACT
Publications on the prevalence of unicompartmental knee arthroplasty in the United States using a single database may have underestimated the “true” number of cases performed, given that several unicondylar knee arthroplasty (UKA) patients are <65 years and have private insurance. The prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations was evaluated using the 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases, respectively. The prevalence of UKA was stratified by age, gender, census region, Charlson comorbidity index, Medicare buy-in status, and diagnosis. The annual rate of change in the UKA rate was examined using Poisson regression to evaluate temporal changes considering year as a covariate.
A total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. The rates of UKA generally increased until 2008, after which there was a decline. In both cohorts, gender and year of operation were found to be significantly associated with UKA rate. Analysis of data obtained over the past few years revealed that males 55 to 64 years, 65 to 69 years, and 70 to 74 years were the only age-gender groups whose UKA rates appeared to be trending upward.
From 2002 to 2011, the rate of UKAs performed in the United States has increased, and a significant proportion of the surgeries were performed in younger (<65 years) patients.
Continue to: Unicondylar knee arthroplasty...
Unicondylar knee arthroplasty (UKA) is an effective surgical treatment for symptomatic degenerative joint disease of a single compartment of the knee, providing improved functional outcomes compared with total knee arthroplasty (TKA).1-3 It has been estimated that the proportion of patients undergoing TKA, who meet the criteria for UKA, varies between 21% and 47%.4,5 However, it has been variably estimated that the usage of UKA ranges from 0% to 50% (mean, 8%) of all primary knee arthroplasties.5-8 It is believed that this discrepancy between the percentage of patients who meet indications for the surgery and those who receive it is associated with various factors, including surgeon training and experiences, diverse indications, economic factors, as well as acknowledgment of the reportedly higher revision rates of UKA than those of TKA in national joint registries.7,9-11
According to their classic article, Kozinn and Scott12 outlined the indications for UKA that, in their experience, led to the most successful outcomes, including age >60 years, weight <82 kg, low physical demand, localized arthritis with no full-thickness chondromalacia elsewhere in the joint, intact anterior cruciate ligament, minimal deformity, and flexion >90°. More recently, indications have been expanded to include younger and more active patients, higher body mass index, and some patterns of patellofemoral chondromalacia, with an increasing number of publications reporting successful clinical outcomes in these cohorts as well.13-17 Taken together, it is clear that the “classic” strict indications for UKA can be safely expanded, which have and will result in an increased number of these procedures being performed above and beyond that which might be predicted based on demographic trends alone.
A growing body of literature has been published on the prevalence and projections of orthopedic procedures in the United States.18-20 Several studies have focused their analysis on 1 of several large administrative databases, including the Nationwide Inpatient Sample, the 5% Medicare Part B database, and the National Hospital Discharge Survey.18,20-23 A concern with limiting an analysis of the prevalence of unicompartmental knee arthroplasty to these particular databases is that it may underestimate the “true” number of cases performed in the United States, given that several UKA patients are <65 years and have private insurance, and therefore, would not be captured statistically by a database that collects data on patients ≥65 years.
The purpose of this study was to quantify the current prevalence and epidemiology of UKA in the U.S. patient population. Our hypothesis was that the number of procedures and the procedural rate of UKA are increasing over time. Furthermore, this increase may be attributed to an increase in select age- or gender-based segments of the population. To test this hypothesis, we analyzed 2 separate large claims databases to capture patients over a spectrum of age and inclusive of both private and public payers, including the 5% Medicare Part B database (2002–2011) for patients ≥65 years and the MarketScan database (2004 to June 2011) for patients <65 years. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, and physicians.
MATERIALS AND METHODS
The 2002 to 2011 5% sample of the Medicare data (Part B) and the 2004 to June 2012 MarketScan Commercial and Medicare Supplemental databases were used to evaluate the prevalence of UKA in elderly (≥65 years) and younger (<65 years) populations, respectively. The UKA procedures were identified using the CPT code 27446.
The prevalence of UKA was stratified by age, gender, census region, Charlson Comorbidity Index, Medicare buy-in status, and diagnosis. The buy-in status is a proxy for the socioeconomic status as it reflects the state subsidizing the health insurance premium for the beneficiary. The Charlson Comorbidity Index is a composite score that has been used to assess the comorbidity level of a patient by taking into account the number and the severity of comorbid conditions.24 For the elderly population, the rate of UKA was subsequently evaluated based on the number of beneficiaries for that particular age-gender group and year in both databases. Poisson regression was used to evaluate the annual rate of change in the UKA rate for assessing temporal changes considering year as a covariate. Age and gender, as well as 2-way interaction terms for age, gender, and year, were also considered as covariates.
Continue to: RESULTS...
RESULTS
For the time periods analyzed, a total of 5235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively. A peak in the prevalence appeared around 2008 for the elderly population and in 2009 for the younger population (Figure 1). When normalized by the size of the population segment, the rate of UKA was found to be approximately 5 times greater in the elderly population, increasing from 369 in 2002 to 639 in 2008, but plateauing to 561 in 2011. Extrapolating to the 100% Medicare population, these numbers increased to 7380, 12,780, and 11,220, respectively. Temporal changes in the UKA rate were significant, increasing from 24.5 UKAs per 100,000 persons in 2002 to 43.1 UKAs in 2008, followed by a decline to 36.5 in 2011 (P < .0001) (Figure 2). The rates of UKA generally increased from 2002 to 2008 for both males and females in the Medicare cohort; however, the rates of UKA in female patients continuously declined from 2008 onward, whereas the UKA rates in male patients decreased in 2009, followed by an increase in 2010 and 2011 (Figure 2). For the younger population, there was a slight increase in the rate of UKA from 2004 to approximately 2009, after which the rates for both males and females remained relatively steady. When put in the context of the prevalence of TKA, the prevalence of UKA fluctuated during the same time period. In the Medicare population, the prevalence of UKA ranged from 4.3% (2005) to 5.9% (2008) of the TKA prevalence between 2002 and 2011. In the younger MarketScan population, the prevalence of UKA ranged from 6.7% (2005) to 8.9% (2008) between 2004 and June 2012.
The UKA rate differed significantly according to gender (P = .0209), with higher rates for males. Although there were no age-related differences (P = .3723), age–gender interactions were found to be significant (P < .0001). For males, the largest rate of UKA in the most recent year of data was observed in the 70- to 74-year-old group, followed by the 75- to 79- and the 65- to 69-year-old groups (Figure 3). For females, those in the 65- to 69- and the 70- to 74-year-old groups had the highest rate of UKA. In the younger cohort, there were increases in the UKA rates since 2004. These rates appeared to be relatively stable from the 2008 or 2009 period onward, except for females 55–64 years, which demonstrated a steady decline since 2008. Analysis of data obtained over the past few years showed that males 55–64, 65–69, and 70–74 years were the only age–gender groups whose UKA rates appeared to be trending upward.
The vast majority of elderly UKA patients were white (95.5%), and when stratified by census region, the highest proportion of UKA procedures was observed in the South and the Midwest (Figure 4). Furthermore, among patients <65 years, 64.2% had a Charlson score of 0 compared to 40.8% in the elderly group (Figure 5). For the Medicare population, based on their receipt of state subsidies for their insurance premiums, 5.1% of patients were of lower socioeconomic status. Osteoarthritis was diagnosed in 99.4% and 97.3% of the MarketScan and Medicare cohorts, respectively.
In the Medicare cohort, gender (P = .0209) and year of operation (P < .0001) were found to be significantly associated with the rate of UKA, along with age-gender (P < .0001) and gender-year (P = .0202) interaction terms. In the MarketScan cohort, age (P = .0173), gender (P = .0017), and year of operation (P = .0002) were found to be significantly associated with UKA rate. Two-way interactions between age-gender (P = .0018), age–year (P = .0207), and gender-year (P = .0017) were also found to be statistically significant factors.
Continue to: DISCUSSION...
DISCUSSION
The results of our study indicate that between 2002 and 2011, a steadily increasing number of UKA procedures was performed in the United States, and a significant proportion of the surgeries was performed on patients <65 years. Without the MarketScan database data, we would have missed more than 23,000 UKA cases performed during this 10-year time period. This finding validates our research methodology that incorporated data on privately insured younger (<65 years) patients, which is something that has not been done when examining the epidemiology of UKA.
To our knowledge, there are only 2 other publications attempting to quantify the incidence of UKA procedures performed in the United States. Bolognesi and colleagues23 used the Medicare 5% sample to assess trends in the use of knee arthroplasty from 2000 to 2009. The authors reported that a total of 68,603 patients underwent unilateral total knee arthroplasty (n = 65,505) or unicompartmental knee arthroplasty (n = 3098) over this 10-year time period. Given that there is substantial overlap of our time periods, it is not surprising that our Medicare numbers are similar (3098 vs 5235). In their study, the use of TKA increased 1.7-fold, whereas the use of UKA increased 6.2-fold23. In our analysis of the Medicare (2011 vs 2002) and MarketScan (2011 vs 2004) databases, there was a 1.3-fold and a 3.4-fold increase in the number of TKAs performed. Concomitantly, the use of UKA increased 1.5-fold and 2.8-fold, respectively, in these databases over the same time periods. The reason for the slight discrepancy in the numbers may be attributable to the peak occurring in 2008. The other publication on the subject by Riddle and colleagues8 focused on the time period 1998 to 2005 and used implant manufacturer’s sales data cross-referenced to a database of 44 hospitals to derive their national estimates. Using their unique methodology, the authors calculated an incidence of UKA, ranging from 6570 implants in 1998 to 44,990 in 2005. They reported that UKA use during the study period increased by 3 times the rate of TKA in the United States, with an average yearly percentage increase in the number of UKA procedures of 32.5% compared to 9.4% for TKA procedures. It is difficult to account for the discrepancy in the number of UKAs performed reported between our current study and that of Riddle and colleagues;8 however, the fact that the authors used implant manufacturer’s individual sales numbers may indicate that a portion of UKA patients was not captured in either the Medicare 5% or the MarketScan database. Nonetheless, in our analysis, the annual increase in the number of UKA procedures performed during the time periods studied averaged 5.8% in the older population and 25.4% in the younger population compared to the increase in the number of TKA procedures, which averaged 3.6% and 33.9% in the older and younger populations, respectively. In addition, in our study, the percentage of UKAs performed relative to the number of TKAs during the time intervals studied varied from a low of 4.3% to a high of 5.9% in the older population and from a low of 6.7% to a high of 8.9% in the younger population.
During the 10-year period of this study, a general upward trend appeared in the total number of unicompartmental knee arthroplasties performed in both the Medicare and the MarketScan databases. The rate at which the procedure was performed increased in the Medicare population from 24.5 to 36.5 (per 100,000 persons) over a 10-year time period and in the MarketScan cohort from 5.9 to 7.4 (per 100,000 persons) over an 8.5-year time period. This indicates both a larger absolute and a relative rate increase in UKA procedures in the elderly population. Around 2008 and 2009, the data showed a slight dip in the rate of UKA in the Medicare population and a plateau in the rate in the MarketScan database. Although this may be a spurious finding in the data that would be smoothed out with a longer time period investigated, it is interesting that this finding coincided with a national economic downturn. Although it might be expected that macroeconomics may affect the utilization of elective surgery such as total joint replacement, Kurtz and colleagues25 investigated this particular question and found that neither the economic downturns of 2001 or those of 2008 and 2009 had a significant impact on the incidence of total joint replacement surgeries.
Incorporation of the MarketScan database data indicated that a significant proportion of patients undergoing UKA were <65 years and that there was a slight but increasing rate of procedures performed on this age cohort over the past decade. A similar finding has been reported in the Finnish Arthroplasty Registry. Leskinen and colleagues26 reported that the incidence of UKAs among individuals 30 to 59 years increased from 0.2 (per 100,000 persons) to 10 (per 100,000 persons) from 1980 to 2006 and that most of the increase occurred among patients 50 to 59 years. The fact that younger age is no longer observed as a relative contraindication to this procedure is supported by several clinical investigations. Cartier and colleagues27 reported 93% survival at 10 years in patients with a mean age of 65 years, but included patients as young as 28 years, claiming that the results for younger patients were no worse than those for older patients in the series. Pandit and colleagues17 compared the results of 245 young patients (<60 years) to those of 755 older patients (>60 years) and found a survival rate of 97% at 10 years, with no significant difference in mean functional outcomes, failure rate, or survival between the groups at >5 years of follow-up. Given that patients <65 years now account for approximately half of the TKAs performed each year, with the greatest increase in volume among patients between 45 and 54, it is clear that investigations on the epidemiology of UKA must take into account this increasingly relevant younger patient cohort.28
Continue to: Our data indicate...
Our data indicate that only approximately 5% of UKA patients were non-white and another 5% were from lower socioeconomic status. These findings have been observed in multiple other studies looking at the epidemiology of total joint replacement in the United States.29 Bolognesi and colleagues23 reported that although “non-white race” patients made up 12% of the general Medicare sample they were analyzing, these patients accounted for only 5% and 3% of the total knee arthroplasty and unicompartmental knee arthroplasty populations, respectively. Although it is beyond the scope of this paper to delve into the reasons for this discrepancy, it may be related to differences in access to care, healthcare literacy, and trust of patients in the healthcare system.30,31
Our study, like all those based on administrative claims, has several notable inherent limitations. Coding inaccuracies as well as the potential for systematic bias (eg, underreporting) may affect the accuracy of our results. Although the MarketScan Commercial Research Database (Truven Health Analytics) includes nationally representative information for >180 million patients covered with private insurance, it is possible that we have missed some patients who underwent UKA during the time period investigated. However, we feel that the number missed is probably small and does not affect our conclusions in any meaningful manner.
CONCLUSION
This novel analysis of 2 separate administrative claims databases, which more accurately captures all patients undergoing UKA, indicates that there has been a steady increase in the rate of the procedure over the past decade and that a significant proportion of the surgeries were performed in younger (<65 years) patients. Understanding the accurate trends in the use of UKA on a national scale is important for legislative bodies, healthcare administrators, as well as physicians. Furthermore, given the increasing rates of UKA in patients <65 years old, and the increased burden on implants for withstanding increased activities and repetitive loads, it remains imperative to strive to optimize materials, implant designs, and surgical techniques to enhance implant durability.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979. doi: 10.1007/s00167-008-0596-9.
- Lygre SH, Espehaug B, Havelin LI, Furnes O, Vollset SE. Pain and function in patients after primary unicompartmental and total knee arthroplasty. J Bone Joint Surg, (Am). 2010;92(18):2890-2897. doi: 10.2106/JBJS.I.00917.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. doi: 10.1302/0301-620X.97B6.35155.
- Arno S, Maffei D, Walker PS, Schwarzkopf R, Desai P, Steiner GC. Retrospective analysis of total knee arthroplasty cases for visual, histological, and clinical eligibility of unicompartmental knee arthroplasties. J Arthroplast. 2011;26(8):1396-1403. doi: 10.1016/j.arth.2010.12.023.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee. 2009;16(6):473-478. doi: 10.1016/j.knee.2009.04.006.
- Murray DW, Liddle AD, Dodd CA, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J. 2015;97-B(10 Suppl. A):3-8. doi: 10.1302/0301-620X.97B10.36542.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet. 2014;384(9952):1437-1445. doi: 10.1016/S0140-6736(14)60419-0.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23(3):408-412. doi: 10.1016/j.arth.2007.04.012.
- Argenson JN, Blanc G, Aubaniac JM, Parratte S. Modern unicompartmental knee arthroplasty with cement: a concise follow-up, at a mean of twenty years, of a previous report. J Bone Joint Surg, (Am). 2013;95(10):905-909. doi: 10.2106/JBJS.L.00963.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507. doi: 10.1080/17453670710015490.
- Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65(4):375-386. doi: 10.3109/17453679408995475.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg, (Am). 1989;71(1):145-150. doi: 10.2106/00004623-198971010-00023.
- Pennington DW. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg, (Am). 2003;85-A(10):1968-1973. doi: 10.2106/00004623-200310000-00016.
- Biswas D, Van Thiel GS, Wetters NG, Pack BJ, Berger RA, Della Valle CJ. Medial unicompartmental knee arthroplasty in patients less than 55 years old: minimum of two years of follow-up. J Arthroplast. 2014;29(1):101-105. doi: 10.1016/j.arth.2013.04.046.
- Murray DW, Pandit H, Weston-Simons JS, et al. Does body mass index affect the outcome of unicompartmental knee replacement? Knee. 2013;20(6):461-465. doi: 10.1016/j.knee.2012.09.017.
- Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg Br. 2011;93(4):476-478. doi: 10.1302/0301-620X.93B4.25562.
- Pandit H, Jenkins C, Gill HS, et al. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br. 2011;93(5):622-628. doi: 10.1302/0301-620X.93B5.26214.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497. doi: 10.2106/JBJS.D.02441.
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg, (Am). 2007;89(Suppl. 3):144-151. doi: 10.2106/JBJS.G.00587.
- Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: 10.1016/j.jse.2010.02.009.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg, (Am). 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222.
- Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplast. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035.
- Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg, (Am). 2013;95(22):e174. doi: 10.2106/JBJS.L.00652.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8.
- Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg, (Am). 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285.
- Leskinen J, Eskelinen A, Huhtala H, Paavolainen P, Remes V. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64(2):423-428. doi: 10.1002/art.33367.
- Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast. 1996;11(7):782-788. doi: 10.1016/S0883-5403(96)80177-X.
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. doi: 10.1007/s11999-009-0834-6.
- Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107-2115. doi: 10.1136/annrheumdis-2013-203494.
- Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12): e1139-e1146. doi: 10.3928/01477447-20151123-05.
- Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817-1823. doi: 10.1007/s11999-011-1885-z.
TAKE-HOME POINTS
- Prior publications on prevalence of unicondylar knee arthroplasty (UKA) in the United States using a single database may have underestimated the “true” number of cases performed.
- For the time periods analyzed, a total of 5,235 and 23,310 UKA procedures were identified from the 5% Medicare and MarketScan databases, respectively.
- Rates of UKA generally increased until 2008, after which there was a decline through 2012.
- Gender and year of operation were found to be significantly associated with UKA rate.
- Males ages 55-64, 65-69, and 70-74 were the only age-gender groups whose UKA rates appear to be trending upward.
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
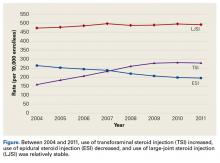
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.