User login
Does Ozempic cause hair loss?
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nevus Sebaceus With Novel HRAS Sequence Variant Mutation Misdiagnosed as Alopecia Areata
To the Editor:
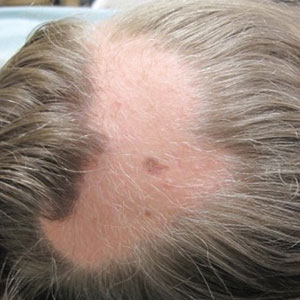
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).
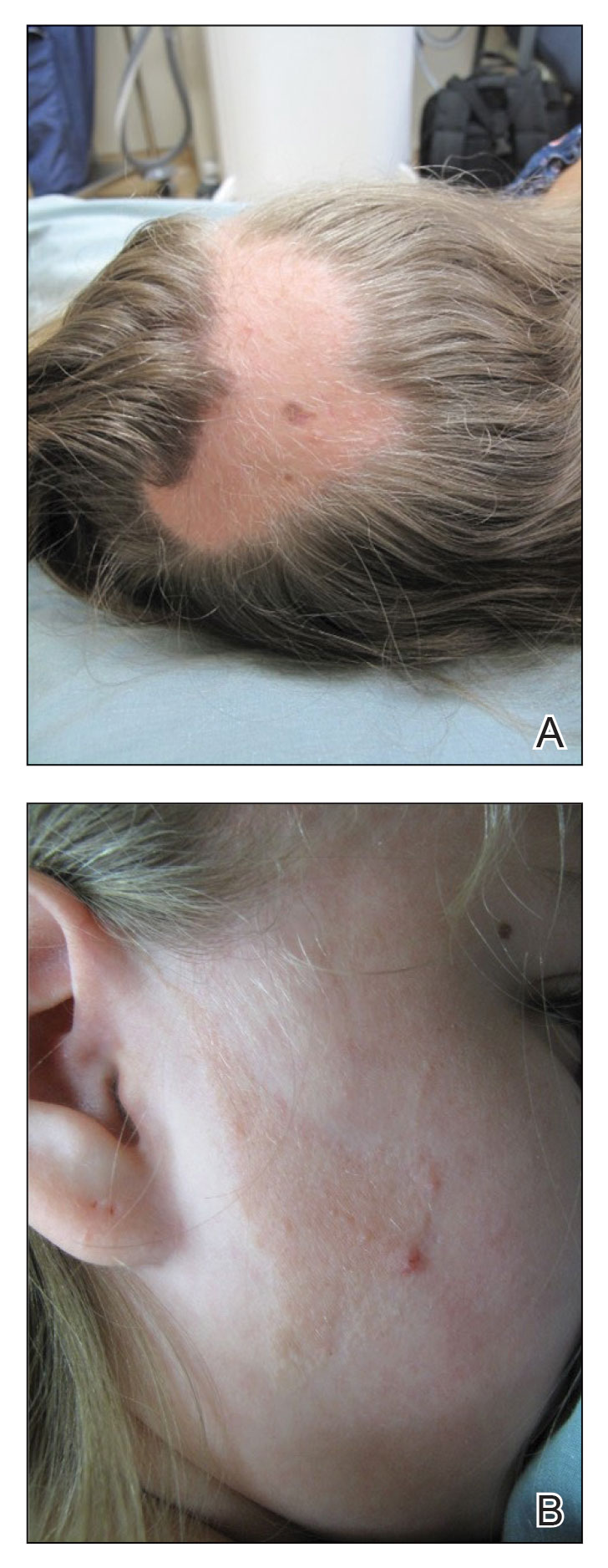
The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.
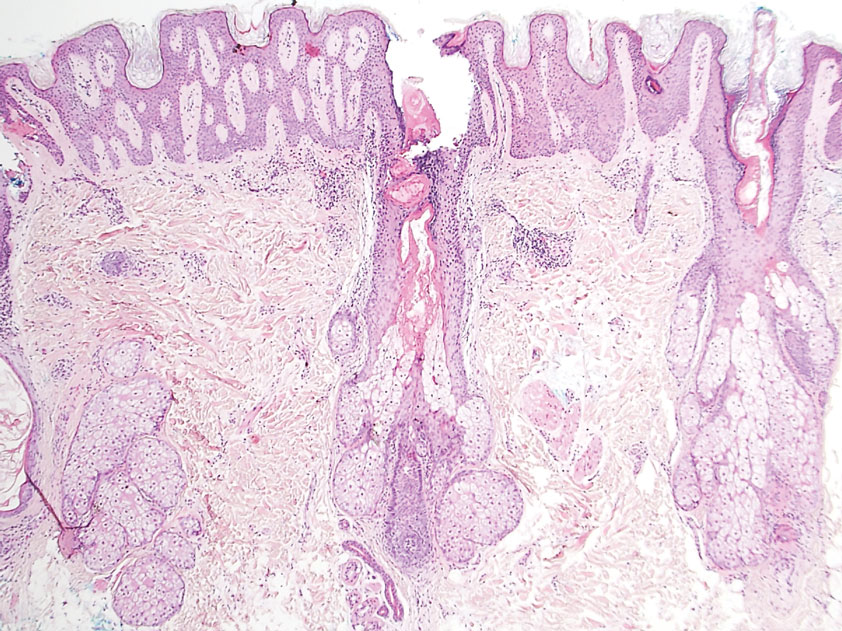
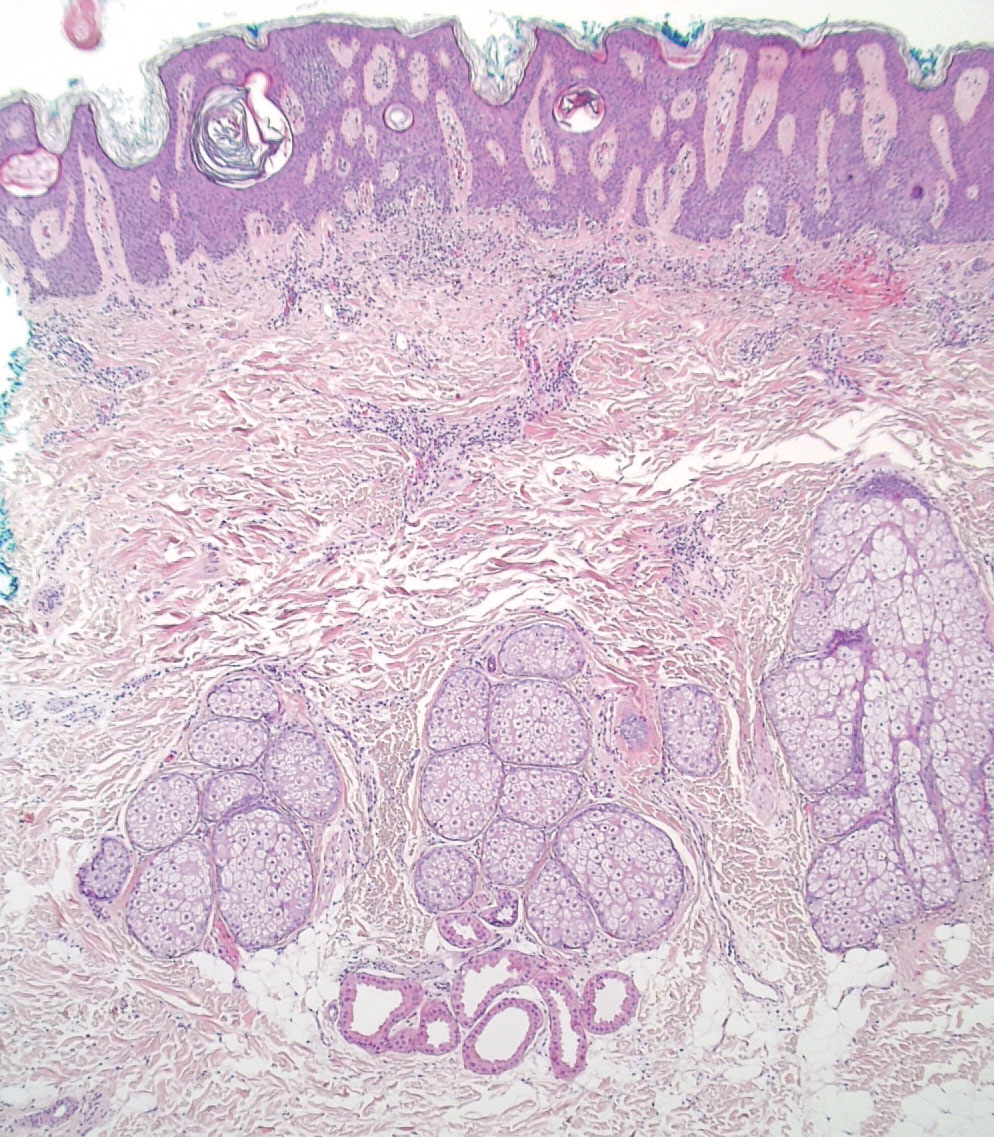
A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.
Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
Practice Points
- Nevus sebaceus (NS), commonly referred to as NS of Jadassohn or organoid nevus, is a benign skin hamartoma that consists of epidermal, sebaceous, and apocrine elements and is caused by a congenital defect in the pilosebaceous follicular unit.
- Early stages of NS can be mistaken for alopecia areata.
- Once the diagnosis of NS is confirmed, the presence of associated syndromes should be evaluated.
- The definitive treatment of NS is surgical excision; however, multiple variables must be considered when determining treatment, including patient age, risk for developing malignancy, and surgery-associated risks.
FDA puts partial hold on investigational alopecia areata drug deuruxolitinib
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
Gray hair and aging: Could ‘stuck’ stem cells be to blame?
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Cutaneous Signs of Malnutrition Secondary to Eating Disorders
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
Practice Points
- Cutaneous manifestations of malnutrition may be the presenting sign of disordered eating.
- Dermatologists have a unique opportunity for early recognition and intervention in patients with eating disorders (EDs).
- Rapid identification and multidisciplinary management of EDs may improve patient outcomes and potentially attenuate the risk of irreversible damage from malnutrition.
Polyurethane Tubing to Minimize Pain During Nail Injections
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.
What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
Scalp Nodule Associated With Hair Loss
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1
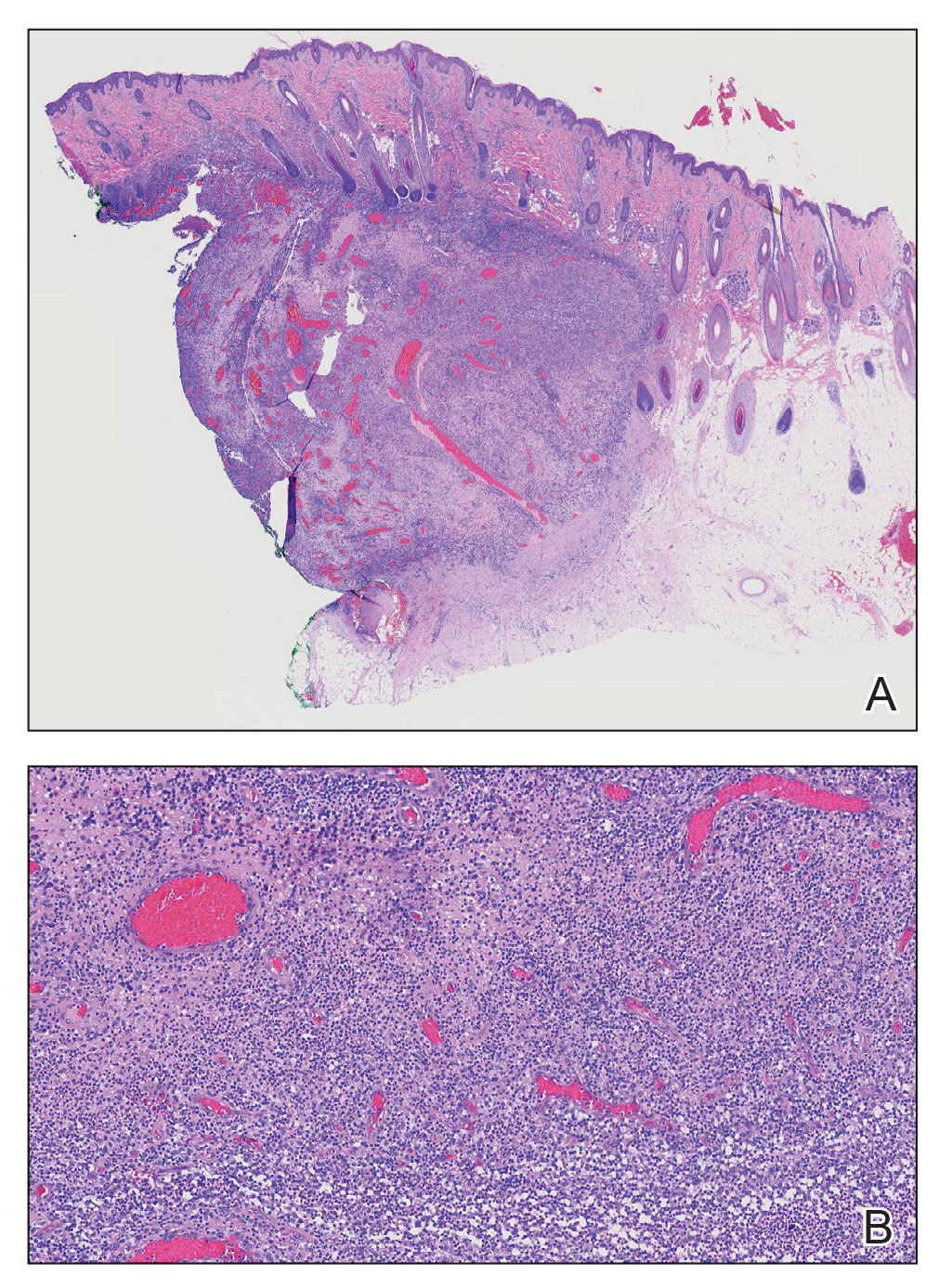
Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
A 9-year-old boy presented with a soft subcutaneous nodule with overlying alopecia on the right parietal scalp of 5 months’ duration that had grown in size, became increasingly alopecic, and was complicated by intermittent pain. An excisional biopsy of the nodule revealed deep dermal mixed inflammation with scattered granulomas. No foreign material, definitive cystic spaces, or cyst wall lining was identified. Special stains including periodic acid– Schiff, Fite acid-fast, and Twort Gram were negative for infectious organisms. His postoperative course was uneventful, and no recurrence of the nodule was reported.

Alzheimer’s drug may ease hair pulling, skin-picking disorders
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
The earlier baricitinib for severe alopecia areata is started, the better
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
AT AAD 2023
Treatment of Frontal Fibrosing Alopecia in Black Patients: A Systematic Review
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.
The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.
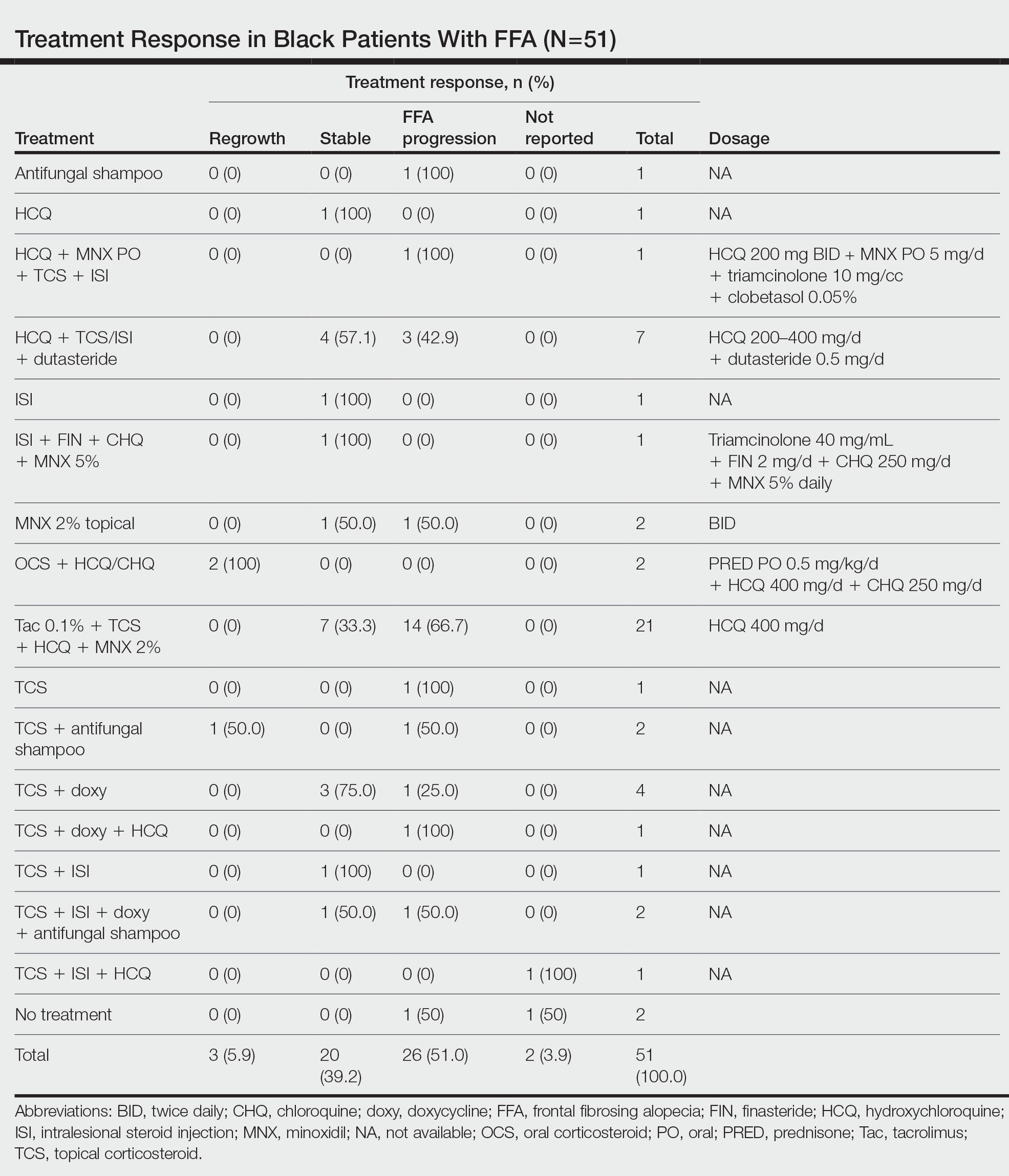
Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.

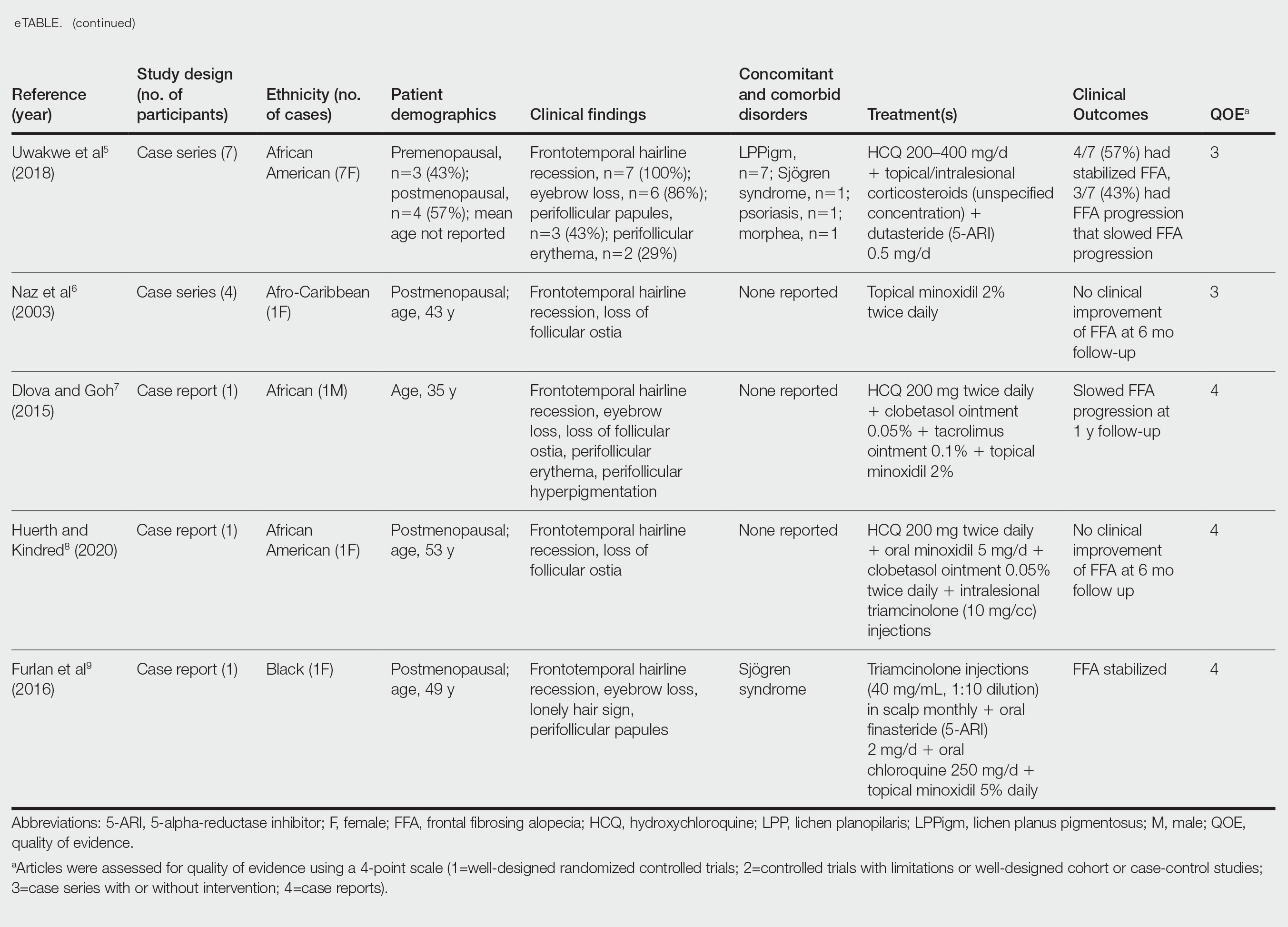
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Practice Points
- Treatment of frontal fibrosing alopecia (FFA) is challenging, and there are no evidence-based treatment guidelines available. Patients with skin of color (SOC) may have varying responses to treatment modalities.
- Special consideration should be taken when treating FFA in patients with SOC.
- Histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.