User login
Cervical cancer recurrence patterns differ after laparoscopic and open hysterectomy
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
FROM AAGL GLOBAL CONGRESS
Two-layer vaginal cuff closure may protect against laparoscopic hysterectomy complications
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
FROM AAGL GLOBAL CONGRESS
Researchers evaluate gynecology-specific laparoscopic simulator
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
FROM AAGL GLOBAL CONGRESS
Is a pelvic examination necessary 6 weeks after hysterectomy?
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
FROM AAGL GLOBAL CONGRESS
Adenomyosis: An update on imaging, medical, and surgical treatment
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at obnews@mdedge.com.
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at obnews@mdedge.com.
Adenomyosis is a benign disorder, present in 20%-35% of women and characterized by the presence of endometrial glands and stroma within the myometrium. The ectopic endometrial tissue appears to cause hypertrophy in the myometrium, resulting in an enlarged globular uterus.
Adenomyosis may present as diffuse or focal involvement within the uterus. When the focal lesion appears to be well defined, it is referred to as an adenomyoma. It is not encapsulated like a fibroid. There may be involvement of the junctional zone of the myometrium – the area between the subendometrial myometrium and the outer myometrium. While the pathogenesis of adenomyosis is unknown, two rigorous theories exist: endomyometrial invagination of the endometrium and de novo from Müllerian rests.
For this installment of the Master Class in Gynecologic Surgery, I have enlisted Keith B. Isaacson, MD, to discuss the clinical presentation, diagnosis, and medical and surgical treatment of adenomyosis.
Dr. Isaacson is the director of minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, Newton, Mass., and associate professor of obstetrics and gynecology at Harvard Medical School, Boston. He is currently in practice specializing in minimally invasive gynecologic surgery and infertility at Newton-Wellesley Hospital, where he is the director of the AAGL Fellowship in Minimally Invasive Gynecologic Surgery. Dr. Isaacson is a past president of both the AAGL and the Society of Reproductive Surgeons, as well as a published clinical researcher and surgical innovator.
It is a true honor to welcome Dr. Isaacson to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is professor of obstetrics & gynecology in the Department of Clinical Sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Dr. Miller reported that he has no relevant disclosures. Email him at obnews@mdedge.com.
Adenomyosis: While a last resort, surgery remains an option
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
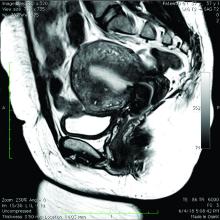
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
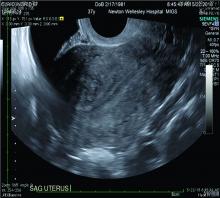
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
POP surgeries not tied to decreased sexual functioning
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.
In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
FROM OBSTETRICS & GYNECOLOGY
Endometriosis, surgical approach impact risk of bowel injury in hysterectomy
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
FROM OBSTETRICS & GYNECOLOGY
Enhanced recovery program improves outcomes after cesarean delivery
according to recent research published in Obstetrics & Gynecology.
Luciana Mullman, MPH, of Saint Barnabas Medical Center in Livingston, N.J., and colleagues used a pre-post study design to evaluate the effectiveness of ERAS at a tertiary care institution after implementing the program for patients undergoing scheduled or emergent cesarean delivery between December 2018 and August 2019. The researchers compared the rates of opioid use, length of stay, and costs of care for patients undergoing cesarean section after ERAS was implemented with those outcomes for cesarean deliveries at the center prior to ERAS between January 2018 and December 2018.
The ERAS program
ERAS was described in the study as incorporating a preoperative strategy, intraoperative management and postoperative care for cesarean delivery. The preoperative strategy consisted of a patient guidebook and a personal meeting for patient education on what to expect for preoperative and postoperative experiences as well as instructions leading up to the surgery.
For intraoperative management, intravenous opioids were minimized and replaced with neuraxial opioids when appropriate. The patient’s body temperature was monitored and controlled during the intraoperative pathway, and fluid balance was maintained. To prevent postoperative nausea and vomiting, IV ondansetron at a dose of 4 mg was started at the beginning of the cesarean delivery. When the cesarean delivery was complete, an anesthesiologist administered transversus abdominis plane blocks with 0.3% ropivacaine 30 mL on each side before the patient moved to the recovery area.
Postoperatively, the patient’s catheter was removed in the recovery room, and then transferred to postpartum floors if appropriate based on patient status. Patients began resuming a clear liquid diet 1 hour after cesarean delivery and a regular diet 6 hours after delivery. At 6 hours after surgery, the patient was out of bed and moving; walks around the nursing unit were scheduled three times per day at minimum. For pain, patients were given a 1,000-mg acetaminophen tablet every 8 hours, a 600-mg ibuprofen tablet every 6 hours, and dextromethorphan 30 mg/mL every 8 hours, with oral oxycodone 5 mg administered after physician evaluation for breakthrough pain.
Overall, there were 3,679 cesarean deliveries in the study, which included 2,171 deliveries prior to ERAS implementation and 1,508 cesarean deliveries after implementation. Patients with a scheduled cesarean delivery prior to ERAS implementation received no consistent educational program for anticipating cesarean delivery. After implementation, those patients with scheduled cesarean delivery received the full preoperative, intraoperative, and postoperative pathway, while emergent cesarean cases included the intraoperative management and postoperative care, but did not contain the preoperative component.
Improved outcomes after ERAS
The researchers found a significant decrease in the use of opioids after implementing ERAS at the center, with 24% of patients receiving opioids after ERAS, compared with 84% of patients prior to ERAS (odds ratio, 16.8; 95% confidence interval, 14.3-19.9; P < .001). These reductions in opioid use from the pre- and postimplementation periods were similar for patients with scheduled cesarean deliveries (85% vs. 27%; OR, 14.9; 95% CI, 12.2-18.3; P < .001) and emergent cesarean deliveries (83% vs. 19%; OR, 21.4; 95% CI, 16.1-28.7; P < .001).
There was also a significant reduction in total morphine milligram equivalents (MME) for patients who received opioids after ERAS (median, 15.0 MME), compared with before (median, 56.5 MME) implementing ERAS (mean relative change, 0.32; 95% CI, 0.28-0.35; P < .001). These results also were significant among both scheduled (median 59.9 vs. 15.0 MME; mean relative change, 0.31; 95% CI, 0.27-0.36; P < .001) and emergent (median 56.5 vs. 15.0 MME; mean relative change, 0.95; 95% CI, 0.89-1.01; P < .001) cesarean deliveries.
The overall length of stay after cesarean delivery significantly decreased after ERAS from an average of 3.2 days to 2.7 days (mean relative change, 0.82, 95% CI, 0.80-0.83; P < .001), and was significant in both scheduled (3.2 vs. 2.7 days; mean relative change, 0.83; 95% CI, 0.81-0.85; P < .001) and emergent (3.1 vs. 2.5 days; mean relative change, 0.80; 95% CI, 0.77-0.82; P < .001) groups. While the number of patients discharged within 2 days increased from 9% to 49% after ERAS implementation, there was no significant difference overall or in either group regarding 30-day readmission. The researchers also noted the median direct costs of cesarean delivery decreased by $349 per case after starting ERAS (mean relative change, 0.93; 95% CI, 0.91-0.95).
ERAS implementation lagging in obstetrics
In an interview, Iris Krishna, MD, MPH, a maternal-fetal medicine specialist at Emory University, Atlanta, said the ERAS approach has been used successfully in other surgical specialties but has “lagged” in obstetrics. “To date, there has been less attention in improving perioperative outcomes for women undergoing cesarean delivery, the most common abdominal surgery for women.”
Dr. Krishna said this study shows ERAS can be used in obstetrics to improve outcomes after cesarean section without increasing readmission rates. “Overall, this study demonstrates that ERAS can be successfully implemented for cesarean delivery as it has been for a variety of surgical specialties. ERAS for cesarean delivery can improve the quality of patient care while reducing health care costs.”
Women in the postpartum and postoperative period could benefit from ERAS as they recover from surgery and adjust to becoming a new mother, Dr. Krishna noted. “The goal of ERAS is to help patients return to physiological functioning as quickly as possible. Improving postoperative recovery can help with mother-infant bonding and breastfeeding.
“Implementation of a standardized approach for cesarean delivery has the potential to reduce health disparities and the disproportionately high rates of maternal morbidity and mortality in the United States,” she added. “ERAS for cesarean delivery also has the potential to address the opioid epidemic amongst reproductive-age women by improving postcesarean pain management and reducing opioid prescribing.”
Dr. Krishna also explained that an ERAS program would be feasible to implement in most centers. “It will require a shift of some elements of care from the inpatient to outpatient setting, but theoretically feasible as pregnant women frequently undergo many clinic visits during their pregnancy course.
“Education on ERAS for cesarean delivery can be implemented into prenatal care visits. ERAS implementation will also require a multidisciplinary team approach that includes obstetrics, anesthesia, nursing, pharmacy, pediatrics – all key stakeholders that will need to ‘buy in’ or be willing to support the protocol to ensure its success. As in this study, it would be helpful for hospitals to have an ERAS coordinator to champion and ensure compliance of protocol.”
Dr. Miller reported that he has received payments from the Coventus Professional Liability Insurance: Risk Management Committee and the New Jersey Board of Medical Examiners. The other authors reported no relevant conflicts of interest. Dr. Krishna reported no relevant conflicts of interest.
SOURCE: Mullman L et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004023.
according to recent research published in Obstetrics & Gynecology.
Luciana Mullman, MPH, of Saint Barnabas Medical Center in Livingston, N.J., and colleagues used a pre-post study design to evaluate the effectiveness of ERAS at a tertiary care institution after implementing the program for patients undergoing scheduled or emergent cesarean delivery between December 2018 and August 2019. The researchers compared the rates of opioid use, length of stay, and costs of care for patients undergoing cesarean section after ERAS was implemented with those outcomes for cesarean deliveries at the center prior to ERAS between January 2018 and December 2018.
The ERAS program
ERAS was described in the study as incorporating a preoperative strategy, intraoperative management and postoperative care for cesarean delivery. The preoperative strategy consisted of a patient guidebook and a personal meeting for patient education on what to expect for preoperative and postoperative experiences as well as instructions leading up to the surgery.
For intraoperative management, intravenous opioids were minimized and replaced with neuraxial opioids when appropriate. The patient’s body temperature was monitored and controlled during the intraoperative pathway, and fluid balance was maintained. To prevent postoperative nausea and vomiting, IV ondansetron at a dose of 4 mg was started at the beginning of the cesarean delivery. When the cesarean delivery was complete, an anesthesiologist administered transversus abdominis plane blocks with 0.3% ropivacaine 30 mL on each side before the patient moved to the recovery area.
Postoperatively, the patient’s catheter was removed in the recovery room, and then transferred to postpartum floors if appropriate based on patient status. Patients began resuming a clear liquid diet 1 hour after cesarean delivery and a regular diet 6 hours after delivery. At 6 hours after surgery, the patient was out of bed and moving; walks around the nursing unit were scheduled three times per day at minimum. For pain, patients were given a 1,000-mg acetaminophen tablet every 8 hours, a 600-mg ibuprofen tablet every 6 hours, and dextromethorphan 30 mg/mL every 8 hours, with oral oxycodone 5 mg administered after physician evaluation for breakthrough pain.
Overall, there were 3,679 cesarean deliveries in the study, which included 2,171 deliveries prior to ERAS implementation and 1,508 cesarean deliveries after implementation. Patients with a scheduled cesarean delivery prior to ERAS implementation received no consistent educational program for anticipating cesarean delivery. After implementation, those patients with scheduled cesarean delivery received the full preoperative, intraoperative, and postoperative pathway, while emergent cesarean cases included the intraoperative management and postoperative care, but did not contain the preoperative component.
Improved outcomes after ERAS
The researchers found a significant decrease in the use of opioids after implementing ERAS at the center, with 24% of patients receiving opioids after ERAS, compared with 84% of patients prior to ERAS (odds ratio, 16.8; 95% confidence interval, 14.3-19.9; P < .001). These reductions in opioid use from the pre- and postimplementation periods were similar for patients with scheduled cesarean deliveries (85% vs. 27%; OR, 14.9; 95% CI, 12.2-18.3; P < .001) and emergent cesarean deliveries (83% vs. 19%; OR, 21.4; 95% CI, 16.1-28.7; P < .001).
There was also a significant reduction in total morphine milligram equivalents (MME) for patients who received opioids after ERAS (median, 15.0 MME), compared with before (median, 56.5 MME) implementing ERAS (mean relative change, 0.32; 95% CI, 0.28-0.35; P < .001). These results also were significant among both scheduled (median 59.9 vs. 15.0 MME; mean relative change, 0.31; 95% CI, 0.27-0.36; P < .001) and emergent (median 56.5 vs. 15.0 MME; mean relative change, 0.95; 95% CI, 0.89-1.01; P < .001) cesarean deliveries.
The overall length of stay after cesarean delivery significantly decreased after ERAS from an average of 3.2 days to 2.7 days (mean relative change, 0.82, 95% CI, 0.80-0.83; P < .001), and was significant in both scheduled (3.2 vs. 2.7 days; mean relative change, 0.83; 95% CI, 0.81-0.85; P < .001) and emergent (3.1 vs. 2.5 days; mean relative change, 0.80; 95% CI, 0.77-0.82; P < .001) groups. While the number of patients discharged within 2 days increased from 9% to 49% after ERAS implementation, there was no significant difference overall or in either group regarding 30-day readmission. The researchers also noted the median direct costs of cesarean delivery decreased by $349 per case after starting ERAS (mean relative change, 0.93; 95% CI, 0.91-0.95).
ERAS implementation lagging in obstetrics
In an interview, Iris Krishna, MD, MPH, a maternal-fetal medicine specialist at Emory University, Atlanta, said the ERAS approach has been used successfully in other surgical specialties but has “lagged” in obstetrics. “To date, there has been less attention in improving perioperative outcomes for women undergoing cesarean delivery, the most common abdominal surgery for women.”
Dr. Krishna said this study shows ERAS can be used in obstetrics to improve outcomes after cesarean section without increasing readmission rates. “Overall, this study demonstrates that ERAS can be successfully implemented for cesarean delivery as it has been for a variety of surgical specialties. ERAS for cesarean delivery can improve the quality of patient care while reducing health care costs.”
Women in the postpartum and postoperative period could benefit from ERAS as they recover from surgery and adjust to becoming a new mother, Dr. Krishna noted. “The goal of ERAS is to help patients return to physiological functioning as quickly as possible. Improving postoperative recovery can help with mother-infant bonding and breastfeeding.
“Implementation of a standardized approach for cesarean delivery has the potential to reduce health disparities and the disproportionately high rates of maternal morbidity and mortality in the United States,” she added. “ERAS for cesarean delivery also has the potential to address the opioid epidemic amongst reproductive-age women by improving postcesarean pain management and reducing opioid prescribing.”
Dr. Krishna also explained that an ERAS program would be feasible to implement in most centers. “It will require a shift of some elements of care from the inpatient to outpatient setting, but theoretically feasible as pregnant women frequently undergo many clinic visits during their pregnancy course.
“Education on ERAS for cesarean delivery can be implemented into prenatal care visits. ERAS implementation will also require a multidisciplinary team approach that includes obstetrics, anesthesia, nursing, pharmacy, pediatrics – all key stakeholders that will need to ‘buy in’ or be willing to support the protocol to ensure its success. As in this study, it would be helpful for hospitals to have an ERAS coordinator to champion and ensure compliance of protocol.”
Dr. Miller reported that he has received payments from the Coventus Professional Liability Insurance: Risk Management Committee and the New Jersey Board of Medical Examiners. The other authors reported no relevant conflicts of interest. Dr. Krishna reported no relevant conflicts of interest.
SOURCE: Mullman L et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004023.
according to recent research published in Obstetrics & Gynecology.
Luciana Mullman, MPH, of Saint Barnabas Medical Center in Livingston, N.J., and colleagues used a pre-post study design to evaluate the effectiveness of ERAS at a tertiary care institution after implementing the program for patients undergoing scheduled or emergent cesarean delivery between December 2018 and August 2019. The researchers compared the rates of opioid use, length of stay, and costs of care for patients undergoing cesarean section after ERAS was implemented with those outcomes for cesarean deliveries at the center prior to ERAS between January 2018 and December 2018.
The ERAS program
ERAS was described in the study as incorporating a preoperative strategy, intraoperative management and postoperative care for cesarean delivery. The preoperative strategy consisted of a patient guidebook and a personal meeting for patient education on what to expect for preoperative and postoperative experiences as well as instructions leading up to the surgery.
For intraoperative management, intravenous opioids were minimized and replaced with neuraxial opioids when appropriate. The patient’s body temperature was monitored and controlled during the intraoperative pathway, and fluid balance was maintained. To prevent postoperative nausea and vomiting, IV ondansetron at a dose of 4 mg was started at the beginning of the cesarean delivery. When the cesarean delivery was complete, an anesthesiologist administered transversus abdominis plane blocks with 0.3% ropivacaine 30 mL on each side before the patient moved to the recovery area.
Postoperatively, the patient’s catheter was removed in the recovery room, and then transferred to postpartum floors if appropriate based on patient status. Patients began resuming a clear liquid diet 1 hour after cesarean delivery and a regular diet 6 hours after delivery. At 6 hours after surgery, the patient was out of bed and moving; walks around the nursing unit were scheduled three times per day at minimum. For pain, patients were given a 1,000-mg acetaminophen tablet every 8 hours, a 600-mg ibuprofen tablet every 6 hours, and dextromethorphan 30 mg/mL every 8 hours, with oral oxycodone 5 mg administered after physician evaluation for breakthrough pain.
Overall, there were 3,679 cesarean deliveries in the study, which included 2,171 deliveries prior to ERAS implementation and 1,508 cesarean deliveries after implementation. Patients with a scheduled cesarean delivery prior to ERAS implementation received no consistent educational program for anticipating cesarean delivery. After implementation, those patients with scheduled cesarean delivery received the full preoperative, intraoperative, and postoperative pathway, while emergent cesarean cases included the intraoperative management and postoperative care, but did not contain the preoperative component.
Improved outcomes after ERAS
The researchers found a significant decrease in the use of opioids after implementing ERAS at the center, with 24% of patients receiving opioids after ERAS, compared with 84% of patients prior to ERAS (odds ratio, 16.8; 95% confidence interval, 14.3-19.9; P < .001). These reductions in opioid use from the pre- and postimplementation periods were similar for patients with scheduled cesarean deliveries (85% vs. 27%; OR, 14.9; 95% CI, 12.2-18.3; P < .001) and emergent cesarean deliveries (83% vs. 19%; OR, 21.4; 95% CI, 16.1-28.7; P < .001).
There was also a significant reduction in total morphine milligram equivalents (MME) for patients who received opioids after ERAS (median, 15.0 MME), compared with before (median, 56.5 MME) implementing ERAS (mean relative change, 0.32; 95% CI, 0.28-0.35; P < .001). These results also were significant among both scheduled (median 59.9 vs. 15.0 MME; mean relative change, 0.31; 95% CI, 0.27-0.36; P < .001) and emergent (median 56.5 vs. 15.0 MME; mean relative change, 0.95; 95% CI, 0.89-1.01; P < .001) cesarean deliveries.
The overall length of stay after cesarean delivery significantly decreased after ERAS from an average of 3.2 days to 2.7 days (mean relative change, 0.82, 95% CI, 0.80-0.83; P < .001), and was significant in both scheduled (3.2 vs. 2.7 days; mean relative change, 0.83; 95% CI, 0.81-0.85; P < .001) and emergent (3.1 vs. 2.5 days; mean relative change, 0.80; 95% CI, 0.77-0.82; P < .001) groups. While the number of patients discharged within 2 days increased from 9% to 49% after ERAS implementation, there was no significant difference overall or in either group regarding 30-day readmission. The researchers also noted the median direct costs of cesarean delivery decreased by $349 per case after starting ERAS (mean relative change, 0.93; 95% CI, 0.91-0.95).
ERAS implementation lagging in obstetrics
In an interview, Iris Krishna, MD, MPH, a maternal-fetal medicine specialist at Emory University, Atlanta, said the ERAS approach has been used successfully in other surgical specialties but has “lagged” in obstetrics. “To date, there has been less attention in improving perioperative outcomes for women undergoing cesarean delivery, the most common abdominal surgery for women.”
Dr. Krishna said this study shows ERAS can be used in obstetrics to improve outcomes after cesarean section without increasing readmission rates. “Overall, this study demonstrates that ERAS can be successfully implemented for cesarean delivery as it has been for a variety of surgical specialties. ERAS for cesarean delivery can improve the quality of patient care while reducing health care costs.”
Women in the postpartum and postoperative period could benefit from ERAS as they recover from surgery and adjust to becoming a new mother, Dr. Krishna noted. “The goal of ERAS is to help patients return to physiological functioning as quickly as possible. Improving postoperative recovery can help with mother-infant bonding and breastfeeding.
“Implementation of a standardized approach for cesarean delivery has the potential to reduce health disparities and the disproportionately high rates of maternal morbidity and mortality in the United States,” she added. “ERAS for cesarean delivery also has the potential to address the opioid epidemic amongst reproductive-age women by improving postcesarean pain management and reducing opioid prescribing.”
Dr. Krishna also explained that an ERAS program would be feasible to implement in most centers. “It will require a shift of some elements of care from the inpatient to outpatient setting, but theoretically feasible as pregnant women frequently undergo many clinic visits during their pregnancy course.
“Education on ERAS for cesarean delivery can be implemented into prenatal care visits. ERAS implementation will also require a multidisciplinary team approach that includes obstetrics, anesthesia, nursing, pharmacy, pediatrics – all key stakeholders that will need to ‘buy in’ or be willing to support the protocol to ensure its success. As in this study, it would be helpful for hospitals to have an ERAS coordinator to champion and ensure compliance of protocol.”
Dr. Miller reported that he has received payments from the Coventus Professional Liability Insurance: Risk Management Committee and the New Jersey Board of Medical Examiners. The other authors reported no relevant conflicts of interest. Dr. Krishna reported no relevant conflicts of interest.
SOURCE: Mullman L et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004023.
FROM OBSTETRICS & GYNECOLOGY
FDA updates info on postmarketing surveillance study of Essure
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.