User login
MD-IQ only
Endometriosis and Abnormal Uterine Bleeding
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
New guidelines for cannabis in chronic pain management released
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANNABIS AND CANNABINOID RESEARCH
Prepare for endometriosis excision surgery with a multidisciplinary approach
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
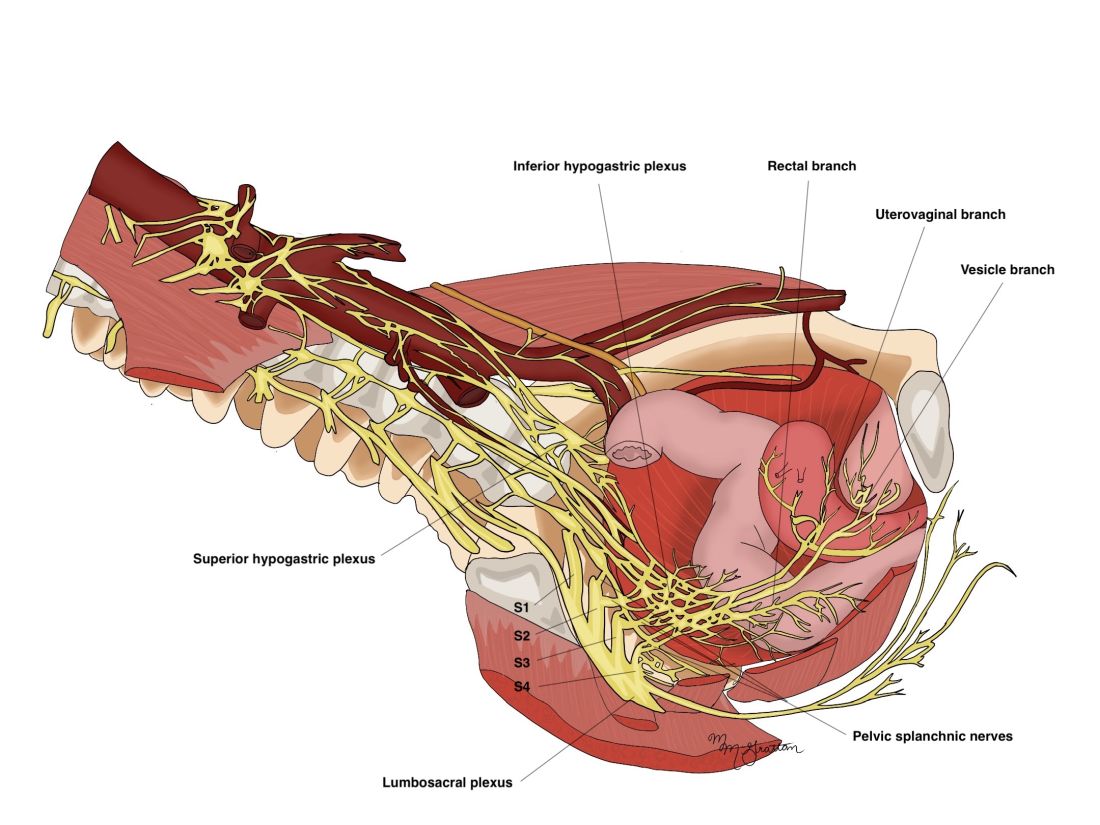
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Endometriosis: Whole-Body Effects, Treatments and Infertility
How does endometriosis affect the whole body, and how often is it misunderstood for another condition?
Dr. Taylor: Far too often, we think about endometriosis as just a cause of bad menstrual cramps. So many times, we miss the signs and falsely attribute symptoms to other diseases. I cannot tell you the number of people who have seen multiple practitioners for other conditions, when the underlying problem was actually endometriosis.
Endometriosis affects the whole body. It can affect the intestines, the bladder, and body weight, and the brain and mood. Endometriosis causes fatigue and inflammation, and in the long run it can lead to an increased risk for cardiovascular disease. When we as physicians do surgery or laparoscopy, we find these little blue and brown lesions in the pelvis, and they certainly do cause pain, but that is not the whole disease.
Endometriosis heightens pain and nerve sensitivity for patients, and we should not dismiss the debilitating effect of these symptoms. Things actually do hurt more. In fact, the pain can spread from beyond the time of the menstrual period and spread to other areas besides the uterus.
We cannot ignore the totality of the effects of the disease and only focus on one part of the problem. More importantly, we cannot be distracted and discount endometriosis or mistake it for another condition. I have seen many patients who went to a gastroenterologist first and may even have had a colonoscopy because of some bowel symptoms, but then we come to find out it was endometriosis irritating the bowels at the time of their period and not another primary disease.
Another example is that the patient may see a urologist and have a cystoscopy to assess bladder pain, especially if the pain comes on around the time of menses. However, that pain is probably due to endometriosis irritating the bladder, not a primary bladder problem.
I have even had patients who were sent to a psychiatrist first because of anxiety that was actually being caused by the endometriosis. It is important to understand that treating the primary problem—endometriosis—should be our focus.
Which effects of this chronic disease can have the most long-term impact?
Dr. Taylor: It is important to understand that endometriosis has long-term effects on the entire body. For example, it affects the brain, increasing anxiety and depression. It causes pain sensitization, fatigue, and body inflammation. Endometriosis can also damage blood vessels or cause atherosclerosis.
A quick diagnosis is crucial because often this disease affects women at the most critical points in their life—either when they are in school, or in the early part of their career, when they need to be able to focus. It is important to get their endometriosis under control early and prevent long-term complications. Unfortunately, the long-term impacts are an aspect we do not focus on enough.
Infertility is another common long-term consequence of endometriosis. The sooner we can diagnose endometriosis, the sooner we can begin treatment and the more likely we can preserve someone's fertility. Our goal is to catch endometriosis early enough, preserve the patient’s fertility, and prevent any damage, so they hopefully will not have trouble getting pregnant or need medical intervention to get pregnant.
What methods do you use to diagnose endometriosis as quickly as possible?
Dr. Taylor: The sooner we can pinpoint the correct diagnosis and begin treatment, the more we can not only relieve patients of pain, but also stop that inflammation and all of these other manifestations of endometriosis so that they are not saddled with this for life.
We used to say you needed a laparoscopy to accurately diagnose endometriosis, and that statement is still true. You cannot see the most common types of endometriosis on an ultrasound or MRI. The endometriosis has to be pretty bad before you see it on an MRI or an ultrasound, and at that point it is often a big cyst in the ovary or a big nodule that is invasive.
However, you can diagnose endometriosis just by listening to your patients. If they have extremely painful menstrual cycles, dysmenorrhea, or painful menstrual cramps that get worse over the years, the problem is most likely endometriosis. You can rule out a few other things, and you can make that empiric clinical diagnosis of endometriosis. You can know with confidence that somebody likely has endometriosis. The treatments are benign. The first-line therapy would be to try a birth control pill. If we had to perform a laparoscopy before beginning endometriosis treatment, I think we would be doing our patients a huge disservice.
In addition to birth control pills, what are the most common therapeutic treatments you use in day-to-day practice?
Dr. Taylor: Birth control pills are still the first-line therapy. We use birth control pills because they are easy, well-tolerated, and inexpensive, but about a third of women will be resistant. Birth control pills are a great option when they work, but they do not always work 100% of the time.
We have a couple of other hormonal treatment options. Rarely, but occasionally, we use something called danazol, which is a mild male hormone. Side effects can be acne or hair growth, but it works well and is inexpensive. We used to give injectable agents, like leuprolide. Leuprolide is a harsh medication with once-a-month injections, and it puts someone in a temporary menopausal state with hot flashes and the possibility of decreased bone mineral density.
Today, we have the new class of GnRH antagonists that are a milder, gentler version of those injectable medications. They are oral, and you do not have to fully suppress estrogen levels all the way down to menopause. Patients can take the GnRH antagonists, stop treatment, and try to get pregnant at their next cycle.
Occasionally, we find that someone does not respond to any medical therapies, so surgery still has an important role. The usual reason for surgery is that you may suppress the active disease with medications, but the old damage is still there causing some pain, which can only be removed with surgery. Surgery is a good way to relieve that pain and it helps improve pregnancy rates for people with endometriosis wishing to conceive.
What does the future look like for endometriosis-related infertility, particularly related to in vitro fertilization (IVF)?
Dr. Taylor: We currently have an IVF trial in the works, in which we are using a hormone-suppressing GnRH (gonadotropin-releasing hormone) antagonist, elagolix, which suppresses endometriosis. The medication is administered before IVF. The goal is to determine if this approach leads to a better pregnancy rate in IVF cycles.
We are also working on nonhormonal medications in the laboratory, but these strategies are not yet ready for human clinical trials. These laboratory trials are investigating the basic biology of endometriosis, with the goal of learning what makes endometrial tissue grow in the wrong place, what makes it grow aggressively, and how it signals to other organs to cause damage.
We are looking at some additional nonhormonal medications that may possibly be used in someone trying to conceive, so that we can increase their chances of becoming pregnant. We are testing these medications in mice, but eventually we hope they progress to human clinical trials. The future for endometriosis therapy is nonhormonal treatments that can be used in somebody trying to conceive. That is what we have on the horizon.
Unfortunately for people with endometriosis wishing to conceive, all the classic first-line medications used to treat the condition are reproductive hormones that interfere with the ability to become pregnant. When we stop these medications, many women with endometriosis get pregnant spontaneously always suggest patients with endometriosis wishing to conceive try this approach, unless we know the endometriosis is very extensive. However, IVF is a good way to correct even the worst cases of endometriosis. As long as somebody has not waited until they are in their 40s and they have run out of eggs, IVF will usually correct endometriosis-related infertility.
Overall, how we treat endometriosis has been revolutionized and we have much better options than we had just 5 years ago. In my day-to-day practice, I recognize the importance of talking more openly about endometriosis, understanding the different symptoms, and not dismissing those connections. We should be able to talk about this important medical problem and all of its manifestations. I published a paper in The Lancet at the end of 2021 about the concept of endometriosis as a systemic whole-body disease and its effects. I think open conversations with patients about endometriosis is making a world of difference for the women with this disease.
How does endometriosis affect the whole body, and how often is it misunderstood for another condition?
Dr. Taylor: Far too often, we think about endometriosis as just a cause of bad menstrual cramps. So many times, we miss the signs and falsely attribute symptoms to other diseases. I cannot tell you the number of people who have seen multiple practitioners for other conditions, when the underlying problem was actually endometriosis.
Endometriosis affects the whole body. It can affect the intestines, the bladder, and body weight, and the brain and mood. Endometriosis causes fatigue and inflammation, and in the long run it can lead to an increased risk for cardiovascular disease. When we as physicians do surgery or laparoscopy, we find these little blue and brown lesions in the pelvis, and they certainly do cause pain, but that is not the whole disease.
Endometriosis heightens pain and nerve sensitivity for patients, and we should not dismiss the debilitating effect of these symptoms. Things actually do hurt more. In fact, the pain can spread from beyond the time of the menstrual period and spread to other areas besides the uterus.
We cannot ignore the totality of the effects of the disease and only focus on one part of the problem. More importantly, we cannot be distracted and discount endometriosis or mistake it for another condition. I have seen many patients who went to a gastroenterologist first and may even have had a colonoscopy because of some bowel symptoms, but then we come to find out it was endometriosis irritating the bowels at the time of their period and not another primary disease.
Another example is that the patient may see a urologist and have a cystoscopy to assess bladder pain, especially if the pain comes on around the time of menses. However, that pain is probably due to endometriosis irritating the bladder, not a primary bladder problem.
I have even had patients who were sent to a psychiatrist first because of anxiety that was actually being caused by the endometriosis. It is important to understand that treating the primary problem—endometriosis—should be our focus.
Which effects of this chronic disease can have the most long-term impact?
Dr. Taylor: It is important to understand that endometriosis has long-term effects on the entire body. For example, it affects the brain, increasing anxiety and depression. It causes pain sensitization, fatigue, and body inflammation. Endometriosis can also damage blood vessels or cause atherosclerosis.
A quick diagnosis is crucial because often this disease affects women at the most critical points in their life—either when they are in school, or in the early part of their career, when they need to be able to focus. It is important to get their endometriosis under control early and prevent long-term complications. Unfortunately, the long-term impacts are an aspect we do not focus on enough.
Infertility is another common long-term consequence of endometriosis. The sooner we can diagnose endometriosis, the sooner we can begin treatment and the more likely we can preserve someone's fertility. Our goal is to catch endometriosis early enough, preserve the patient’s fertility, and prevent any damage, so they hopefully will not have trouble getting pregnant or need medical intervention to get pregnant.
What methods do you use to diagnose endometriosis as quickly as possible?
Dr. Taylor: The sooner we can pinpoint the correct diagnosis and begin treatment, the more we can not only relieve patients of pain, but also stop that inflammation and all of these other manifestations of endometriosis so that they are not saddled with this for life.
We used to say you needed a laparoscopy to accurately diagnose endometriosis, and that statement is still true. You cannot see the most common types of endometriosis on an ultrasound or MRI. The endometriosis has to be pretty bad before you see it on an MRI or an ultrasound, and at that point it is often a big cyst in the ovary or a big nodule that is invasive.
However, you can diagnose endometriosis just by listening to your patients. If they have extremely painful menstrual cycles, dysmenorrhea, or painful menstrual cramps that get worse over the years, the problem is most likely endometriosis. You can rule out a few other things, and you can make that empiric clinical diagnosis of endometriosis. You can know with confidence that somebody likely has endometriosis. The treatments are benign. The first-line therapy would be to try a birth control pill. If we had to perform a laparoscopy before beginning endometriosis treatment, I think we would be doing our patients a huge disservice.
In addition to birth control pills, what are the most common therapeutic treatments you use in day-to-day practice?
Dr. Taylor: Birth control pills are still the first-line therapy. We use birth control pills because they are easy, well-tolerated, and inexpensive, but about a third of women will be resistant. Birth control pills are a great option when they work, but they do not always work 100% of the time.
We have a couple of other hormonal treatment options. Rarely, but occasionally, we use something called danazol, which is a mild male hormone. Side effects can be acne or hair growth, but it works well and is inexpensive. We used to give injectable agents, like leuprolide. Leuprolide is a harsh medication with once-a-month injections, and it puts someone in a temporary menopausal state with hot flashes and the possibility of decreased bone mineral density.
Today, we have the new class of GnRH antagonists that are a milder, gentler version of those injectable medications. They are oral, and you do not have to fully suppress estrogen levels all the way down to menopause. Patients can take the GnRH antagonists, stop treatment, and try to get pregnant at their next cycle.
Occasionally, we find that someone does not respond to any medical therapies, so surgery still has an important role. The usual reason for surgery is that you may suppress the active disease with medications, but the old damage is still there causing some pain, which can only be removed with surgery. Surgery is a good way to relieve that pain and it helps improve pregnancy rates for people with endometriosis wishing to conceive.
What does the future look like for endometriosis-related infertility, particularly related to in vitro fertilization (IVF)?
Dr. Taylor: We currently have an IVF trial in the works, in which we are using a hormone-suppressing GnRH (gonadotropin-releasing hormone) antagonist, elagolix, which suppresses endometriosis. The medication is administered before IVF. The goal is to determine if this approach leads to a better pregnancy rate in IVF cycles.
We are also working on nonhormonal medications in the laboratory, but these strategies are not yet ready for human clinical trials. These laboratory trials are investigating the basic biology of endometriosis, with the goal of learning what makes endometrial tissue grow in the wrong place, what makes it grow aggressively, and how it signals to other organs to cause damage.
We are looking at some additional nonhormonal medications that may possibly be used in someone trying to conceive, so that we can increase their chances of becoming pregnant. We are testing these medications in mice, but eventually we hope they progress to human clinical trials. The future for endometriosis therapy is nonhormonal treatments that can be used in somebody trying to conceive. That is what we have on the horizon.
Unfortunately for people with endometriosis wishing to conceive, all the classic first-line medications used to treat the condition are reproductive hormones that interfere with the ability to become pregnant. When we stop these medications, many women with endometriosis get pregnant spontaneously always suggest patients with endometriosis wishing to conceive try this approach, unless we know the endometriosis is very extensive. However, IVF is a good way to correct even the worst cases of endometriosis. As long as somebody has not waited until they are in their 40s and they have run out of eggs, IVF will usually correct endometriosis-related infertility.
Overall, how we treat endometriosis has been revolutionized and we have much better options than we had just 5 years ago. In my day-to-day practice, I recognize the importance of talking more openly about endometriosis, understanding the different symptoms, and not dismissing those connections. We should be able to talk about this important medical problem and all of its manifestations. I published a paper in The Lancet at the end of 2021 about the concept of endometriosis as a systemic whole-body disease and its effects. I think open conversations with patients about endometriosis is making a world of difference for the women with this disease.
How does endometriosis affect the whole body, and how often is it misunderstood for another condition?
Dr. Taylor: Far too often, we think about endometriosis as just a cause of bad menstrual cramps. So many times, we miss the signs and falsely attribute symptoms to other diseases. I cannot tell you the number of people who have seen multiple practitioners for other conditions, when the underlying problem was actually endometriosis.
Endometriosis affects the whole body. It can affect the intestines, the bladder, and body weight, and the brain and mood. Endometriosis causes fatigue and inflammation, and in the long run it can lead to an increased risk for cardiovascular disease. When we as physicians do surgery or laparoscopy, we find these little blue and brown lesions in the pelvis, and they certainly do cause pain, but that is not the whole disease.
Endometriosis heightens pain and nerve sensitivity for patients, and we should not dismiss the debilitating effect of these symptoms. Things actually do hurt more. In fact, the pain can spread from beyond the time of the menstrual period and spread to other areas besides the uterus.
We cannot ignore the totality of the effects of the disease and only focus on one part of the problem. More importantly, we cannot be distracted and discount endometriosis or mistake it for another condition. I have seen many patients who went to a gastroenterologist first and may even have had a colonoscopy because of some bowel symptoms, but then we come to find out it was endometriosis irritating the bowels at the time of their period and not another primary disease.
Another example is that the patient may see a urologist and have a cystoscopy to assess bladder pain, especially if the pain comes on around the time of menses. However, that pain is probably due to endometriosis irritating the bladder, not a primary bladder problem.
I have even had patients who were sent to a psychiatrist first because of anxiety that was actually being caused by the endometriosis. It is important to understand that treating the primary problem—endometriosis—should be our focus.
Which effects of this chronic disease can have the most long-term impact?
Dr. Taylor: It is important to understand that endometriosis has long-term effects on the entire body. For example, it affects the brain, increasing anxiety and depression. It causes pain sensitization, fatigue, and body inflammation. Endometriosis can also damage blood vessels or cause atherosclerosis.
A quick diagnosis is crucial because often this disease affects women at the most critical points in their life—either when they are in school, or in the early part of their career, when they need to be able to focus. It is important to get their endometriosis under control early and prevent long-term complications. Unfortunately, the long-term impacts are an aspect we do not focus on enough.
Infertility is another common long-term consequence of endometriosis. The sooner we can diagnose endometriosis, the sooner we can begin treatment and the more likely we can preserve someone's fertility. Our goal is to catch endometriosis early enough, preserve the patient’s fertility, and prevent any damage, so they hopefully will not have trouble getting pregnant or need medical intervention to get pregnant.
What methods do you use to diagnose endometriosis as quickly as possible?
Dr. Taylor: The sooner we can pinpoint the correct diagnosis and begin treatment, the more we can not only relieve patients of pain, but also stop that inflammation and all of these other manifestations of endometriosis so that they are not saddled with this for life.
We used to say you needed a laparoscopy to accurately diagnose endometriosis, and that statement is still true. You cannot see the most common types of endometriosis on an ultrasound or MRI. The endometriosis has to be pretty bad before you see it on an MRI or an ultrasound, and at that point it is often a big cyst in the ovary or a big nodule that is invasive.
However, you can diagnose endometriosis just by listening to your patients. If they have extremely painful menstrual cycles, dysmenorrhea, or painful menstrual cramps that get worse over the years, the problem is most likely endometriosis. You can rule out a few other things, and you can make that empiric clinical diagnosis of endometriosis. You can know with confidence that somebody likely has endometriosis. The treatments are benign. The first-line therapy would be to try a birth control pill. If we had to perform a laparoscopy before beginning endometriosis treatment, I think we would be doing our patients a huge disservice.
In addition to birth control pills, what are the most common therapeutic treatments you use in day-to-day practice?
Dr. Taylor: Birth control pills are still the first-line therapy. We use birth control pills because they are easy, well-tolerated, and inexpensive, but about a third of women will be resistant. Birth control pills are a great option when they work, but they do not always work 100% of the time.
We have a couple of other hormonal treatment options. Rarely, but occasionally, we use something called danazol, which is a mild male hormone. Side effects can be acne or hair growth, but it works well and is inexpensive. We used to give injectable agents, like leuprolide. Leuprolide is a harsh medication with once-a-month injections, and it puts someone in a temporary menopausal state with hot flashes and the possibility of decreased bone mineral density.
Today, we have the new class of GnRH antagonists that are a milder, gentler version of those injectable medications. They are oral, and you do not have to fully suppress estrogen levels all the way down to menopause. Patients can take the GnRH antagonists, stop treatment, and try to get pregnant at their next cycle.
Occasionally, we find that someone does not respond to any medical therapies, so surgery still has an important role. The usual reason for surgery is that you may suppress the active disease with medications, but the old damage is still there causing some pain, which can only be removed with surgery. Surgery is a good way to relieve that pain and it helps improve pregnancy rates for people with endometriosis wishing to conceive.
What does the future look like for endometriosis-related infertility, particularly related to in vitro fertilization (IVF)?
Dr. Taylor: We currently have an IVF trial in the works, in which we are using a hormone-suppressing GnRH (gonadotropin-releasing hormone) antagonist, elagolix, which suppresses endometriosis. The medication is administered before IVF. The goal is to determine if this approach leads to a better pregnancy rate in IVF cycles.
We are also working on nonhormonal medications in the laboratory, but these strategies are not yet ready for human clinical trials. These laboratory trials are investigating the basic biology of endometriosis, with the goal of learning what makes endometrial tissue grow in the wrong place, what makes it grow aggressively, and how it signals to other organs to cause damage.
We are looking at some additional nonhormonal medications that may possibly be used in someone trying to conceive, so that we can increase their chances of becoming pregnant. We are testing these medications in mice, but eventually we hope they progress to human clinical trials. The future for endometriosis therapy is nonhormonal treatments that can be used in somebody trying to conceive. That is what we have on the horizon.
Unfortunately for people with endometriosis wishing to conceive, all the classic first-line medications used to treat the condition are reproductive hormones that interfere with the ability to become pregnant. When we stop these medications, many women with endometriosis get pregnant spontaneously always suggest patients with endometriosis wishing to conceive try this approach, unless we know the endometriosis is very extensive. However, IVF is a good way to correct even the worst cases of endometriosis. As long as somebody has not waited until they are in their 40s and they have run out of eggs, IVF will usually correct endometriosis-related infertility.
Overall, how we treat endometriosis has been revolutionized and we have much better options than we had just 5 years ago. In my day-to-day practice, I recognize the importance of talking more openly about endometriosis, understanding the different symptoms, and not dismissing those connections. We should be able to talk about this important medical problem and all of its manifestations. I published a paper in The Lancet at the end of 2021 about the concept of endometriosis as a systemic whole-body disease and its effects. I think open conversations with patients about endometriosis is making a world of difference for the women with this disease.
Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
Scientists create ‘vagina on a chip’: What to know
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
FROM MICROBIOME
Decoding endometriosis: Recent research fosters hope
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
Endometriosis and infertility – Combining a chronic physical and emotional pain
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A hypogastric nerve-focused approach to nerve-sparing endometriosis surgery
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.
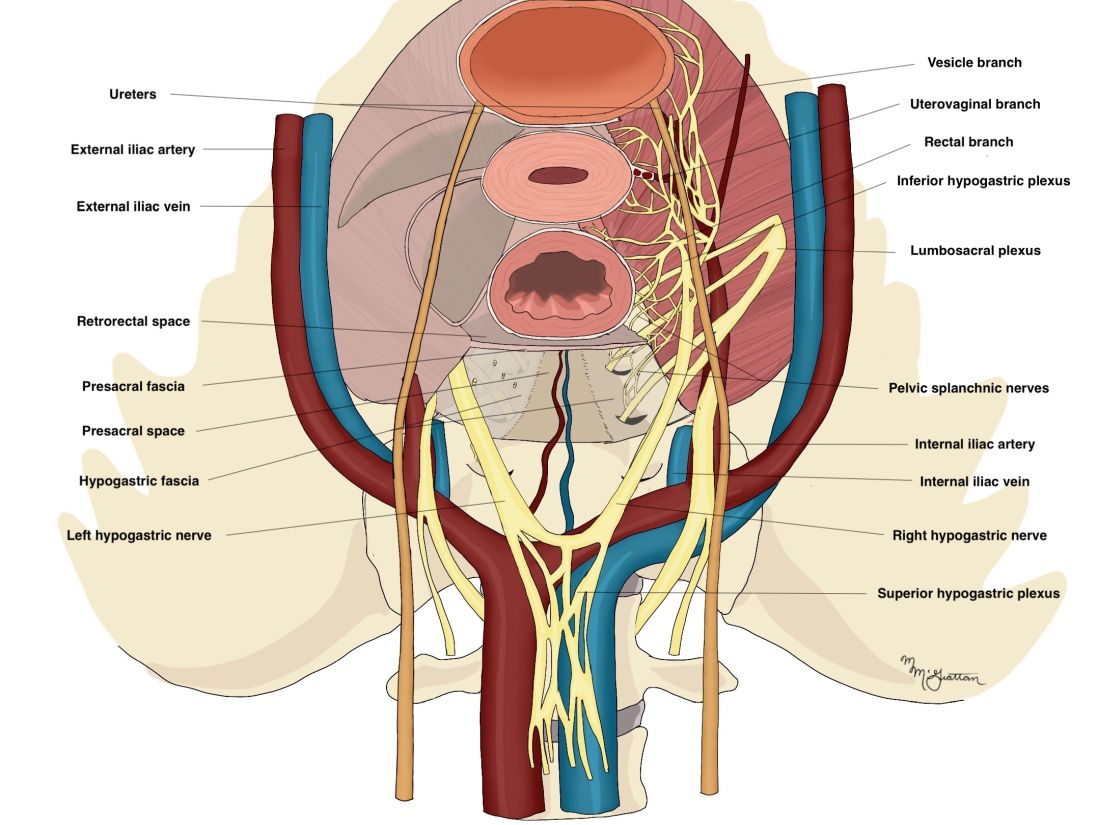
The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Spare the nerves in deep infiltrative endometriosis surgery
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.