User login
What Every Provider Should Know About Type 1 Diabetes
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
PPI Prophylaxis Prevents GI Bleed in Ventilated Patients
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
How Drones Are Reducing Emergency Response Times
The drones are coming.
Starting in September, if someone in Clemmons, North Carolina, calls 911 to report a cardiac arrest, the first responder on the scene may be a drone carrying an automated external defibrillator, or AED.
“The idea is for the drone to get there several minutes before first responders,” such as an emergency medical technician or an ambulance, said Daniel Crews, a spokesperson for the sheriff’s office in Forsyth County, where Clemmons is located. The sheriff’s office is partnering on the project with local emergency services, the Clinical Research Institute at Duke University, and the drone consulting firm Hovecon. “The ultimate goal is to save lives and improve life expectancy for someone experiencing a cardiac episode,” Mr. Crews said.
The Forsyth County program is one of a growing number of efforts by public safety and healthcare organizations across the country to use drones to speed up lifesaving treatment in situations in which every second counts.
More than 356,000 people have a cardiac arrest outside of a hospital setting every year in the United States, according to the American Heart Association. Most people are at home when it happens, and about 90% die because they don’t get immediate help from first responders or bystanders. Every minute that passes without medical intervention decreases the odds of survival by 10%.
“We’ve never been able to move the needle for cardiac arrest in private settings, and this technology could meet that need,” said Monique Anderson Starks, MD, a cardiologist and associate professor of medicine at Duke University. Dr. Starks is leading pilot studies in Forsyth County and James City County, Virginia, to test whether drone AED delivery can improve treatment response times. The work is funded by a 4-year grant from the American Heart Association.
Dr. Starks said she believes the drone-delivered AEDs in the pilot study could reduce the time to treatment by 4 minutes compared with first responders.
Unlike a heart attack, which occurs when blood flow to the heart is blocked, a cardiac arrest happens when a heart malfunction causes it to stop beating, typically because of an arrhythmia or an electrical problem. Eighty percent of cardiac arrests start as heart attacks. The only way to get the heart restarted is with CPR and a defibrillator.
In Forsyth County, a drone pilot from the sheriff’s department will listen in on 911 calls. If there’s a suspected cardiac arrest, the pilot can dispatch the drone even before emergency medical services are contacted. The drone, which weighs 22 pounds and can travel 60 mph, will fly to the location and hover 125 feet in the air before lowering an AED to the ground on a winch. The AED provides simple verbal instructions; the 911 dispatcher on the phone can also help a bystander use the AED.
Eventually there will be six drone bases in Forsyth and James City counties, Dr. Starks said.
While the technology is promising and research has often found that drones arrive faster than first responders, there’s little conclusive evidence that drones improve health outcomes.
A Swedish study published in The Lancet in 2023 compared the response times between drones and ambulances for suspected cardiac arrest in 58 deployments in an area of about 200,000 people. It found that drones beat the ambulance to the scene two thirds of the time, by a median of 3 minutes and 14 seconds.
In the United States, most programs are just getting started, and they are exploring the use of drones to also provide remedies for drug overdoses and major trauma or potential drowning rescues.
In Florida, Tampa General Hospital, Manatee County, and Archer First Response Systems, or AFRS, began a program in May to deliver AEDs, a tourniquet, and Narcan, a nasal spray that can reverse an opioid overdose. The program initially covers a 7-square-mile area, and EMS dispatchers deploy the drones, which are monitored by drone pilots.
There were nearly 108,000 drug overdose deaths in the United States in 2022, according to the National Institute on Drug Abuse.
As of early July, the Tampa program hadn’t yet deployed any drones, said Gordon Folkes, the founder and chief executive of AFRS, which develops and deploys emergency drone logistics systems. One request in June to send a drone to an overdose couldn’t be fulfilled because of a violent thunderstorm, Mr. Folkes said. In the testing area, which covers about 7,000 residents, Mr. Folkes estimates that 10-15 drones might be deployed each year.
“The bread and butter for these systems is suburban areas” like Manatee County that are well-populated and where the drones have the advantage of being able to avoid traffic congestion, Mr. Folkes said.
There are other uses for drones in medical emergencies. The New York Police Department plans to drop emergency flotation devices to struggling swimmers at local beaches. In Chula Vista, California, a police drone was able to pinpoint the location of a burning car, and then officers pulled the driver out, said Sgt. Tony Molina.
Rescue personnel have used drones to locate people who wander away from nursing homes, said James Augustine, a spokesperson for the American College of Emergency Physicians who is the medical director for the International Association of Fire Chiefs.
In the United States, one hurdle for drone programs is that the Federal Aviation Administration typically requires that drones be operated within the operators’ visual line of sight. In May, when Congress passed the FAA reauthorization bill, it gave the FAA 4 months to issue a notice of proposed rule-making on drone operations beyond the visual line of sight.
“The FAA is focused on developing standard rules to make [Beyond Visual Line of Sight] operations routine, scalable, and economically viable,” said Rick Breitenfeldt, an FAA spokesperson.
Some civil liberties groups are concerned that the FAA’s new rules may not provide enough protection from drone cameras for people on the ground.
Jay Stanley, a senior policy analyst at the American Civil Liberties Union, acknowledged the benefits of using drones in emergency situations but said there are issues that need to be addressed.
“The concern is that the FAA is going to significantly loosen the reins of drones without any significant privacy protections,” he said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
The drones are coming.
Starting in September, if someone in Clemmons, North Carolina, calls 911 to report a cardiac arrest, the first responder on the scene may be a drone carrying an automated external defibrillator, or AED.
“The idea is for the drone to get there several minutes before first responders,” such as an emergency medical technician or an ambulance, said Daniel Crews, a spokesperson for the sheriff’s office in Forsyth County, where Clemmons is located. The sheriff’s office is partnering on the project with local emergency services, the Clinical Research Institute at Duke University, and the drone consulting firm Hovecon. “The ultimate goal is to save lives and improve life expectancy for someone experiencing a cardiac episode,” Mr. Crews said.
The Forsyth County program is one of a growing number of efforts by public safety and healthcare organizations across the country to use drones to speed up lifesaving treatment in situations in which every second counts.
More than 356,000 people have a cardiac arrest outside of a hospital setting every year in the United States, according to the American Heart Association. Most people are at home when it happens, and about 90% die because they don’t get immediate help from first responders or bystanders. Every minute that passes without medical intervention decreases the odds of survival by 10%.
“We’ve never been able to move the needle for cardiac arrest in private settings, and this technology could meet that need,” said Monique Anderson Starks, MD, a cardiologist and associate professor of medicine at Duke University. Dr. Starks is leading pilot studies in Forsyth County and James City County, Virginia, to test whether drone AED delivery can improve treatment response times. The work is funded by a 4-year grant from the American Heart Association.
Dr. Starks said she believes the drone-delivered AEDs in the pilot study could reduce the time to treatment by 4 minutes compared with first responders.
Unlike a heart attack, which occurs when blood flow to the heart is blocked, a cardiac arrest happens when a heart malfunction causes it to stop beating, typically because of an arrhythmia or an electrical problem. Eighty percent of cardiac arrests start as heart attacks. The only way to get the heart restarted is with CPR and a defibrillator.
In Forsyth County, a drone pilot from the sheriff’s department will listen in on 911 calls. If there’s a suspected cardiac arrest, the pilot can dispatch the drone even before emergency medical services are contacted. The drone, which weighs 22 pounds and can travel 60 mph, will fly to the location and hover 125 feet in the air before lowering an AED to the ground on a winch. The AED provides simple verbal instructions; the 911 dispatcher on the phone can also help a bystander use the AED.
Eventually there will be six drone bases in Forsyth and James City counties, Dr. Starks said.
While the technology is promising and research has often found that drones arrive faster than first responders, there’s little conclusive evidence that drones improve health outcomes.
A Swedish study published in The Lancet in 2023 compared the response times between drones and ambulances for suspected cardiac arrest in 58 deployments in an area of about 200,000 people. It found that drones beat the ambulance to the scene two thirds of the time, by a median of 3 minutes and 14 seconds.
In the United States, most programs are just getting started, and they are exploring the use of drones to also provide remedies for drug overdoses and major trauma or potential drowning rescues.
In Florida, Tampa General Hospital, Manatee County, and Archer First Response Systems, or AFRS, began a program in May to deliver AEDs, a tourniquet, and Narcan, a nasal spray that can reverse an opioid overdose. The program initially covers a 7-square-mile area, and EMS dispatchers deploy the drones, which are monitored by drone pilots.
There were nearly 108,000 drug overdose deaths in the United States in 2022, according to the National Institute on Drug Abuse.
As of early July, the Tampa program hadn’t yet deployed any drones, said Gordon Folkes, the founder and chief executive of AFRS, which develops and deploys emergency drone logistics systems. One request in June to send a drone to an overdose couldn’t be fulfilled because of a violent thunderstorm, Mr. Folkes said. In the testing area, which covers about 7,000 residents, Mr. Folkes estimates that 10-15 drones might be deployed each year.
“The bread and butter for these systems is suburban areas” like Manatee County that are well-populated and where the drones have the advantage of being able to avoid traffic congestion, Mr. Folkes said.
There are other uses for drones in medical emergencies. The New York Police Department plans to drop emergency flotation devices to struggling swimmers at local beaches. In Chula Vista, California, a police drone was able to pinpoint the location of a burning car, and then officers pulled the driver out, said Sgt. Tony Molina.
Rescue personnel have used drones to locate people who wander away from nursing homes, said James Augustine, a spokesperson for the American College of Emergency Physicians who is the medical director for the International Association of Fire Chiefs.
In the United States, one hurdle for drone programs is that the Federal Aviation Administration typically requires that drones be operated within the operators’ visual line of sight. In May, when Congress passed the FAA reauthorization bill, it gave the FAA 4 months to issue a notice of proposed rule-making on drone operations beyond the visual line of sight.
“The FAA is focused on developing standard rules to make [Beyond Visual Line of Sight] operations routine, scalable, and economically viable,” said Rick Breitenfeldt, an FAA spokesperson.
Some civil liberties groups are concerned that the FAA’s new rules may not provide enough protection from drone cameras for people on the ground.
Jay Stanley, a senior policy analyst at the American Civil Liberties Union, acknowledged the benefits of using drones in emergency situations but said there are issues that need to be addressed.
“The concern is that the FAA is going to significantly loosen the reins of drones without any significant privacy protections,” he said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
The drones are coming.
Starting in September, if someone in Clemmons, North Carolina, calls 911 to report a cardiac arrest, the first responder on the scene may be a drone carrying an automated external defibrillator, or AED.
“The idea is for the drone to get there several minutes before first responders,” such as an emergency medical technician or an ambulance, said Daniel Crews, a spokesperson for the sheriff’s office in Forsyth County, where Clemmons is located. The sheriff’s office is partnering on the project with local emergency services, the Clinical Research Institute at Duke University, and the drone consulting firm Hovecon. “The ultimate goal is to save lives and improve life expectancy for someone experiencing a cardiac episode,” Mr. Crews said.
The Forsyth County program is one of a growing number of efforts by public safety and healthcare organizations across the country to use drones to speed up lifesaving treatment in situations in which every second counts.
More than 356,000 people have a cardiac arrest outside of a hospital setting every year in the United States, according to the American Heart Association. Most people are at home when it happens, and about 90% die because they don’t get immediate help from first responders or bystanders. Every minute that passes without medical intervention decreases the odds of survival by 10%.
“We’ve never been able to move the needle for cardiac arrest in private settings, and this technology could meet that need,” said Monique Anderson Starks, MD, a cardiologist and associate professor of medicine at Duke University. Dr. Starks is leading pilot studies in Forsyth County and James City County, Virginia, to test whether drone AED delivery can improve treatment response times. The work is funded by a 4-year grant from the American Heart Association.
Dr. Starks said she believes the drone-delivered AEDs in the pilot study could reduce the time to treatment by 4 minutes compared with first responders.
Unlike a heart attack, which occurs when blood flow to the heart is blocked, a cardiac arrest happens when a heart malfunction causes it to stop beating, typically because of an arrhythmia or an electrical problem. Eighty percent of cardiac arrests start as heart attacks. The only way to get the heart restarted is with CPR and a defibrillator.
In Forsyth County, a drone pilot from the sheriff’s department will listen in on 911 calls. If there’s a suspected cardiac arrest, the pilot can dispatch the drone even before emergency medical services are contacted. The drone, which weighs 22 pounds and can travel 60 mph, will fly to the location and hover 125 feet in the air before lowering an AED to the ground on a winch. The AED provides simple verbal instructions; the 911 dispatcher on the phone can also help a bystander use the AED.
Eventually there will be six drone bases in Forsyth and James City counties, Dr. Starks said.
While the technology is promising and research has often found that drones arrive faster than first responders, there’s little conclusive evidence that drones improve health outcomes.
A Swedish study published in The Lancet in 2023 compared the response times between drones and ambulances for suspected cardiac arrest in 58 deployments in an area of about 200,000 people. It found that drones beat the ambulance to the scene two thirds of the time, by a median of 3 minutes and 14 seconds.
In the United States, most programs are just getting started, and they are exploring the use of drones to also provide remedies for drug overdoses and major trauma or potential drowning rescues.
In Florida, Tampa General Hospital, Manatee County, and Archer First Response Systems, or AFRS, began a program in May to deliver AEDs, a tourniquet, and Narcan, a nasal spray that can reverse an opioid overdose. The program initially covers a 7-square-mile area, and EMS dispatchers deploy the drones, which are monitored by drone pilots.
There were nearly 108,000 drug overdose deaths in the United States in 2022, according to the National Institute on Drug Abuse.
As of early July, the Tampa program hadn’t yet deployed any drones, said Gordon Folkes, the founder and chief executive of AFRS, which develops and deploys emergency drone logistics systems. One request in June to send a drone to an overdose couldn’t be fulfilled because of a violent thunderstorm, Mr. Folkes said. In the testing area, which covers about 7,000 residents, Mr. Folkes estimates that 10-15 drones might be deployed each year.
“The bread and butter for these systems is suburban areas” like Manatee County that are well-populated and where the drones have the advantage of being able to avoid traffic congestion, Mr. Folkes said.
There are other uses for drones in medical emergencies. The New York Police Department plans to drop emergency flotation devices to struggling swimmers at local beaches. In Chula Vista, California, a police drone was able to pinpoint the location of a burning car, and then officers pulled the driver out, said Sgt. Tony Molina.
Rescue personnel have used drones to locate people who wander away from nursing homes, said James Augustine, a spokesperson for the American College of Emergency Physicians who is the medical director for the International Association of Fire Chiefs.
In the United States, one hurdle for drone programs is that the Federal Aviation Administration typically requires that drones be operated within the operators’ visual line of sight. In May, when Congress passed the FAA reauthorization bill, it gave the FAA 4 months to issue a notice of proposed rule-making on drone operations beyond the visual line of sight.
“The FAA is focused on developing standard rules to make [Beyond Visual Line of Sight] operations routine, scalable, and economically viable,” said Rick Breitenfeldt, an FAA spokesperson.
Some civil liberties groups are concerned that the FAA’s new rules may not provide enough protection from drone cameras for people on the ground.
Jay Stanley, a senior policy analyst at the American Civil Liberties Union, acknowledged the benefits of using drones in emergency situations but said there are issues that need to be addressed.
“The concern is that the FAA is going to significantly loosen the reins of drones without any significant privacy protections,” he said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Use of albumin in critically ill patients
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.
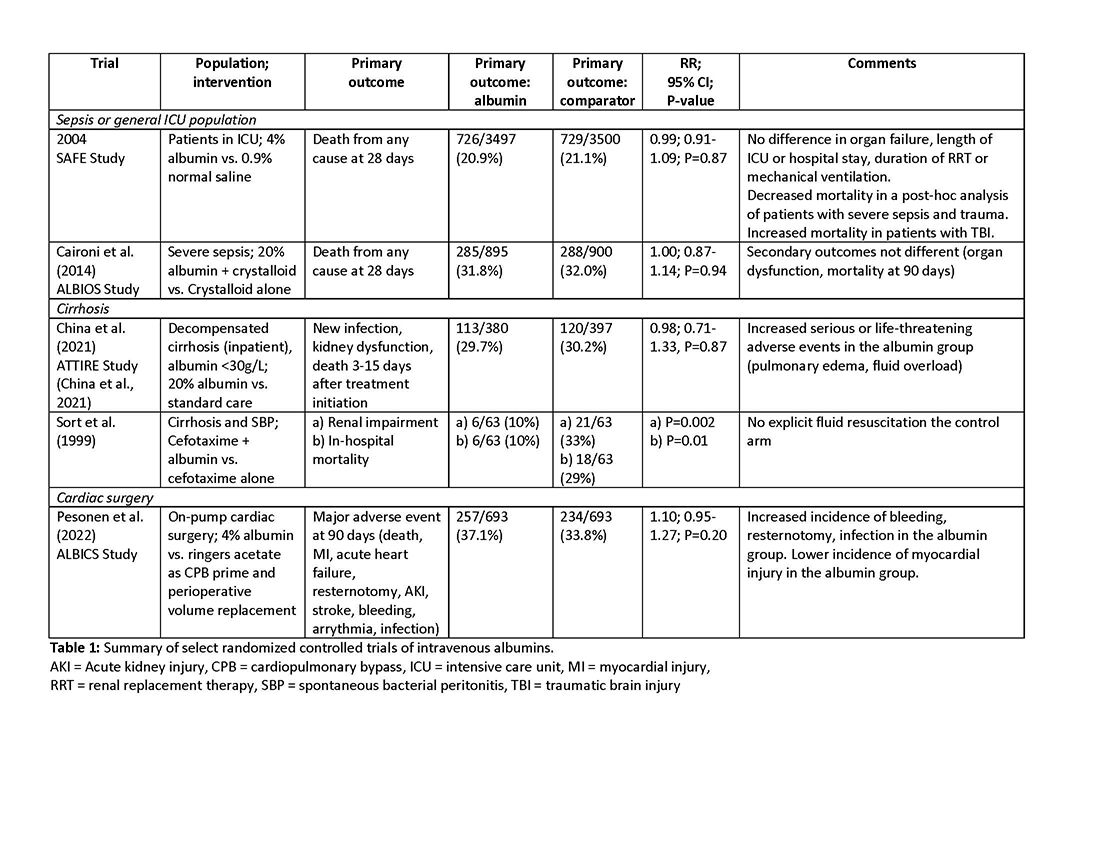
As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.
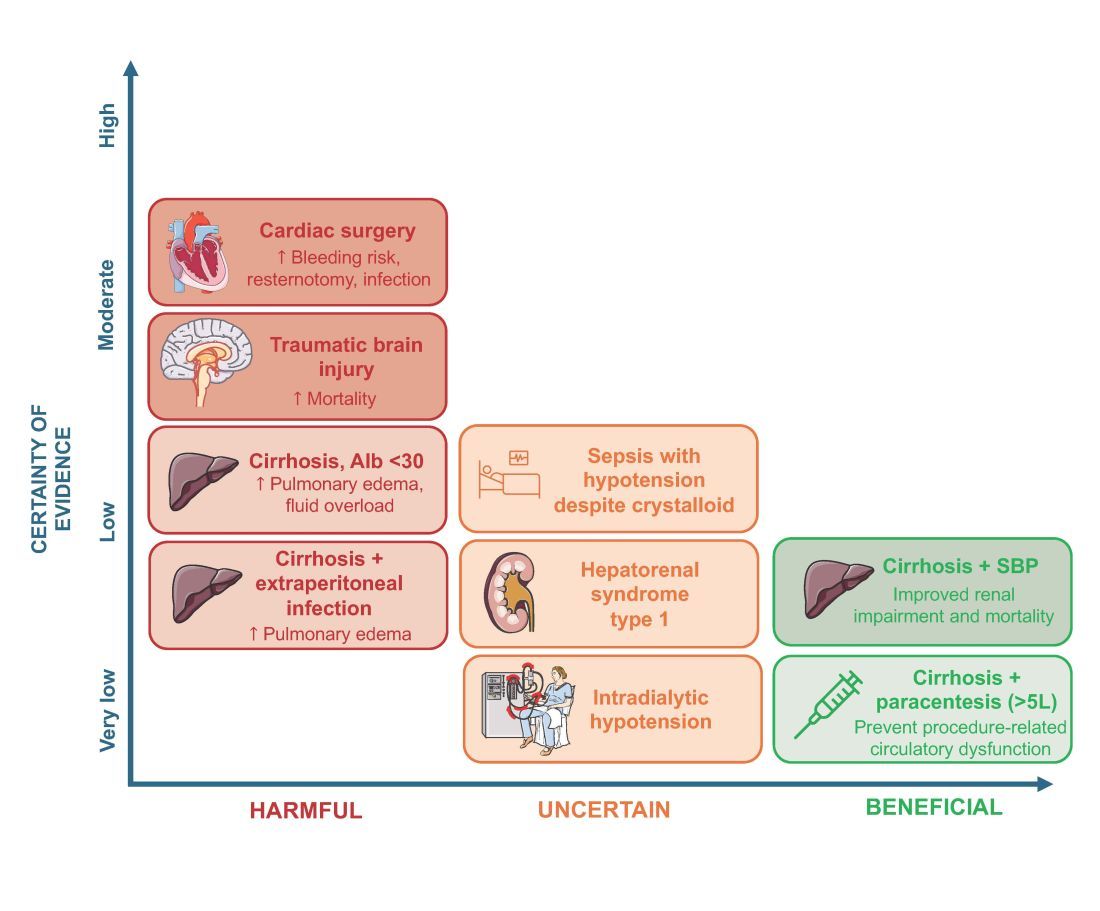
High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.

As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.

High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.

As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.

High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Electrical impedance tomography: Visualization and integration of the impact of mechanical ventilation
CRITICAL CARE NETWORK
Mechanical Ventilation and Airways Management Section
1,2,3 Continuous monitoring of the tidal volume, plateau pressure, and positive end-expiratory pressure (PEEP) is crucial to maintain LPV. Electrical impedance tomography (EIT) is a noninvasive, radiation-free, imaging method of the electrical conductivity distribution inside the human body.4 Integrating EIT into invasive mechanical ventilation allows imaging of the regional lung ventilation as affected by the mechanical ventilation settings as well as the patient position. It can also provide a personalized approach to determining the optimum ventilatory settings based on individual patient conditions.5,6
Optimum PEEP titration is crucial to prevent lung collapse as well as overdistension. In a single-center, randomized, crossover pilot study of 12 patients, optimum PEEP titration was carried out using a high PEEP/FiO2 table vs EIT in moderate to severe ARDS. The primary endpoint was the reduction of mechanical power, which was consistently lower in the EIT group.7 EIT also allows the assessment of regional compliance of the lungs. There are reports regarding the superiority of regional compliance of lung over global compliance in achieving better gas exchange, lung compliance, and weaning of mechanical ventilation.8 EIT could assess the patient’s response to prone positioning by illustrating the change in the functional residual capacity between supine and prone positioning.9 In addition, by visualization of the ventilated areas during spontaneous breathing and reduction of pressure support, EIT could help in weaning off the mechanical ventilation.10
In conclusion, EIT can be a tool to provide safe and personalized mechanical ventilation in patients with respiratory failure. However, there are limited data regarding its use and application, which might become an interesting subject for future clinical research.
– Akram M. Zaaqoq, MD, MPH
Member-at-Large
References
1. Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
2. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
3. Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10):2155-2163.
4. Adler A, Boyle A. Electrical impedance tomography: tissue properties to image measures. IEEE Trans Biomed Eng. 2017;64(11):2494-2504.
5. Jang GY, Ayoub G, Kim YE, et al. Integrated EIT system for functional lung ventilation imaging. Biomed Eng Online. 2019;18(1):83.
6. Sella N, Pettenuzzo T, Zarantonello F, et al. Electrical impedance tomography: a compass for the safe route to optimal PEEP. Respir Med. 2021;187:106555.
7. Jimenez JV, Munroe E, Weirauch AJ, et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care. 2023;27(1):21.
8. Costa ELV, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132-1137.9. Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58(4):589-596.10. Wisse JJ, Goos TG, Jonkman AH, et al. Electrical impedance tomography as a monitoring tool during weaning from mechanical ventilation: an observational study during the spontaneous breathing trial. Respir Res. 2024;25(1):179.
CRITICAL CARE NETWORK
Mechanical Ventilation and Airways Management Section
1,2,3 Continuous monitoring of the tidal volume, plateau pressure, and positive end-expiratory pressure (PEEP) is crucial to maintain LPV. Electrical impedance tomography (EIT) is a noninvasive, radiation-free, imaging method of the electrical conductivity distribution inside the human body.4 Integrating EIT into invasive mechanical ventilation allows imaging of the regional lung ventilation as affected by the mechanical ventilation settings as well as the patient position. It can also provide a personalized approach to determining the optimum ventilatory settings based on individual patient conditions.5,6
Optimum PEEP titration is crucial to prevent lung collapse as well as overdistension. In a single-center, randomized, crossover pilot study of 12 patients, optimum PEEP titration was carried out using a high PEEP/FiO2 table vs EIT in moderate to severe ARDS. The primary endpoint was the reduction of mechanical power, which was consistently lower in the EIT group.7 EIT also allows the assessment of regional compliance of the lungs. There are reports regarding the superiority of regional compliance of lung over global compliance in achieving better gas exchange, lung compliance, and weaning of mechanical ventilation.8 EIT could assess the patient’s response to prone positioning by illustrating the change in the functional residual capacity between supine and prone positioning.9 In addition, by visualization of the ventilated areas during spontaneous breathing and reduction of pressure support, EIT could help in weaning off the mechanical ventilation.10
In conclusion, EIT can be a tool to provide safe and personalized mechanical ventilation in patients with respiratory failure. However, there are limited data regarding its use and application, which might become an interesting subject for future clinical research.
– Akram M. Zaaqoq, MD, MPH
Member-at-Large
References
1. Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
2. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
3. Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10):2155-2163.
4. Adler A, Boyle A. Electrical impedance tomography: tissue properties to image measures. IEEE Trans Biomed Eng. 2017;64(11):2494-2504.
5. Jang GY, Ayoub G, Kim YE, et al. Integrated EIT system for functional lung ventilation imaging. Biomed Eng Online. 2019;18(1):83.
6. Sella N, Pettenuzzo T, Zarantonello F, et al. Electrical impedance tomography: a compass for the safe route to optimal PEEP. Respir Med. 2021;187:106555.
7. Jimenez JV, Munroe E, Weirauch AJ, et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care. 2023;27(1):21.
8. Costa ELV, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132-1137.9. Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58(4):589-596.10. Wisse JJ, Goos TG, Jonkman AH, et al. Electrical impedance tomography as a monitoring tool during weaning from mechanical ventilation: an observational study during the spontaneous breathing trial. Respir Res. 2024;25(1):179.
CRITICAL CARE NETWORK
Mechanical Ventilation and Airways Management Section
1,2,3 Continuous monitoring of the tidal volume, plateau pressure, and positive end-expiratory pressure (PEEP) is crucial to maintain LPV. Electrical impedance tomography (EIT) is a noninvasive, radiation-free, imaging method of the electrical conductivity distribution inside the human body.4 Integrating EIT into invasive mechanical ventilation allows imaging of the regional lung ventilation as affected by the mechanical ventilation settings as well as the patient position. It can also provide a personalized approach to determining the optimum ventilatory settings based on individual patient conditions.5,6
Optimum PEEP titration is crucial to prevent lung collapse as well as overdistension. In a single-center, randomized, crossover pilot study of 12 patients, optimum PEEP titration was carried out using a high PEEP/FiO2 table vs EIT in moderate to severe ARDS. The primary endpoint was the reduction of mechanical power, which was consistently lower in the EIT group.7 EIT also allows the assessment of regional compliance of the lungs. There are reports regarding the superiority of regional compliance of lung over global compliance in achieving better gas exchange, lung compliance, and weaning of mechanical ventilation.8 EIT could assess the patient’s response to prone positioning by illustrating the change in the functional residual capacity between supine and prone positioning.9 In addition, by visualization of the ventilated areas during spontaneous breathing and reduction of pressure support, EIT could help in weaning off the mechanical ventilation.10
In conclusion, EIT can be a tool to provide safe and personalized mechanical ventilation in patients with respiratory failure. However, there are limited data regarding its use and application, which might become an interesting subject for future clinical research.
– Akram M. Zaaqoq, MD, MPH
Member-at-Large
References
1. Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
2. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
3. Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10):2155-2163.
4. Adler A, Boyle A. Electrical impedance tomography: tissue properties to image measures. IEEE Trans Biomed Eng. 2017;64(11):2494-2504.
5. Jang GY, Ayoub G, Kim YE, et al. Integrated EIT system for functional lung ventilation imaging. Biomed Eng Online. 2019;18(1):83.
6. Sella N, Pettenuzzo T, Zarantonello F, et al. Electrical impedance tomography: a compass for the safe route to optimal PEEP. Respir Med. 2021;187:106555.
7. Jimenez JV, Munroe E, Weirauch AJ, et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care. 2023;27(1):21.
8. Costa ELV, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132-1137.9. Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58(4):589-596.10. Wisse JJ, Goos TG, Jonkman AH, et al. Electrical impedance tomography as a monitoring tool during weaning from mechanical ventilation: an observational study during the spontaneous breathing trial. Respir Res. 2024;25(1):179.
Hospital-onset sepsis: Why the brouhaha?
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 o F) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4 Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 o F) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4 Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 o F) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4 Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
Hold the antianaerobics in the ICU whenever possible
SAN DIEGO —
“You may not be personally moved by a 2- to 5-percent absolute difference in mortality, but sepsis is so common and so lethal that even small differences in outcomes can actually translate into enormous public health implications,” said Robert P. Dickson, MD a pulmonary and critical care specialist at the University of Michigan in Ann Arbor.
If instead of prescribing piperacillin-tazobactam (Zosyn; pip-tazo) for sepsis critical care specialists were to switch to cefepime “even if you make very conservative assumptions like a modest effect size, you’re still talking about [saving] thousands of lives a year,” he said in a scientific symposium at the American Thoracic Society’s international conference.
“This is why I say this isn’t really over the horizon; this is microbiome modulation that’s happening all the time,” he said.
Most patients with sepsis in a medical ICU with respiratory, urinary or bloodstream sources of infection do not have indications for antianaerobic antibiotics, and there are no head-to-head clinical trials demonstrating a benefit for one anti-sepsis antibiotic strategy over another he said.
“In contrast, every observational study between antianaerobic and non-antianaerobic shows benefits to the anaerobe-sparing [drugs], and it’s been shown with animal models too. So to my mind, it’s already practice changing. I need to be talked into giving antianaerobic antibiotics for septic patients” he said.
Targeting gut microbiota
There are three basic approaches to focusing on the gut microbiome as a therapeutic target. The hardest is attempting to engineer an ecosystem — a fiendishly complex task with unpredictable results that has never been shown to work in either the gut or in the ICU, Dr. Dickson said.
A second approach, the use of probiotics to repopulate the gut with beneficial bacteria, is largely futile in the ICU, as the large majority of patients are on antibiotics and can’t be safely weaned off of them while in critical care. In this situation, giving probiotics would be akin to try to repopulate a forest while a forest fire is raging, he said.
The third and easiest approach is to minimize dysbiosis — imbalance of organisms in the gut — in the first place.
Anaerobic bacteria in the gut have been shown in several different disease states and animals models to be protective against pneumonia, organ failure, and death.
To see whether antianaerobic antibiotics could increase risk for adverse outcomes in the ICU, Dr. Dickson and colleagues previously conducted a retrospective study of 3032 mechanically ventilated patients in their center who received antibiotics either with or without anaerobic coverage in the first 72 hours.
They found that patients treated with early antianaerobic antibiotics had decreased ventilator-associated pneumonia-free survival (hazard ratio [HR] 1.24), infection-free survival (HR 1.22), and overall survival (HR 1.14) compared with patients who received antibiotics without anaerobic cover (all comparisons statistically significant by confidence intervals).
In a subcohort of 116 patients for whom gut microbiota data compositions were available, those who received antianaerobic antibiotics had decreased initial gut bacterial density (P = .00038), increased microbiome expansion during hospitalization (P = .011), and domination of the microbiome by Enterobacteriaceae species (P = .045). They also found that Enterobacteriaceae were enriched among respiratory pathogens in antianaerobic treated patients, and that in murine models, treatment with antianaerobic antibiotics increased susceptibility to Enterobacteriaceae pneumonia and increased the risk of death from non-infectious injuries.
Pip-tazo vs. cefepime
In the ACORN (Antibiotic Choice on Renal Outcomes) trial, results of which were reported by this news organization in November 2023, there were no differences in the highest stage of acute kidney injury or death in the first 14 days between piperacillin-tazobactam and cefepime. Remarking on the results, lead investigator Edward T. Qian, MD, MSc from Vanderbilt University in Nashville, Tennessee, said “I think the big takeaway is that you should feel comfortable starting or using pip-tazo for your patients who are coming into the hospital and receiving empiric antibiotics for acute infection.”
But as Dr. Dickson’s group reported more recently in JAMA Internal Medicine, a 15-month pip-tazo shortage allowed the investigators to conduct a natural experiment comparing 90-day outcomes among 7569 patients with sepsis who received vancomycin plus either pip-tazo or cefepime.
They found in an instrumental variable analysis that piperacillin-tazobactam was associated with an absolute increase in mortality at 90 days of 5.0%, and that patients who received this antianaerobic antibiotic had 2.1 fewer organ failure–free days, 1.1 fewer ventilator-free days, and 1.5 fewer vasopressor-free days.
“Our study reveals the potential risks associated with empirical piperacillin-tazobactam in patients with sepsis without a specific indication for antianaerobic therapy. These findings should prompt reconsideration and further study of the widespread use of empirical antianaerobic antibiotics in sepsis,” the investigators concluded.
Who gets what?
In the question-and-answer at the end of the session, comoderator Christina Sarah Thornton, MD, PhD, FRCPC from the University of Calgary, Alberta, asked Dr. Dickson whether the question of antianaerobic overuse in the ICU “is a function that we aren’t able yet from a diagnostic perspective to identify the group that may need antianaerobes? Because we often don’t get culture data back in time for a critically ill patient. Do you think there could maybe be a more rapid diagnostic for these patients?”
He replied that “a lot of our problems would be solved if we had really good, reliable rapid diagnostics for infection,” but noted that most of the patients in the study mentioned above did not have indications for antianaerobics.
Asked by this reporter whether Dr. Dickson’s presentation changed her mind about the use of piperacillin-tazobactam in her patients, Dr. Thornton replied “Yes! It did for me.”
She noted that although in Canada respirologists don’t work in intensive care units, “it makes me wonder about just giving pip-tazo to patients that are really sick. It definitely changed my mind.”
The work of Dr. Dickson and colleagues is supported by National Institute of Health and Agency for Healthcare Research and Quality grants. He reported no other relevant disclosures. Dr. Thornton had no relevant disclosures.
SAN DIEGO —
“You may not be personally moved by a 2- to 5-percent absolute difference in mortality, but sepsis is so common and so lethal that even small differences in outcomes can actually translate into enormous public health implications,” said Robert P. Dickson, MD a pulmonary and critical care specialist at the University of Michigan in Ann Arbor.
If instead of prescribing piperacillin-tazobactam (Zosyn; pip-tazo) for sepsis critical care specialists were to switch to cefepime “even if you make very conservative assumptions like a modest effect size, you’re still talking about [saving] thousands of lives a year,” he said in a scientific symposium at the American Thoracic Society’s international conference.
“This is why I say this isn’t really over the horizon; this is microbiome modulation that’s happening all the time,” he said.
Most patients with sepsis in a medical ICU with respiratory, urinary or bloodstream sources of infection do not have indications for antianaerobic antibiotics, and there are no head-to-head clinical trials demonstrating a benefit for one anti-sepsis antibiotic strategy over another he said.
“In contrast, every observational study between antianaerobic and non-antianaerobic shows benefits to the anaerobe-sparing [drugs], and it’s been shown with animal models too. So to my mind, it’s already practice changing. I need to be talked into giving antianaerobic antibiotics for septic patients” he said.
Targeting gut microbiota
There are three basic approaches to focusing on the gut microbiome as a therapeutic target. The hardest is attempting to engineer an ecosystem — a fiendishly complex task with unpredictable results that has never been shown to work in either the gut or in the ICU, Dr. Dickson said.
A second approach, the use of probiotics to repopulate the gut with beneficial bacteria, is largely futile in the ICU, as the large majority of patients are on antibiotics and can’t be safely weaned off of them while in critical care. In this situation, giving probiotics would be akin to try to repopulate a forest while a forest fire is raging, he said.
The third and easiest approach is to minimize dysbiosis — imbalance of organisms in the gut — in the first place.
Anaerobic bacteria in the gut have been shown in several different disease states and animals models to be protective against pneumonia, organ failure, and death.
To see whether antianaerobic antibiotics could increase risk for adverse outcomes in the ICU, Dr. Dickson and colleagues previously conducted a retrospective study of 3032 mechanically ventilated patients in their center who received antibiotics either with or without anaerobic coverage in the first 72 hours.
They found that patients treated with early antianaerobic antibiotics had decreased ventilator-associated pneumonia-free survival (hazard ratio [HR] 1.24), infection-free survival (HR 1.22), and overall survival (HR 1.14) compared with patients who received antibiotics without anaerobic cover (all comparisons statistically significant by confidence intervals).
In a subcohort of 116 patients for whom gut microbiota data compositions were available, those who received antianaerobic antibiotics had decreased initial gut bacterial density (P = .00038), increased microbiome expansion during hospitalization (P = .011), and domination of the microbiome by Enterobacteriaceae species (P = .045). They also found that Enterobacteriaceae were enriched among respiratory pathogens in antianaerobic treated patients, and that in murine models, treatment with antianaerobic antibiotics increased susceptibility to Enterobacteriaceae pneumonia and increased the risk of death from non-infectious injuries.
Pip-tazo vs. cefepime
In the ACORN (Antibiotic Choice on Renal Outcomes) trial, results of which were reported by this news organization in November 2023, there were no differences in the highest stage of acute kidney injury or death in the first 14 days between piperacillin-tazobactam and cefepime. Remarking on the results, lead investigator Edward T. Qian, MD, MSc from Vanderbilt University in Nashville, Tennessee, said “I think the big takeaway is that you should feel comfortable starting or using pip-tazo for your patients who are coming into the hospital and receiving empiric antibiotics for acute infection.”
But as Dr. Dickson’s group reported more recently in JAMA Internal Medicine, a 15-month pip-tazo shortage allowed the investigators to conduct a natural experiment comparing 90-day outcomes among 7569 patients with sepsis who received vancomycin plus either pip-tazo or cefepime.
They found in an instrumental variable analysis that piperacillin-tazobactam was associated with an absolute increase in mortality at 90 days of 5.0%, and that patients who received this antianaerobic antibiotic had 2.1 fewer organ failure–free days, 1.1 fewer ventilator-free days, and 1.5 fewer vasopressor-free days.
“Our study reveals the potential risks associated with empirical piperacillin-tazobactam in patients with sepsis without a specific indication for antianaerobic therapy. These findings should prompt reconsideration and further study of the widespread use of empirical antianaerobic antibiotics in sepsis,” the investigators concluded.
Who gets what?
In the question-and-answer at the end of the session, comoderator Christina Sarah Thornton, MD, PhD, FRCPC from the University of Calgary, Alberta, asked Dr. Dickson whether the question of antianaerobic overuse in the ICU “is a function that we aren’t able yet from a diagnostic perspective to identify the group that may need antianaerobes? Because we often don’t get culture data back in time for a critically ill patient. Do you think there could maybe be a more rapid diagnostic for these patients?”
He replied that “a lot of our problems would be solved if we had really good, reliable rapid diagnostics for infection,” but noted that most of the patients in the study mentioned above did not have indications for antianaerobics.
Asked by this reporter whether Dr. Dickson’s presentation changed her mind about the use of piperacillin-tazobactam in her patients, Dr. Thornton replied “Yes! It did for me.”
She noted that although in Canada respirologists don’t work in intensive care units, “it makes me wonder about just giving pip-tazo to patients that are really sick. It definitely changed my mind.”
The work of Dr. Dickson and colleagues is supported by National Institute of Health and Agency for Healthcare Research and Quality grants. He reported no other relevant disclosures. Dr. Thornton had no relevant disclosures.
SAN DIEGO —
“You may not be personally moved by a 2- to 5-percent absolute difference in mortality, but sepsis is so common and so lethal that even small differences in outcomes can actually translate into enormous public health implications,” said Robert P. Dickson, MD a pulmonary and critical care specialist at the University of Michigan in Ann Arbor.
If instead of prescribing piperacillin-tazobactam (Zosyn; pip-tazo) for sepsis critical care specialists were to switch to cefepime “even if you make very conservative assumptions like a modest effect size, you’re still talking about [saving] thousands of lives a year,” he said in a scientific symposium at the American Thoracic Society’s international conference.
“This is why I say this isn’t really over the horizon; this is microbiome modulation that’s happening all the time,” he said.
Most patients with sepsis in a medical ICU with respiratory, urinary or bloodstream sources of infection do not have indications for antianaerobic antibiotics, and there are no head-to-head clinical trials demonstrating a benefit for one anti-sepsis antibiotic strategy over another he said.
“In contrast, every observational study between antianaerobic and non-antianaerobic shows benefits to the anaerobe-sparing [drugs], and it’s been shown with animal models too. So to my mind, it’s already practice changing. I need to be talked into giving antianaerobic antibiotics for septic patients” he said.
Targeting gut microbiota
There are three basic approaches to focusing on the gut microbiome as a therapeutic target. The hardest is attempting to engineer an ecosystem — a fiendishly complex task with unpredictable results that has never been shown to work in either the gut or in the ICU, Dr. Dickson said.
A second approach, the use of probiotics to repopulate the gut with beneficial bacteria, is largely futile in the ICU, as the large majority of patients are on antibiotics and can’t be safely weaned off of them while in critical care. In this situation, giving probiotics would be akin to try to repopulate a forest while a forest fire is raging, he said.
The third and easiest approach is to minimize dysbiosis — imbalance of organisms in the gut — in the first place.
Anaerobic bacteria in the gut have been shown in several different disease states and animals models to be protective against pneumonia, organ failure, and death.
To see whether antianaerobic antibiotics could increase risk for adverse outcomes in the ICU, Dr. Dickson and colleagues previously conducted a retrospective study of 3032 mechanically ventilated patients in their center who received antibiotics either with or without anaerobic coverage in the first 72 hours.
They found that patients treated with early antianaerobic antibiotics had decreased ventilator-associated pneumonia-free survival (hazard ratio [HR] 1.24), infection-free survival (HR 1.22), and overall survival (HR 1.14) compared with patients who received antibiotics without anaerobic cover (all comparisons statistically significant by confidence intervals).
In a subcohort of 116 patients for whom gut microbiota data compositions were available, those who received antianaerobic antibiotics had decreased initial gut bacterial density (P = .00038), increased microbiome expansion during hospitalization (P = .011), and domination of the microbiome by Enterobacteriaceae species (P = .045). They also found that Enterobacteriaceae were enriched among respiratory pathogens in antianaerobic treated patients, and that in murine models, treatment with antianaerobic antibiotics increased susceptibility to Enterobacteriaceae pneumonia and increased the risk of death from non-infectious injuries.
Pip-tazo vs. cefepime
In the ACORN (Antibiotic Choice on Renal Outcomes) trial, results of which were reported by this news organization in November 2023, there were no differences in the highest stage of acute kidney injury or death in the first 14 days between piperacillin-tazobactam and cefepime. Remarking on the results, lead investigator Edward T. Qian, MD, MSc from Vanderbilt University in Nashville, Tennessee, said “I think the big takeaway is that you should feel comfortable starting or using pip-tazo for your patients who are coming into the hospital and receiving empiric antibiotics for acute infection.”
But as Dr. Dickson’s group reported more recently in JAMA Internal Medicine, a 15-month pip-tazo shortage allowed the investigators to conduct a natural experiment comparing 90-day outcomes among 7569 patients with sepsis who received vancomycin plus either pip-tazo or cefepime.
They found in an instrumental variable analysis that piperacillin-tazobactam was associated with an absolute increase in mortality at 90 days of 5.0%, and that patients who received this antianaerobic antibiotic had 2.1 fewer organ failure–free days, 1.1 fewer ventilator-free days, and 1.5 fewer vasopressor-free days.
“Our study reveals the potential risks associated with empirical piperacillin-tazobactam in patients with sepsis without a specific indication for antianaerobic therapy. These findings should prompt reconsideration and further study of the widespread use of empirical antianaerobic antibiotics in sepsis,” the investigators concluded.
Who gets what?
In the question-and-answer at the end of the session, comoderator Christina Sarah Thornton, MD, PhD, FRCPC from the University of Calgary, Alberta, asked Dr. Dickson whether the question of antianaerobic overuse in the ICU “is a function that we aren’t able yet from a diagnostic perspective to identify the group that may need antianaerobes? Because we often don’t get culture data back in time for a critically ill patient. Do you think there could maybe be a more rapid diagnostic for these patients?”
He replied that “a lot of our problems would be solved if we had really good, reliable rapid diagnostics for infection,” but noted that most of the patients in the study mentioned above did not have indications for antianaerobics.
Asked by this reporter whether Dr. Dickson’s presentation changed her mind about the use of piperacillin-tazobactam in her patients, Dr. Thornton replied “Yes! It did for me.”
She noted that although in Canada respirologists don’t work in intensive care units, “it makes me wonder about just giving pip-tazo to patients that are really sick. It definitely changed my mind.”
The work of Dr. Dickson and colleagues is supported by National Institute of Health and Agency for Healthcare Research and Quality grants. He reported no other relevant disclosures. Dr. Thornton had no relevant disclosures.
FROM ATS 2024
Vacationing Doctors Fight to Revive a Drowned Child
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Transesophageal ultrasound: The future of ultrasound in the ICU
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074


















