User login
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
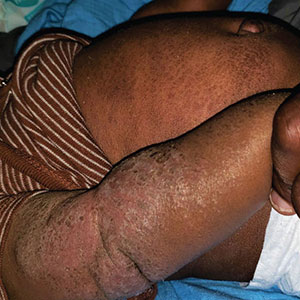
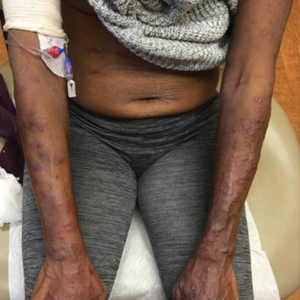
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10
magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).
Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
PRACTICE POINTS
- Lyngbya majuscula causes seaweed dermatitis in swimmers and can be prevented by avoiding rough turbid waters in areas known to have L majuscula blooms.
- Seaweed dermatitis should be included in the differential diagnosis for erythematous papulovesicular rashes manifesting in patients who recently have spent time in the ocean.
Hypopigmented Cutaneous Langerhans Cell Histiocytosis in a Hispanic Infant
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
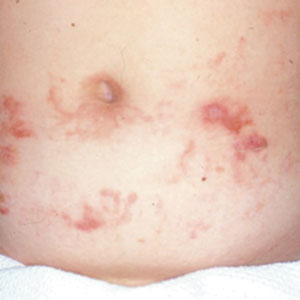
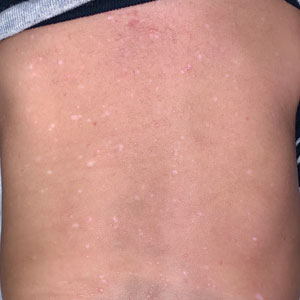
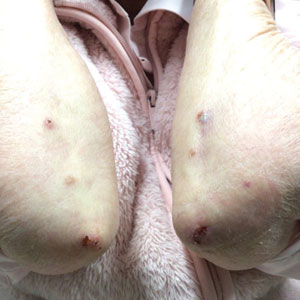
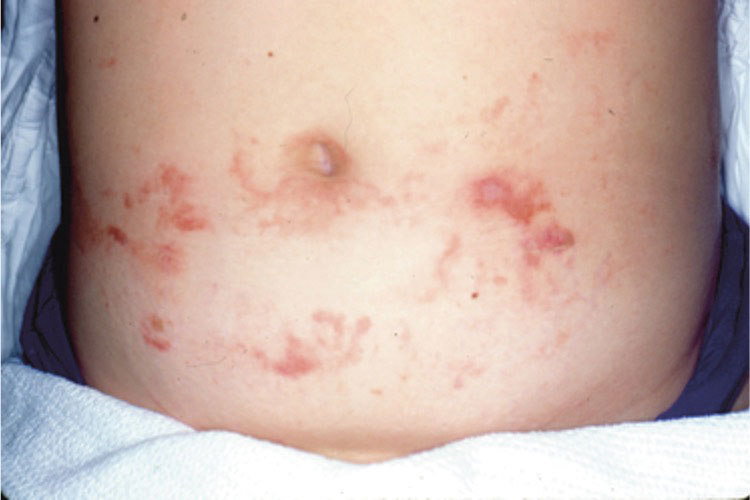
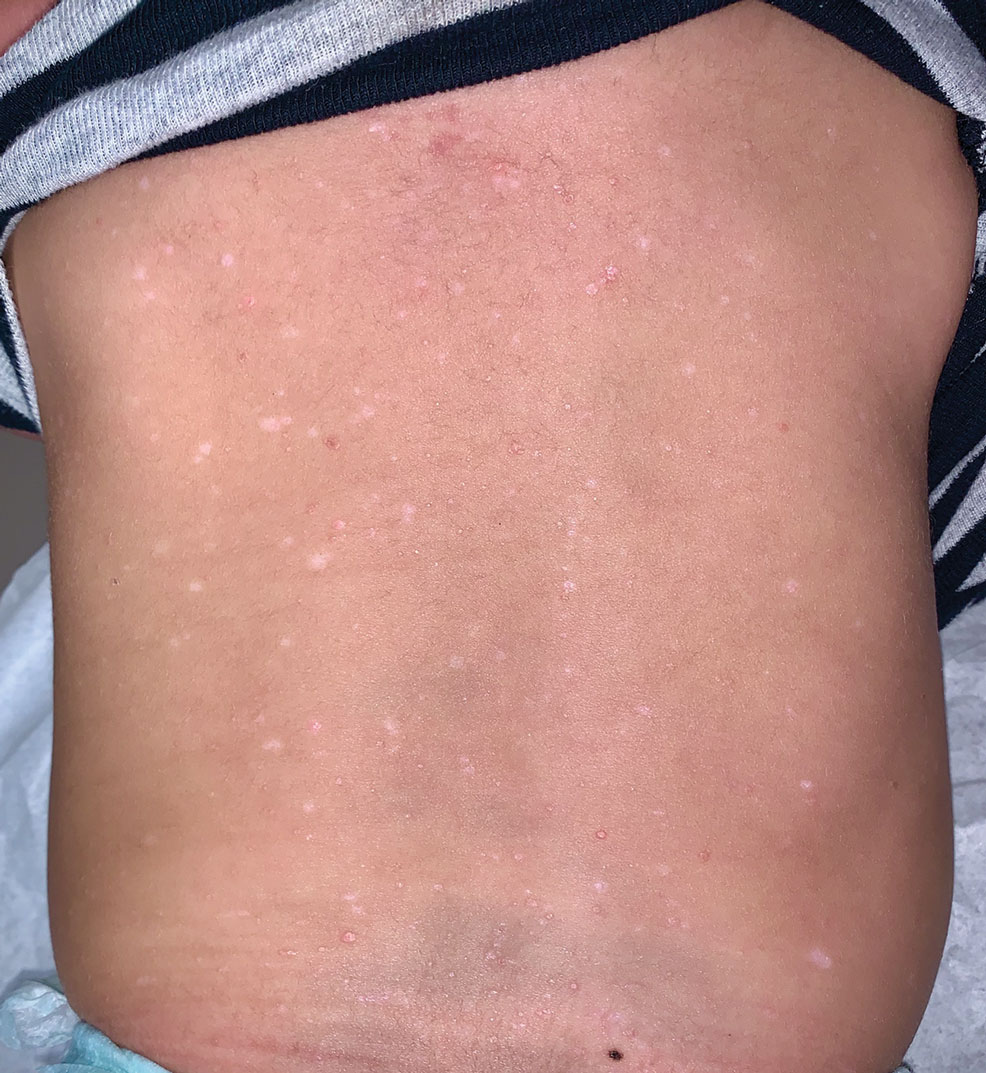
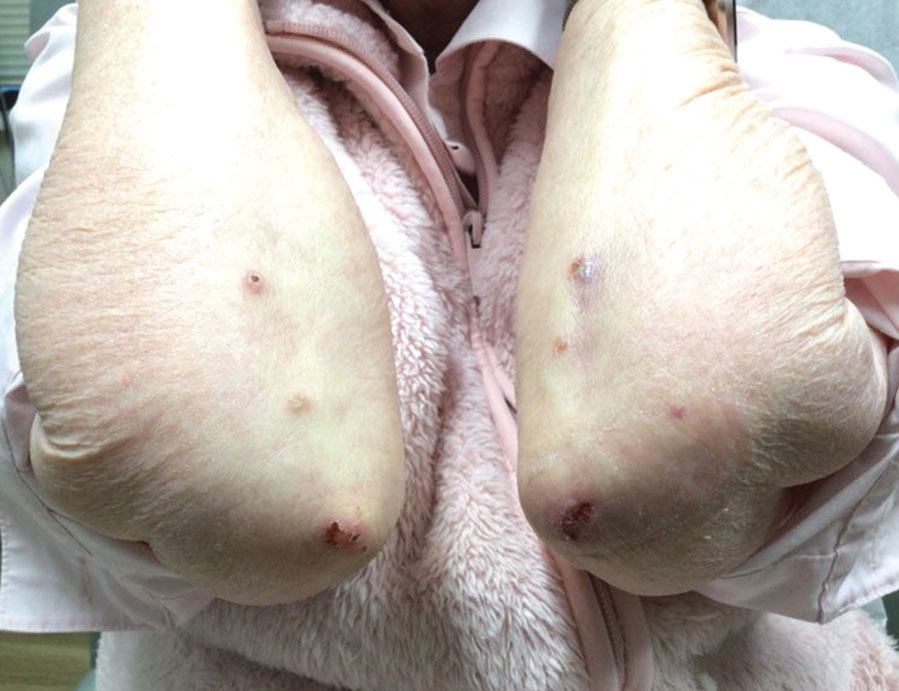
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
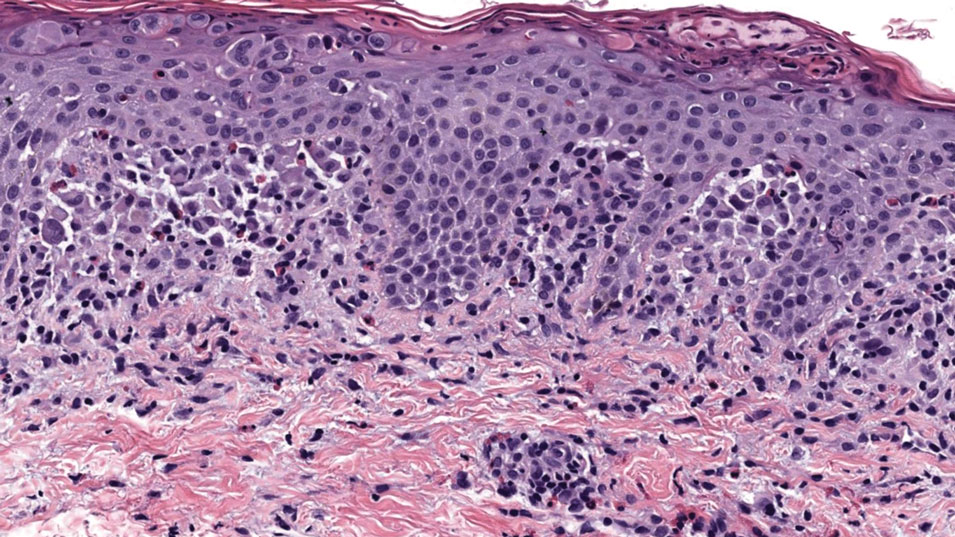
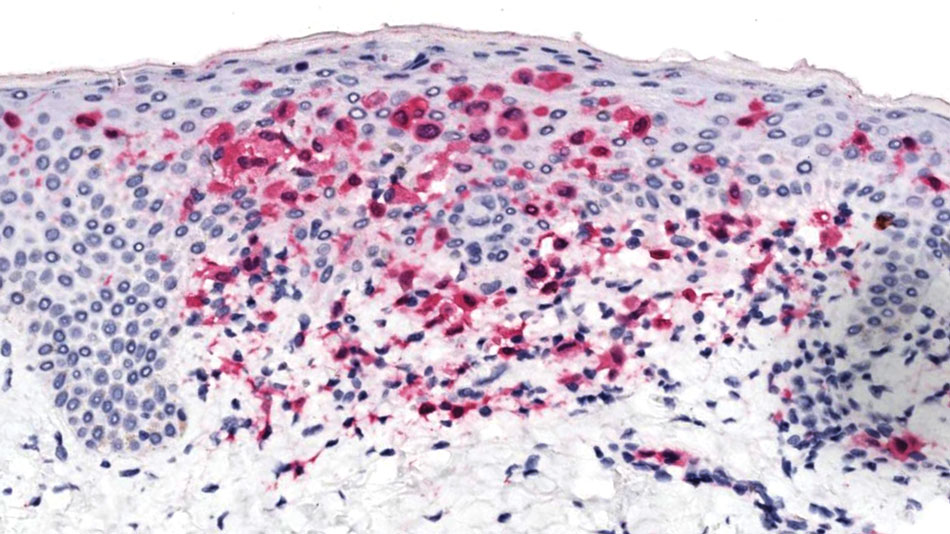
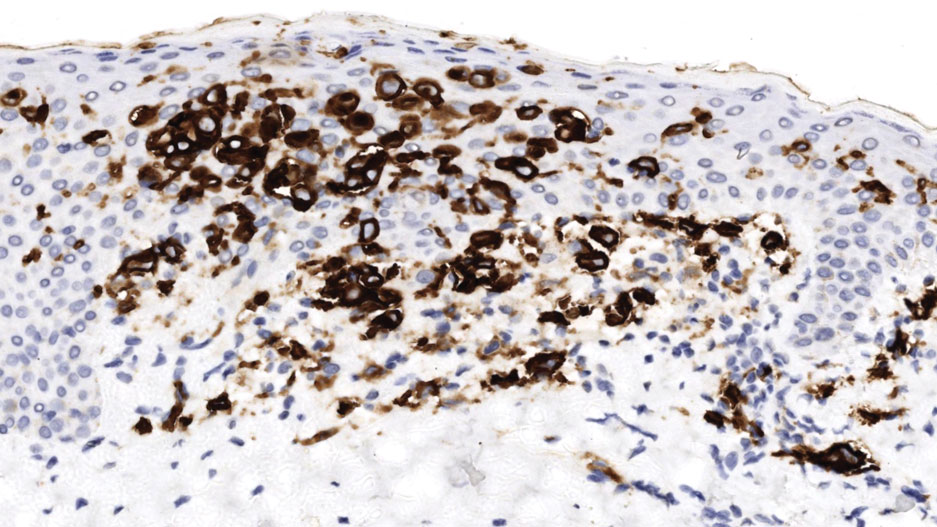
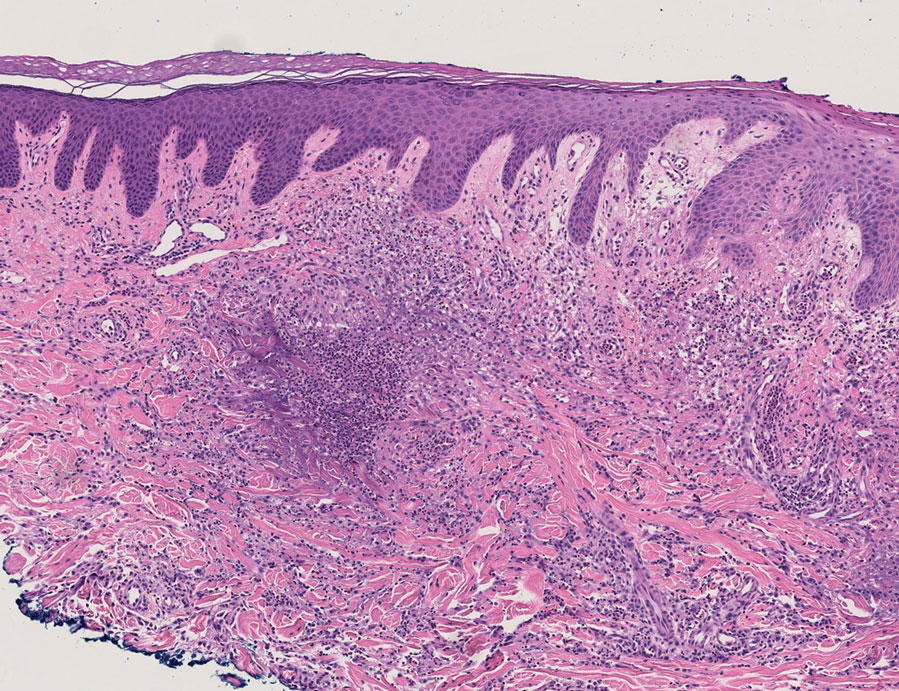
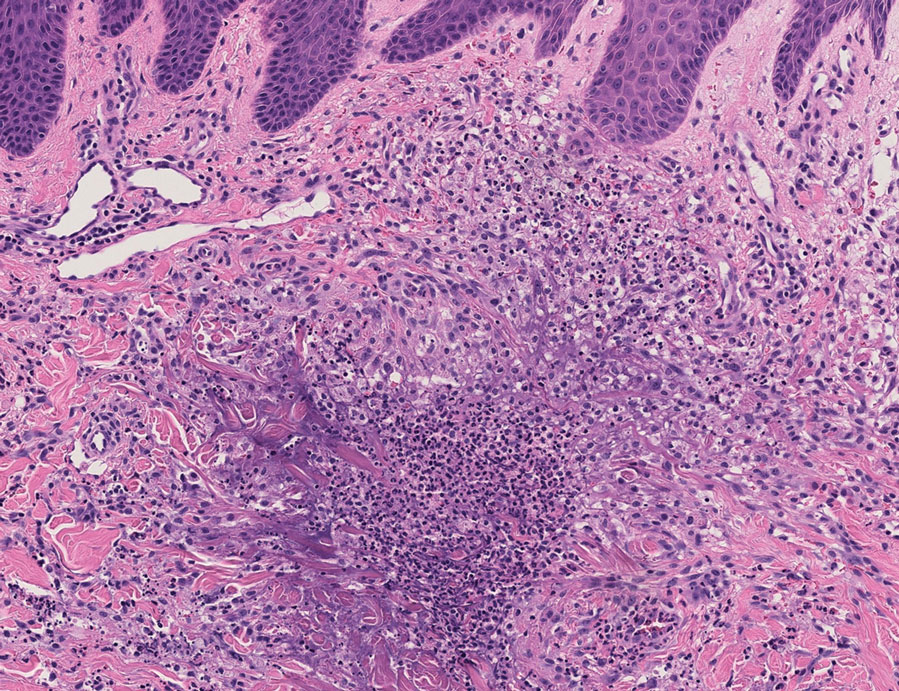
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.
The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
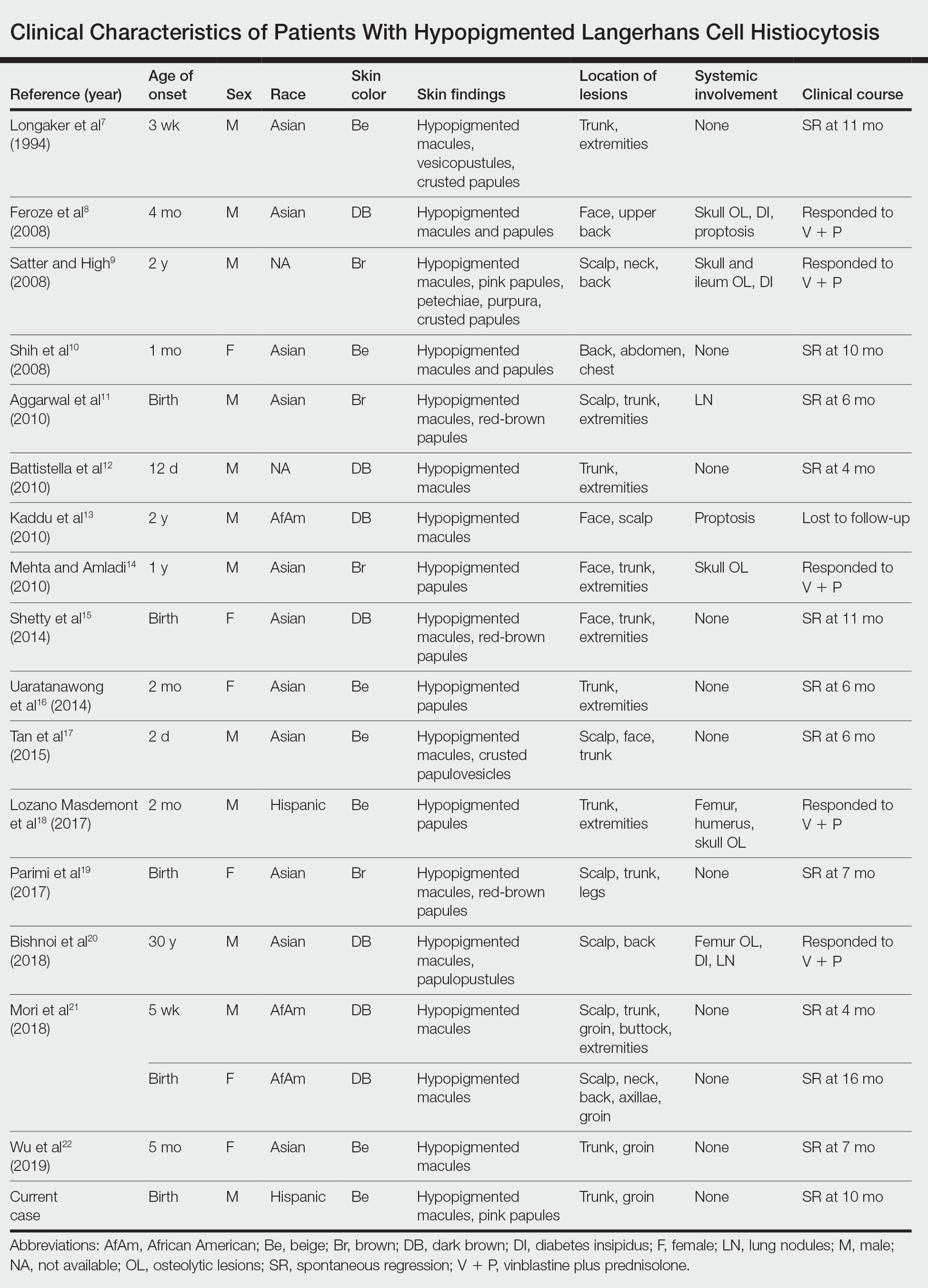
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
Practice Points
- Dermatologists should be aware of the hypopigmented variant of cutaneous Langerhans cell histiocytosis (LCH), which has been reported exclusively in patients with skin of color.
- Langerhans cell histiocytosis should be included in the differential diagnosis of hypopigmented macules, which may be the only cutaneous manifestation or may coincide with typical lesions of LCH.
- Hypopigmented cutaneous LCH may be more common in newborns and associated with a better prognosis.
Reactive Granulomatous Dermatitis: Variability of the Predominant Inflammatory Cell Type
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
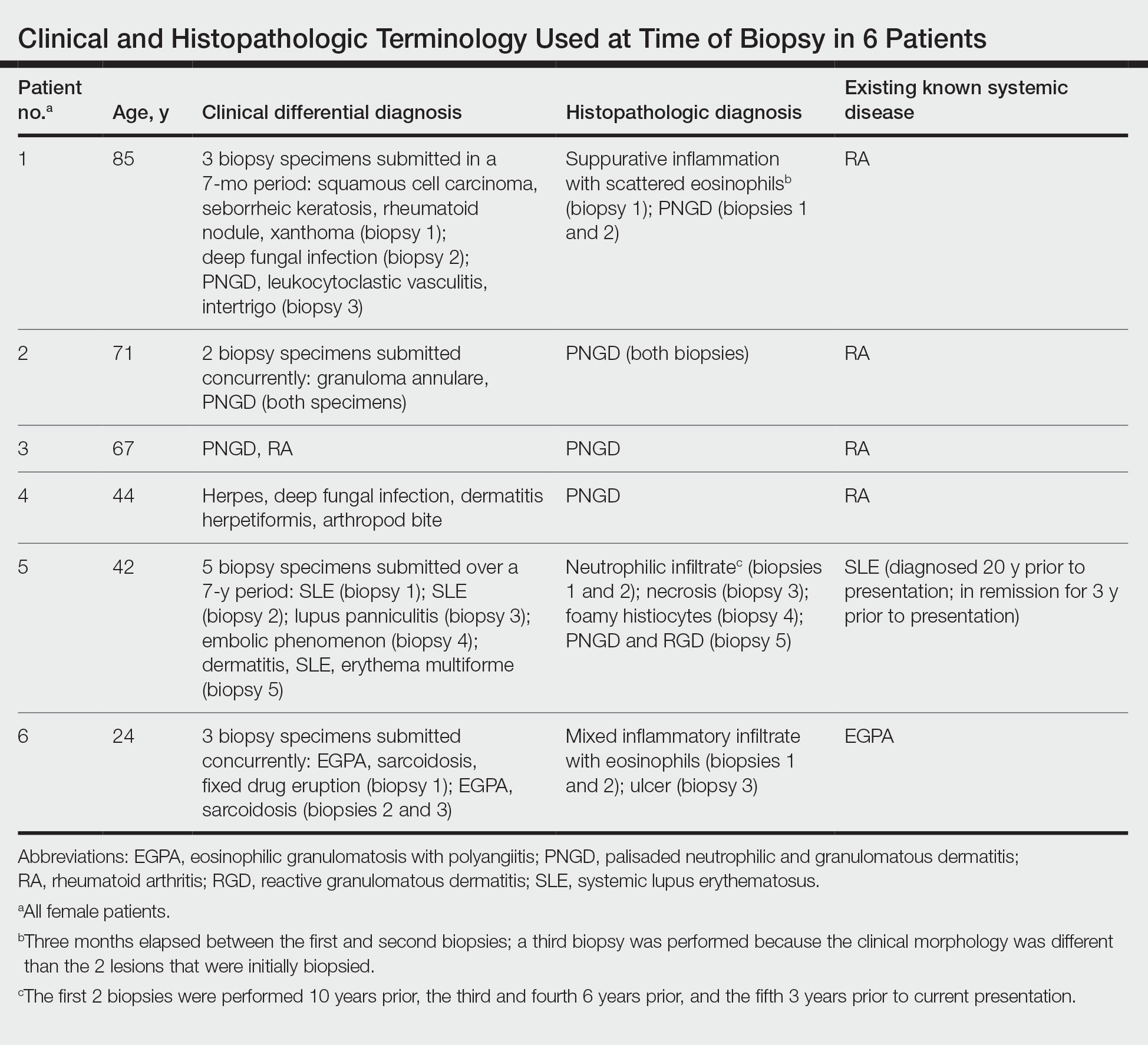
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.
The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.
In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.
The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
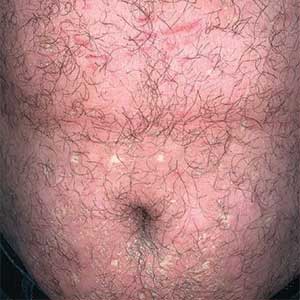
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
Practice Points
- The term reactive granulomatous dermatitis (RGD) provides a unifying rubric for palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction.
- Reactive granulomatous dermatitis can have a variable infiltrate that includes neutrophils, histiocytes, and/or eosinophils.
- Awareness of the variability in inflammatory cell type is important for the diagnosis of RGD.
Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed
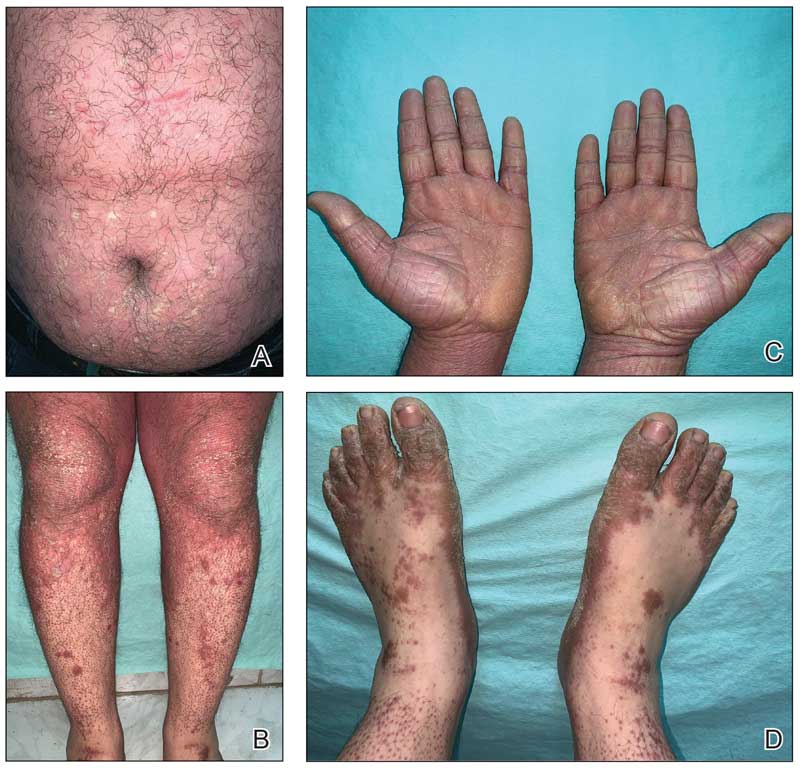
Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed
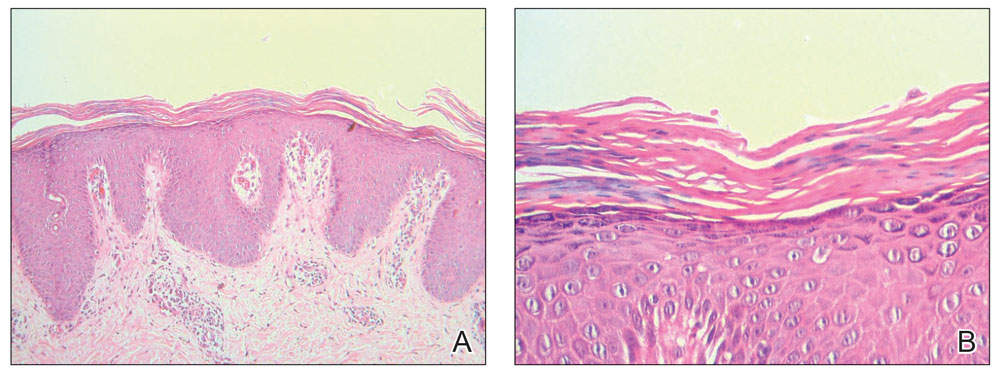
Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
Practice Points
- Dermatologists must be aware of the possibility of COVID-19 vaccine–induced pityriasis rubra pilaris (PRP), especially in de novo cases.
- Management of these cases usually follows similar standards for PRP cases.
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
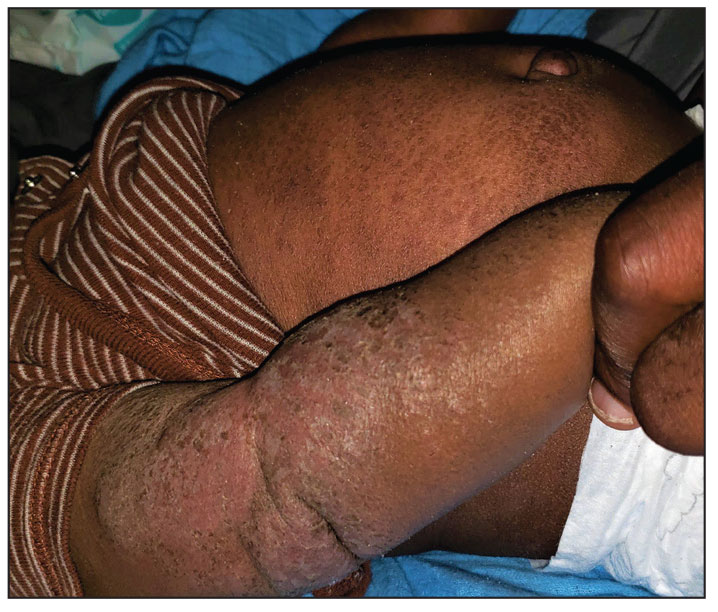
Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.
Micronutrient Deficiencies in Patients With Inflammatory Bowel Disease
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.
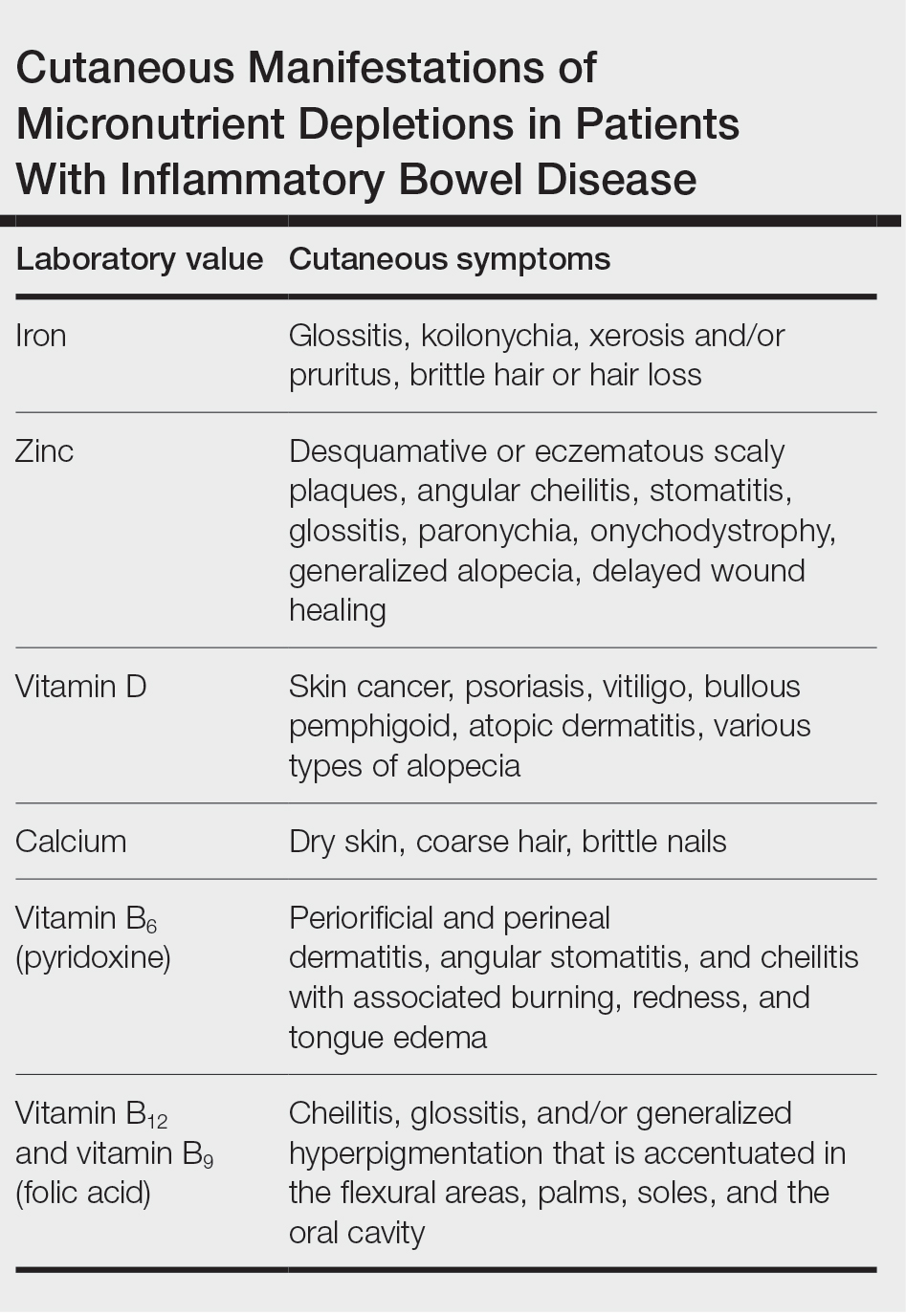
Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.

Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.

Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
Practice Points
- Patients with inflammatory bowel disease (IBD) are at increased risk for vitamin and nutrient deficiencies that may be identified first through cutaneous manifestations.
- Because active inflammation in IBD may skew routine laboratory values used for screening of micronutrient deficiencies, be cautious when interpreting these values.
- Patients taking systemic therapies for IBD such as corticosteroids and methotrexate are at higher risk for nutritional deficiencies.
Eosinophilic Pustular Folliculitis in the Setting of Untreated Chronic Lymphocytic Leukemia
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.
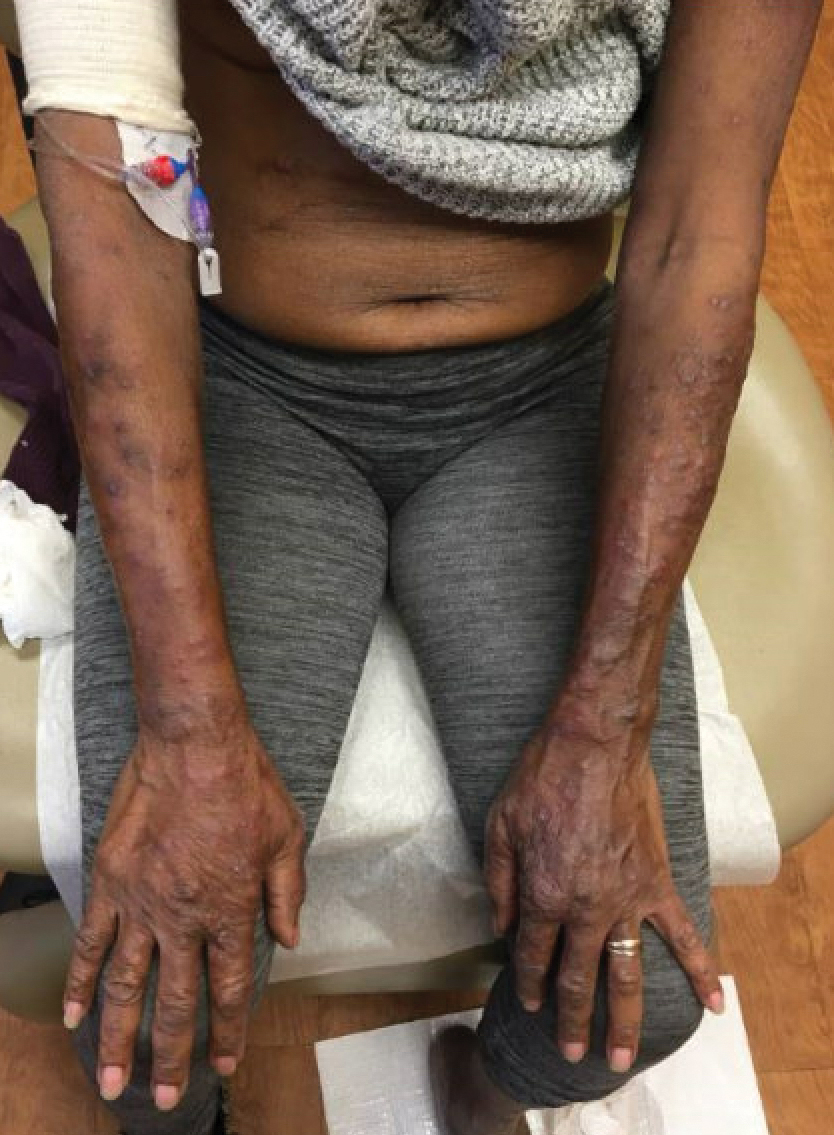
A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.
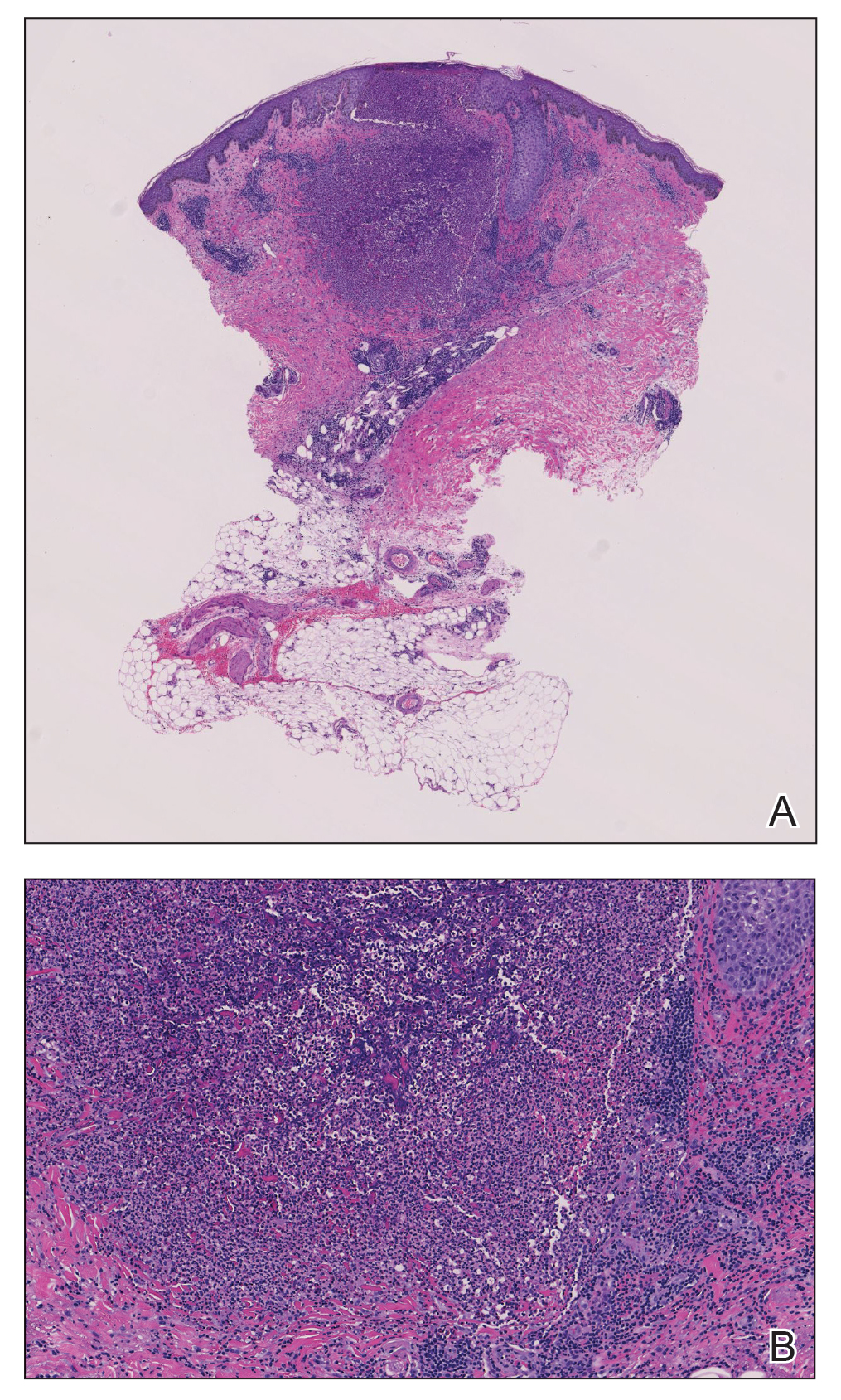
Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
Practice Points
- Eosinophilic pustular folliculitis (EPF) is associated with an immunosuppressed state, as in patients with underlying hematologic malignancy.
- Topical corticosteroids remain the first-line treatment for EPF; however, antimicrobial agents have been used with moderate success when topical therapies have failed.
Allergens Present in Most ‘Hypoallergenic’ Baby Cleansers, Study Finds
TOPLINE:
METHODOLOGY:
- Many baby cleansers are marketed as “hypoallergenic,” but these claims are not validated.
- This study assessed the potential allergens and marketing claims in best-selling baby cleansers.
- The researchers collected ingredients and marketing claims of the top 50 best-selling baby body wash products sold on Amazon on April 4, 2023.
- Ingredient lists were checked for potential allergens using the 2020 American Contact Dermatitis Society (ACDS) core allergen series, which lists 90 common allergens for adults and children.
TAKEAWAY:
- In the 50 cleansers tested, 10 allergens were identified. Overall, 94% of the cleansers contained at least one allergen, averaging 2.9 allergens per product; cocamidopropyl betaine (72%), fragrance (64%), and sodium benzoate (54%) were the most common allergens.
- All cleansers had at least five marketing claims, with an average of 10.9 claims per product; the most common claims were “paraben-free” (88%), “phthalate-free” (84%), “tear-free” (74%), and “hypoallergenic” or “allergy-tested” (74%).
- There was no significant difference in the number of allergens in the cleansers marketed as “hypoallergenic” or “allergy tested” compared with cleansers that did not have these claims (P = .843).
- Fewer allergens were found in cleansers endorsed by the National Eczema Association (P = .004) or labeled “synthetic fragrance-free” (P = .003).
- There was a positive correlation between a greater number of allergens and an increased number of marketing claims (r = 0.547, P < .001) and a negative correlation between cost and number of allergens (r = −0.450, P = .001).
IN PRACTICE:
Because marketing claims like “hypoallergenic” may be misleading, “clinicians should counsel parents to carefully examine cleanser ingredients or consider selecting cleansers” endorsed by the National Eczema Association or another international eczema organization, especially for infants and children with a history of atopic dermatitis, the authors wrote.
SOURCE:
The study, led by Sasan D. Noveir, BA, from the University of California, Los Angeles, and coauthors from the division of dermatology at UCLA, was published online in Pediatric Dermatology.
LIMITATIONS:
The study only evaluated top-selling products from a single online source at a specific time, which may limit generalizability. Potential allergens not included in the ACDS core series may be present.
DISCLOSURES:
The study did not disclose any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Many baby cleansers are marketed as “hypoallergenic,” but these claims are not validated.
- This study assessed the potential allergens and marketing claims in best-selling baby cleansers.
- The researchers collected ingredients and marketing claims of the top 50 best-selling baby body wash products sold on Amazon on April 4, 2023.
- Ingredient lists were checked for potential allergens using the 2020 American Contact Dermatitis Society (ACDS) core allergen series, which lists 90 common allergens for adults and children.
TAKEAWAY:
- In the 50 cleansers tested, 10 allergens were identified. Overall, 94% of the cleansers contained at least one allergen, averaging 2.9 allergens per product; cocamidopropyl betaine (72%), fragrance (64%), and sodium benzoate (54%) were the most common allergens.
- All cleansers had at least five marketing claims, with an average of 10.9 claims per product; the most common claims were “paraben-free” (88%), “phthalate-free” (84%), “tear-free” (74%), and “hypoallergenic” or “allergy-tested” (74%).
- There was no significant difference in the number of allergens in the cleansers marketed as “hypoallergenic” or “allergy tested” compared with cleansers that did not have these claims (P = .843).
- Fewer allergens were found in cleansers endorsed by the National Eczema Association (P = .004) or labeled “synthetic fragrance-free” (P = .003).
- There was a positive correlation between a greater number of allergens and an increased number of marketing claims (r = 0.547, P < .001) and a negative correlation between cost and number of allergens (r = −0.450, P = .001).
IN PRACTICE:
Because marketing claims like “hypoallergenic” may be misleading, “clinicians should counsel parents to carefully examine cleanser ingredients or consider selecting cleansers” endorsed by the National Eczema Association or another international eczema organization, especially for infants and children with a history of atopic dermatitis, the authors wrote.
SOURCE:
The study, led by Sasan D. Noveir, BA, from the University of California, Los Angeles, and coauthors from the division of dermatology at UCLA, was published online in Pediatric Dermatology.
LIMITATIONS:
The study only evaluated top-selling products from a single online source at a specific time, which may limit generalizability. Potential allergens not included in the ACDS core series may be present.
DISCLOSURES:
The study did not disclose any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Many baby cleansers are marketed as “hypoallergenic,” but these claims are not validated.
- This study assessed the potential allergens and marketing claims in best-selling baby cleansers.
- The researchers collected ingredients and marketing claims of the top 50 best-selling baby body wash products sold on Amazon on April 4, 2023.
- Ingredient lists were checked for potential allergens using the 2020 American Contact Dermatitis Society (ACDS) core allergen series, which lists 90 common allergens for adults and children.
TAKEAWAY:
- In the 50 cleansers tested, 10 allergens were identified. Overall, 94% of the cleansers contained at least one allergen, averaging 2.9 allergens per product; cocamidopropyl betaine (72%), fragrance (64%), and sodium benzoate (54%) were the most common allergens.
- All cleansers had at least five marketing claims, with an average of 10.9 claims per product; the most common claims were “paraben-free” (88%), “phthalate-free” (84%), “tear-free” (74%), and “hypoallergenic” or “allergy-tested” (74%).
- There was no significant difference in the number of allergens in the cleansers marketed as “hypoallergenic” or “allergy tested” compared with cleansers that did not have these claims (P = .843).
- Fewer allergens were found in cleansers endorsed by the National Eczema Association (P = .004) or labeled “synthetic fragrance-free” (P = .003).
- There was a positive correlation between a greater number of allergens and an increased number of marketing claims (r = 0.547, P < .001) and a negative correlation between cost and number of allergens (r = −0.450, P = .001).
IN PRACTICE:
Because marketing claims like “hypoallergenic” may be misleading, “clinicians should counsel parents to carefully examine cleanser ingredients or consider selecting cleansers” endorsed by the National Eczema Association or another international eczema organization, especially for infants and children with a history of atopic dermatitis, the authors wrote.
SOURCE:
The study, led by Sasan D. Noveir, BA, from the University of California, Los Angeles, and coauthors from the division of dermatology at UCLA, was published online in Pediatric Dermatology.
LIMITATIONS:
The study only evaluated top-selling products from a single online source at a specific time, which may limit generalizability. Potential allergens not included in the ACDS core series may be present.
DISCLOSURES:
The study did not disclose any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Sulfites Selected as ACDS Allergen of the Year
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ACDS 2024