User login
VIDEO: Ischemic-stroke thrombectomy use widens and refines
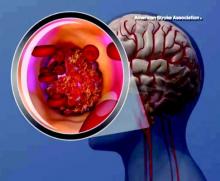
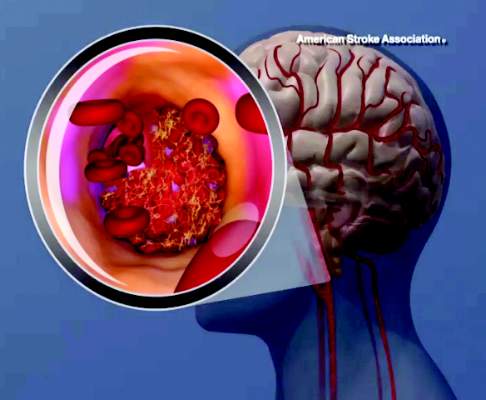
LOS ANGELES – The use of endovascular thrombectomy in the United States to treat appropriate patients with acute ischemic stroke mushroomed during the past year, following several early-2015 reports that collectively documented the dramatic clinical benefit of the treatment.
As endovascular thrombectomy use grows, stroke centers are also refining and reshaping delivery of the treatment in concert with administration of intravenous tissue plasminogen activator (TPA; alteplase; Activase), which remains a key partner in producing best outcomes for acute ischemic-stroke patients with a proximal occlusion of a large cerebral artery. Collapsing delivery of the two treatments into a more seamless and streamlined process shaves critical minutes to treatment delivery, an approach called parallel processing. Recent findings have also emboldened stroke specialists to seriously consider simplifying the brain imaging that stroke patients receive prior to these treatments, a step that could further cut time to intervention while also making thrombectomy even more widely available.
Use of thrombectomy surges
The biggest endovascular thrombectomy news of the past year is how it has taken off for treating selected patients with acute ischemic stroke. “The rollout over the past year has been explosive. Everything pretty much shut down after the negative trial results in 2013, but now more hospitals are offering thrombectomy,” said Dr. Thomas A. Kent, professor of neurology and director of stroke research and education at Baylor College of Medicine in Houston, in an interview at the International Stroke Conference sponsored by the American Heart Association.
The best documentation of this surge came in a poster presented at the conference by researchers at the University of California, San Francisco. They analyzed data on treatment of 357,973 patients with acute ischemic stroke who were hospitalized at any one of 161 U.S. academic medical centers during October 2009-July 2015 and included in the University Healthsystem Consortium database. They tracked the percentage of patients treated endovascularly during each calendar quarter of the study period.
During 2009-2013, use of endovascular treatment rose steadily but gradually, from 1.5% of stroke patients in 2009 to 3.1% during the fourth quarter of 2012. Then, following three reports of no benefit from endovascular treatment presented at the International Stroke Conference in February 2013 – the IMS III, MR RESCUE, and SYNTHESIS trials – the endovascular rate dropped immediately and quickly bottomed out at a level of 2.6% that remained steady through the third quarter of 2014. But when the positive endovascular results from the MR CLEAN study became public in the final week of 2014, endovascular use began to quickly rise again, and then began to skyrocket during the first quarter of 2015 with three additional positive trial results reported during the Stroke Conference in February 2015. By the end of the second quarter of 2015, usage stood at 4.7%, representing a projected year-over-year increase of about 150% for all of 2015, compared with 2014, reported Dr. Anthony S. Kim, a vascular neurologist and medical director of the Stroke Center at the University of California, San Francisco, and his associates.
To put these percentages in perspective, experts estimate that roughly 10%-15% of all stroke patients qualify for thrombectomy intervention.
Their data also showed that the percentage of hospitals included in the database that performed endovascular therapies for stroke rose steadily from about 40% of centers in 2009 to nearly 60% by mid-2015.
“Endovascular therapy with newer-generation devices is increasingly part of standard treatment for acute ischemic stroke,” they said in their poster. In addition, they cited a “new urgency to evaluate regional access to embolectomy [another name for thrombectomy] nationally and to identify system-based solutions to improve access in underserved areas.”
Several stroke experts interviewed at the conference added their own anecdotal view of thrombectomy’s rapidly expanding use for appropriate acute ischemic stroke patients during 2015, and the need for continued effort to broaden its U.S. availability.
“The number of thrombectomies fell off after the negative 2013 trials and stayed flat until a year ago, but then jumped up. It has been very dramatic,” said Dr. Wade S. Smith, professor of neurology and director of the neurovascular service at the University of California, San Francisco.
“Thrombectomy use tremendously increased since February 2015,” said Dr. Mark J. Alberts, professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas, in a video interview during the conference. But despite this growth, “the major challenge [today] is geography;” that is, reaching patients in suburban and rural areas who are not as close to the primarily urban medical centers that currently offer the procedure.
“We now have about 100 certified comprehensive stroke centers in the U.S.,” and by definition comprehensive stroke centers have the capability of treating patients with endovascular thrombectomy, noted Dr. Jeffrey Saver, professor of neurology and director of the stroke unit at the University of California, Los Angeles.
“Certification of these centers did not begin until about 2-3 years ago. But we probably need 300-400 of these centers” to provide thrombectomy to most U.S. stroke patients, he said. “A lot of additional hospitals are close to certification. I anticipate that over the next 1-2 years we will be in the neighborhood of having the number of centers we need,” Dr. Saver said in an interview.
Making thrombectomy better
In addition to expanding availability, the specifics of how endovascular thrombectomy gets delivered is evolving. A major trend is movement toward a “parallel processing” model, in which patients with an acute clinical presentation of a stroke amenable for endovascular treatment simultaneously undergo CT angiography to confirm and localize the large-artery clot causing their stroke, receive intravenous TPA, and undergo preparation for the endovascular access needed to remove the clot.
A pooled analysis of the recent, positive endovascular thrombectomy trials that was presented at the conference showed how quick you need to be to obtain a benefit from the procedures. “This gives us a starting point to further improve the target metrics for imaging and puncture times,” Dr. Saver said. “We want to shorten door-to-needle times for TPA and door-to-puncture times for thrombectomy, and the processes that need to be addressed for rapid delivery of both of these are very similar. We need for patients to only make a pit stop in the ED; we need to have the catheterization team ready to go in the thrombectomy suite within 30 minutes; and we need to emphasize speed in access to the target clot rather than time-consuming diagnostic angiography.”
“We now face the issue of how to best integrate TPA treatment and clot removal.” Dr. Kent said. “People are still trying to work that out. With parallel processing there is some overuse of resources: Some patients recover with TPA alone and don’t need thrombectomy. We are getting closer to the cardiology model of MI treatment. It’s now clear that there needs to be a simple, safe, effective way to do both TPA treatment and thrombectomy. We need to model ourselves on the cardiology experience.”
“If you can deal with the TPA decision in the same room without moving patients from room to room, from a scanner to a catheterization suite, you can really shorten the time to treatment,” Dr. Smith explained. “This is identical to the model that cardiologists have developed. We should now consider taking stroke patients directly to the angiography room in addition to administering TPA. We still need cross-sectional imaging, but the quality of the image from an angiography suite is probably sufficient to make a TPA decision. So you can start TPA while you are getting arterial access. The idea is simultaneous approaches to the patient instead of serial.”
“The whole system moves at the same time to eliminate wasted time,” Dr. Alberts summed up.
One of the big questions that has come up in this effort to speed up treatment and carve the quickest route to endovascular thrombectomy is whether TPA remains necessary. The skeptics’ position is, why waste time administering TPA if you’re also going to take out the offending clot?
The answer, at least for now, is that all signs indicate that giving TPA helps and is worth delivering.
“The 2015 thrombectomy trials had big differences among them in the dosage of TPA administered, and in the percentage of patients who received TPA. When 100% of patients received TPA they had the best outcomes,” Dr. Kent said. “There was a clear synergistic relationship between thrombectomy and TPA. There has been a trend to think about sending patients straight to thrombectomy and skipping TPA, but my colleagues and I think that we need to hold off on doing that. For now, if a patient is eligible to receive TPA they should get it and then quickly move to endovascular therapy. We are not yet ready to know it’s okay to go straight to endovascular treatment. In SWIFT-PRIME, it was pretty clear that the good outcomes were attributable to both [thrombectomy plus TPA]. Treating patients with TPA helps soften the clot to make it easier to remove, and improves flow through collateral arteries.”
“Our data in Memphis show that patients do better with thrombectomy plus intravenous TPA than on TPA alone,” agreed Dr. Lucas Elijovich, a neurologist at the University of Tennessee Health Science Center in Memphis, in an interview.
Simpler imaging also saves time
Although it’s not yet proven, another new wrinkle in working up acute ischemic-stroke patients for TPA and thrombectomy treatment is the idea that simpler and more widely available CT imaging, especially CT angiography of cerebral arteries, may suffice for confirming and localizing the culprit clot.
This concept received a significant boost at the International Stroke Conference in data reported from the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) trial, yet another study that compared treatment with TPA alone with TPA plus endovascular thrombectomy, this time in 65 randomized patients treated at any of 11 U.K. centers. PISTE had a low enrollment level because the trial stopped prematurely, in July 2015, following the news that several fully completed trials had collectively established the superiority of endovascular thrombectomy plus TPA, thereby making it unethical to continue yet another randomized study.
This premature stoppage prevented PISTE from itself producing a statistically significant difference for its primary efficacy endpoint in favor of the combined treatment, although the results did show a nominal advantage to using thrombectomy plus TPA over TPA alone that was fully consistent with the other studies, Dr. Keith W. Muir reported at the conference.
But what made the PISTE results especially notable was that the trial achieved this consistent outcome with a “simpler” imaging protocol for patients during their workup that used only CT angiography, avoiding the cerebral CT perfusion imaging or MRI used in several of the other TPA-plus-thrombectomy versus TPA-only trials, noted Dr. Muir, professor of neuroscience and head of the stroke imaging group at the University of Glasgow.
“PISTE raises the question of how much imaging is necessary,” Dr. Kent commented.
“The PISTE results are exciting. A lot of us believe that all we need to know is that there is a blockage in a target vessel,” Dr. Smith said. “If we have that information, then we can identify a population of patients who will benefit from [thrombectomy]. CT angiography is simple and can easily fit into work flows.”
“PISTE used a very simple imaging system that makes thrombectomy even more applicable and generalizable to less resourced health systems,” Dr. Saver said. “Although the results from PISTE were not internally statistically significant because the trial ended early, the results were consistent with the external studies of thrombectomy, so it provides further evidence for benefit from thrombectomy.” And because the consistent results were achieved with simpler imaging it suggests simpler imaging may be all that’s needed.
“That’s a major question to wrestle with,” Dr. Saver suggested. “We need addition trials with a head-to-head comparison of simpler and more sophisticated imaging so we can tailor treatment to patients who would benefit from simpler and faster imaging.”
Dr. Kent had no disclosures. Dr. Kim has received research funding from SanBio and Biogen. Dr. Smith served on the data safety and monitoring board for a trial funded by Stryker. Dr. Alberts has been a consultant to Genentech. Dr. Saver has been a consultant to Stryker, Neuravi, Cognition Medical, Boehringer Ingelheim, and Medtronic. Dr. Elijovich has been a consultant to Stryker and Codman and received research support from Siemens. Dr. Muir has received research support from ReNeuron and unrestricted grants from Codman and Covidien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LOS ANGELES – The use of endovascular thrombectomy in the United States to treat appropriate patients with acute ischemic stroke mushroomed during the past year, following several early-2015 reports that collectively documented the dramatic clinical benefit of the treatment.
As endovascular thrombectomy use grows, stroke centers are also refining and reshaping delivery of the treatment in concert with administration of intravenous tissue plasminogen activator (TPA; alteplase; Activase), which remains a key partner in producing best outcomes for acute ischemic-stroke patients with a proximal occlusion of a large cerebral artery. Collapsing delivery of the two treatments into a more seamless and streamlined process shaves critical minutes to treatment delivery, an approach called parallel processing. Recent findings have also emboldened stroke specialists to seriously consider simplifying the brain imaging that stroke patients receive prior to these treatments, a step that could further cut time to intervention while also making thrombectomy even more widely available.
Use of thrombectomy surges
The biggest endovascular thrombectomy news of the past year is how it has taken off for treating selected patients with acute ischemic stroke. “The rollout over the past year has been explosive. Everything pretty much shut down after the negative trial results in 2013, but now more hospitals are offering thrombectomy,” said Dr. Thomas A. Kent, professor of neurology and director of stroke research and education at Baylor College of Medicine in Houston, in an interview at the International Stroke Conference sponsored by the American Heart Association.
The best documentation of this surge came in a poster presented at the conference by researchers at the University of California, San Francisco. They analyzed data on treatment of 357,973 patients with acute ischemic stroke who were hospitalized at any one of 161 U.S. academic medical centers during October 2009-July 2015 and included in the University Healthsystem Consortium database. They tracked the percentage of patients treated endovascularly during each calendar quarter of the study period.
During 2009-2013, use of endovascular treatment rose steadily but gradually, from 1.5% of stroke patients in 2009 to 3.1% during the fourth quarter of 2012. Then, following three reports of no benefit from endovascular treatment presented at the International Stroke Conference in February 2013 – the IMS III, MR RESCUE, and SYNTHESIS trials – the endovascular rate dropped immediately and quickly bottomed out at a level of 2.6% that remained steady through the third quarter of 2014. But when the positive endovascular results from the MR CLEAN study became public in the final week of 2014, endovascular use began to quickly rise again, and then began to skyrocket during the first quarter of 2015 with three additional positive trial results reported during the Stroke Conference in February 2015. By the end of the second quarter of 2015, usage stood at 4.7%, representing a projected year-over-year increase of about 150% for all of 2015, compared with 2014, reported Dr. Anthony S. Kim, a vascular neurologist and medical director of the Stroke Center at the University of California, San Francisco, and his associates.
To put these percentages in perspective, experts estimate that roughly 10%-15% of all stroke patients qualify for thrombectomy intervention.
Their data also showed that the percentage of hospitals included in the database that performed endovascular therapies for stroke rose steadily from about 40% of centers in 2009 to nearly 60% by mid-2015.
“Endovascular therapy with newer-generation devices is increasingly part of standard treatment for acute ischemic stroke,” they said in their poster. In addition, they cited a “new urgency to evaluate regional access to embolectomy [another name for thrombectomy] nationally and to identify system-based solutions to improve access in underserved areas.”
Several stroke experts interviewed at the conference added their own anecdotal view of thrombectomy’s rapidly expanding use for appropriate acute ischemic stroke patients during 2015, and the need for continued effort to broaden its U.S. availability.
“The number of thrombectomies fell off after the negative 2013 trials and stayed flat until a year ago, but then jumped up. It has been very dramatic,” said Dr. Wade S. Smith, professor of neurology and director of the neurovascular service at the University of California, San Francisco.
“Thrombectomy use tremendously increased since February 2015,” said Dr. Mark J. Alberts, professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas, in a video interview during the conference. But despite this growth, “the major challenge [today] is geography;” that is, reaching patients in suburban and rural areas who are not as close to the primarily urban medical centers that currently offer the procedure.
“We now have about 100 certified comprehensive stroke centers in the U.S.,” and by definition comprehensive stroke centers have the capability of treating patients with endovascular thrombectomy, noted Dr. Jeffrey Saver, professor of neurology and director of the stroke unit at the University of California, Los Angeles.
“Certification of these centers did not begin until about 2-3 years ago. But we probably need 300-400 of these centers” to provide thrombectomy to most U.S. stroke patients, he said. “A lot of additional hospitals are close to certification. I anticipate that over the next 1-2 years we will be in the neighborhood of having the number of centers we need,” Dr. Saver said in an interview.
Making thrombectomy better
In addition to expanding availability, the specifics of how endovascular thrombectomy gets delivered is evolving. A major trend is movement toward a “parallel processing” model, in which patients with an acute clinical presentation of a stroke amenable for endovascular treatment simultaneously undergo CT angiography to confirm and localize the large-artery clot causing their stroke, receive intravenous TPA, and undergo preparation for the endovascular access needed to remove the clot.
A pooled analysis of the recent, positive endovascular thrombectomy trials that was presented at the conference showed how quick you need to be to obtain a benefit from the procedures. “This gives us a starting point to further improve the target metrics for imaging and puncture times,” Dr. Saver said. “We want to shorten door-to-needle times for TPA and door-to-puncture times for thrombectomy, and the processes that need to be addressed for rapid delivery of both of these are very similar. We need for patients to only make a pit stop in the ED; we need to have the catheterization team ready to go in the thrombectomy suite within 30 minutes; and we need to emphasize speed in access to the target clot rather than time-consuming diagnostic angiography.”
“We now face the issue of how to best integrate TPA treatment and clot removal.” Dr. Kent said. “People are still trying to work that out. With parallel processing there is some overuse of resources: Some patients recover with TPA alone and don’t need thrombectomy. We are getting closer to the cardiology model of MI treatment. It’s now clear that there needs to be a simple, safe, effective way to do both TPA treatment and thrombectomy. We need to model ourselves on the cardiology experience.”
“If you can deal with the TPA decision in the same room without moving patients from room to room, from a scanner to a catheterization suite, you can really shorten the time to treatment,” Dr. Smith explained. “This is identical to the model that cardiologists have developed. We should now consider taking stroke patients directly to the angiography room in addition to administering TPA. We still need cross-sectional imaging, but the quality of the image from an angiography suite is probably sufficient to make a TPA decision. So you can start TPA while you are getting arterial access. The idea is simultaneous approaches to the patient instead of serial.”
“The whole system moves at the same time to eliminate wasted time,” Dr. Alberts summed up.
One of the big questions that has come up in this effort to speed up treatment and carve the quickest route to endovascular thrombectomy is whether TPA remains necessary. The skeptics’ position is, why waste time administering TPA if you’re also going to take out the offending clot?
The answer, at least for now, is that all signs indicate that giving TPA helps and is worth delivering.
“The 2015 thrombectomy trials had big differences among them in the dosage of TPA administered, and in the percentage of patients who received TPA. When 100% of patients received TPA they had the best outcomes,” Dr. Kent said. “There was a clear synergistic relationship between thrombectomy and TPA. There has been a trend to think about sending patients straight to thrombectomy and skipping TPA, but my colleagues and I think that we need to hold off on doing that. For now, if a patient is eligible to receive TPA they should get it and then quickly move to endovascular therapy. We are not yet ready to know it’s okay to go straight to endovascular treatment. In SWIFT-PRIME, it was pretty clear that the good outcomes were attributable to both [thrombectomy plus TPA]. Treating patients with TPA helps soften the clot to make it easier to remove, and improves flow through collateral arteries.”
“Our data in Memphis show that patients do better with thrombectomy plus intravenous TPA than on TPA alone,” agreed Dr. Lucas Elijovich, a neurologist at the University of Tennessee Health Science Center in Memphis, in an interview.
Simpler imaging also saves time
Although it’s not yet proven, another new wrinkle in working up acute ischemic-stroke patients for TPA and thrombectomy treatment is the idea that simpler and more widely available CT imaging, especially CT angiography of cerebral arteries, may suffice for confirming and localizing the culprit clot.
This concept received a significant boost at the International Stroke Conference in data reported from the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) trial, yet another study that compared treatment with TPA alone with TPA plus endovascular thrombectomy, this time in 65 randomized patients treated at any of 11 U.K. centers. PISTE had a low enrollment level because the trial stopped prematurely, in July 2015, following the news that several fully completed trials had collectively established the superiority of endovascular thrombectomy plus TPA, thereby making it unethical to continue yet another randomized study.
This premature stoppage prevented PISTE from itself producing a statistically significant difference for its primary efficacy endpoint in favor of the combined treatment, although the results did show a nominal advantage to using thrombectomy plus TPA over TPA alone that was fully consistent with the other studies, Dr. Keith W. Muir reported at the conference.
But what made the PISTE results especially notable was that the trial achieved this consistent outcome with a “simpler” imaging protocol for patients during their workup that used only CT angiography, avoiding the cerebral CT perfusion imaging or MRI used in several of the other TPA-plus-thrombectomy versus TPA-only trials, noted Dr. Muir, professor of neuroscience and head of the stroke imaging group at the University of Glasgow.
“PISTE raises the question of how much imaging is necessary,” Dr. Kent commented.
“The PISTE results are exciting. A lot of us believe that all we need to know is that there is a blockage in a target vessel,” Dr. Smith said. “If we have that information, then we can identify a population of patients who will benefit from [thrombectomy]. CT angiography is simple and can easily fit into work flows.”
“PISTE used a very simple imaging system that makes thrombectomy even more applicable and generalizable to less resourced health systems,” Dr. Saver said. “Although the results from PISTE were not internally statistically significant because the trial ended early, the results were consistent with the external studies of thrombectomy, so it provides further evidence for benefit from thrombectomy.” And because the consistent results were achieved with simpler imaging it suggests simpler imaging may be all that’s needed.
“That’s a major question to wrestle with,” Dr. Saver suggested. “We need addition trials with a head-to-head comparison of simpler and more sophisticated imaging so we can tailor treatment to patients who would benefit from simpler and faster imaging.”
Dr. Kent had no disclosures. Dr. Kim has received research funding from SanBio and Biogen. Dr. Smith served on the data safety and monitoring board for a trial funded by Stryker. Dr. Alberts has been a consultant to Genentech. Dr. Saver has been a consultant to Stryker, Neuravi, Cognition Medical, Boehringer Ingelheim, and Medtronic. Dr. Elijovich has been a consultant to Stryker and Codman and received research support from Siemens. Dr. Muir has received research support from ReNeuron and unrestricted grants from Codman and Covidien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LOS ANGELES – The use of endovascular thrombectomy in the United States to treat appropriate patients with acute ischemic stroke mushroomed during the past year, following several early-2015 reports that collectively documented the dramatic clinical benefit of the treatment.
As endovascular thrombectomy use grows, stroke centers are also refining and reshaping delivery of the treatment in concert with administration of intravenous tissue plasminogen activator (TPA; alteplase; Activase), which remains a key partner in producing best outcomes for acute ischemic-stroke patients with a proximal occlusion of a large cerebral artery. Collapsing delivery of the two treatments into a more seamless and streamlined process shaves critical minutes to treatment delivery, an approach called parallel processing. Recent findings have also emboldened stroke specialists to seriously consider simplifying the brain imaging that stroke patients receive prior to these treatments, a step that could further cut time to intervention while also making thrombectomy even more widely available.
Use of thrombectomy surges
The biggest endovascular thrombectomy news of the past year is how it has taken off for treating selected patients with acute ischemic stroke. “The rollout over the past year has been explosive. Everything pretty much shut down after the negative trial results in 2013, but now more hospitals are offering thrombectomy,” said Dr. Thomas A. Kent, professor of neurology and director of stroke research and education at Baylor College of Medicine in Houston, in an interview at the International Stroke Conference sponsored by the American Heart Association.
The best documentation of this surge came in a poster presented at the conference by researchers at the University of California, San Francisco. They analyzed data on treatment of 357,973 patients with acute ischemic stroke who were hospitalized at any one of 161 U.S. academic medical centers during October 2009-July 2015 and included in the University Healthsystem Consortium database. They tracked the percentage of patients treated endovascularly during each calendar quarter of the study period.
During 2009-2013, use of endovascular treatment rose steadily but gradually, from 1.5% of stroke patients in 2009 to 3.1% during the fourth quarter of 2012. Then, following three reports of no benefit from endovascular treatment presented at the International Stroke Conference in February 2013 – the IMS III, MR RESCUE, and SYNTHESIS trials – the endovascular rate dropped immediately and quickly bottomed out at a level of 2.6% that remained steady through the third quarter of 2014. But when the positive endovascular results from the MR CLEAN study became public in the final week of 2014, endovascular use began to quickly rise again, and then began to skyrocket during the first quarter of 2015 with three additional positive trial results reported during the Stroke Conference in February 2015. By the end of the second quarter of 2015, usage stood at 4.7%, representing a projected year-over-year increase of about 150% for all of 2015, compared with 2014, reported Dr. Anthony S. Kim, a vascular neurologist and medical director of the Stroke Center at the University of California, San Francisco, and his associates.
To put these percentages in perspective, experts estimate that roughly 10%-15% of all stroke patients qualify for thrombectomy intervention.
Their data also showed that the percentage of hospitals included in the database that performed endovascular therapies for stroke rose steadily from about 40% of centers in 2009 to nearly 60% by mid-2015.
“Endovascular therapy with newer-generation devices is increasingly part of standard treatment for acute ischemic stroke,” they said in their poster. In addition, they cited a “new urgency to evaluate regional access to embolectomy [another name for thrombectomy] nationally and to identify system-based solutions to improve access in underserved areas.”
Several stroke experts interviewed at the conference added their own anecdotal view of thrombectomy’s rapidly expanding use for appropriate acute ischemic stroke patients during 2015, and the need for continued effort to broaden its U.S. availability.
“The number of thrombectomies fell off after the negative 2013 trials and stayed flat until a year ago, but then jumped up. It has been very dramatic,” said Dr. Wade S. Smith, professor of neurology and director of the neurovascular service at the University of California, San Francisco.
“Thrombectomy use tremendously increased since February 2015,” said Dr. Mark J. Alberts, professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas, in a video interview during the conference. But despite this growth, “the major challenge [today] is geography;” that is, reaching patients in suburban and rural areas who are not as close to the primarily urban medical centers that currently offer the procedure.
“We now have about 100 certified comprehensive stroke centers in the U.S.,” and by definition comprehensive stroke centers have the capability of treating patients with endovascular thrombectomy, noted Dr. Jeffrey Saver, professor of neurology and director of the stroke unit at the University of California, Los Angeles.
“Certification of these centers did not begin until about 2-3 years ago. But we probably need 300-400 of these centers” to provide thrombectomy to most U.S. stroke patients, he said. “A lot of additional hospitals are close to certification. I anticipate that over the next 1-2 years we will be in the neighborhood of having the number of centers we need,” Dr. Saver said in an interview.
Making thrombectomy better
In addition to expanding availability, the specifics of how endovascular thrombectomy gets delivered is evolving. A major trend is movement toward a “parallel processing” model, in which patients with an acute clinical presentation of a stroke amenable for endovascular treatment simultaneously undergo CT angiography to confirm and localize the large-artery clot causing their stroke, receive intravenous TPA, and undergo preparation for the endovascular access needed to remove the clot.
A pooled analysis of the recent, positive endovascular thrombectomy trials that was presented at the conference showed how quick you need to be to obtain a benefit from the procedures. “This gives us a starting point to further improve the target metrics for imaging and puncture times,” Dr. Saver said. “We want to shorten door-to-needle times for TPA and door-to-puncture times for thrombectomy, and the processes that need to be addressed for rapid delivery of both of these are very similar. We need for patients to only make a pit stop in the ED; we need to have the catheterization team ready to go in the thrombectomy suite within 30 minutes; and we need to emphasize speed in access to the target clot rather than time-consuming diagnostic angiography.”
“We now face the issue of how to best integrate TPA treatment and clot removal.” Dr. Kent said. “People are still trying to work that out. With parallel processing there is some overuse of resources: Some patients recover with TPA alone and don’t need thrombectomy. We are getting closer to the cardiology model of MI treatment. It’s now clear that there needs to be a simple, safe, effective way to do both TPA treatment and thrombectomy. We need to model ourselves on the cardiology experience.”
“If you can deal with the TPA decision in the same room without moving patients from room to room, from a scanner to a catheterization suite, you can really shorten the time to treatment,” Dr. Smith explained. “This is identical to the model that cardiologists have developed. We should now consider taking stroke patients directly to the angiography room in addition to administering TPA. We still need cross-sectional imaging, but the quality of the image from an angiography suite is probably sufficient to make a TPA decision. So you can start TPA while you are getting arterial access. The idea is simultaneous approaches to the patient instead of serial.”
“The whole system moves at the same time to eliminate wasted time,” Dr. Alberts summed up.
One of the big questions that has come up in this effort to speed up treatment and carve the quickest route to endovascular thrombectomy is whether TPA remains necessary. The skeptics’ position is, why waste time administering TPA if you’re also going to take out the offending clot?
The answer, at least for now, is that all signs indicate that giving TPA helps and is worth delivering.
“The 2015 thrombectomy trials had big differences among them in the dosage of TPA administered, and in the percentage of patients who received TPA. When 100% of patients received TPA they had the best outcomes,” Dr. Kent said. “There was a clear synergistic relationship between thrombectomy and TPA. There has been a trend to think about sending patients straight to thrombectomy and skipping TPA, but my colleagues and I think that we need to hold off on doing that. For now, if a patient is eligible to receive TPA they should get it and then quickly move to endovascular therapy. We are not yet ready to know it’s okay to go straight to endovascular treatment. In SWIFT-PRIME, it was pretty clear that the good outcomes were attributable to both [thrombectomy plus TPA]. Treating patients with TPA helps soften the clot to make it easier to remove, and improves flow through collateral arteries.”
“Our data in Memphis show that patients do better with thrombectomy plus intravenous TPA than on TPA alone,” agreed Dr. Lucas Elijovich, a neurologist at the University of Tennessee Health Science Center in Memphis, in an interview.
Simpler imaging also saves time
Although it’s not yet proven, another new wrinkle in working up acute ischemic-stroke patients for TPA and thrombectomy treatment is the idea that simpler and more widely available CT imaging, especially CT angiography of cerebral arteries, may suffice for confirming and localizing the culprit clot.
This concept received a significant boost at the International Stroke Conference in data reported from the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) trial, yet another study that compared treatment with TPA alone with TPA plus endovascular thrombectomy, this time in 65 randomized patients treated at any of 11 U.K. centers. PISTE had a low enrollment level because the trial stopped prematurely, in July 2015, following the news that several fully completed trials had collectively established the superiority of endovascular thrombectomy plus TPA, thereby making it unethical to continue yet another randomized study.
This premature stoppage prevented PISTE from itself producing a statistically significant difference for its primary efficacy endpoint in favor of the combined treatment, although the results did show a nominal advantage to using thrombectomy plus TPA over TPA alone that was fully consistent with the other studies, Dr. Keith W. Muir reported at the conference.
But what made the PISTE results especially notable was that the trial achieved this consistent outcome with a “simpler” imaging protocol for patients during their workup that used only CT angiography, avoiding the cerebral CT perfusion imaging or MRI used in several of the other TPA-plus-thrombectomy versus TPA-only trials, noted Dr. Muir, professor of neuroscience and head of the stroke imaging group at the University of Glasgow.
“PISTE raises the question of how much imaging is necessary,” Dr. Kent commented.
“The PISTE results are exciting. A lot of us believe that all we need to know is that there is a blockage in a target vessel,” Dr. Smith said. “If we have that information, then we can identify a population of patients who will benefit from [thrombectomy]. CT angiography is simple and can easily fit into work flows.”
“PISTE used a very simple imaging system that makes thrombectomy even more applicable and generalizable to less resourced health systems,” Dr. Saver said. “Although the results from PISTE were not internally statistically significant because the trial ended early, the results were consistent with the external studies of thrombectomy, so it provides further evidence for benefit from thrombectomy.” And because the consistent results were achieved with simpler imaging it suggests simpler imaging may be all that’s needed.
“That’s a major question to wrestle with,” Dr. Saver suggested. “We need addition trials with a head-to-head comparison of simpler and more sophisticated imaging so we can tailor treatment to patients who would benefit from simpler and faster imaging.”
Dr. Kent had no disclosures. Dr. Kim has received research funding from SanBio and Biogen. Dr. Smith served on the data safety and monitoring board for a trial funded by Stryker. Dr. Alberts has been a consultant to Genentech. Dr. Saver has been a consultant to Stryker, Neuravi, Cognition Medical, Boehringer Ingelheim, and Medtronic. Dr. Elijovich has been a consultant to Stryker and Codman and received research support from Siemens. Dr. Muir has received research support from ReNeuron and unrestricted grants from Codman and Covidien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE INTERNATIONAL STROKE CONFERENCE
ISC: Thrombectomy shown highly cost-effective for stroke
LOS ANGELES – Endovascular thrombectomy is not only clinically the best option for many patients with acute, ischemic strokes involving a proximal occlusion in a large cerebral artery; it’s also highly cost effective, based on follow-up analyses of two of the five randomized trials published in 2015 that collectively established thrombectomy as standard of care for these patients.
Thrombectomy plus administration of intravenous tissue plasminogen activator (TPA), compared with TPA only, “is highly cost effective and economically dominant with lower long-term cost and better outcomes,” Theresa I. Shireman, Ph.D., said at the International Stroke Conference.
And in an independent analysis of data from a totally different trial, endovascular thrombectomy on average reduced patients’ acute length of hospitalization, improved their survival and quality of life, and was cost saving when compared with treatment with intravenous TPA only, which had previously been the standard of care, Dr. Bruce C.V. Campbell reported at the meeting.
The analysis presented by Dr. Shireman used data collected in the SWIFT-PRIME trial, which randomized 196 patients at centers in the United States and Europe to treatment with either intravenous TPA plus endovascular thrombectomy or TPA alone. Average total costs during the index hospitalization ran to roughly $46,000 in the combined-treatment arm and about $29,000 in the TPA-only arm, a difference largely driven by a roughly $15,000 average incremental cost for the thrombectomy procedure, said Dr. Shireman, professor of health services research at Brown University in Providence, R.I.
However, the cost-effectiveness of thrombectomy began to kick in soon after. During the 90 days following index hospitalization, patients who underwent thrombectomy had substantial average reductions in their need for inpatient rehabilitation, time spent in skilled nursing facilities, and in outpatient rehabilitation. Overall, total medical costs during the first 90 days post discharge ran on average close to $5,000 less per patient following thrombectomy. In addition, based on their health status after 90 days, patients treated with thrombectomy were projected to have a greater than 1.7-year average life expectancy than those randomized to TPA only, with a projected net gain of 1.74 quality-adjusted life years (QALY) per patient and with a projected average decrease of roughly $23,000 in total lifetime medical costs.
Based on this average increase in QALYs and decreased long-term cost, adding thrombectomy to TPA for routine treatment of the types of patients enrolled in SWIFT-PRIME was economically dominant, Dr. Shireman said at the meeting sponsored by the American Heart Association. She also projected that despite the higher upfront cost for adding thrombectomy to treatment, the eventual savings in long-term care meant that thrombectomy began producing a net saving once patients survived for more than 22 months following their index hospitalization.
Dr. Campbell reported very similar findings in his analysis of data collected from the EXTEND-IA trial, which randomized 70 patients at 10 centers in Australia and New Zealand. During the first 90 days of treatment, including the index hospitalization, treatment with thrombectomy plus TPA saved an average of roughly $4,000 U.S.per patient, compared with TPA only, even though the average incremental cost for adding thrombectomy was nearly $11,000 U.S. The overall increased total 90-day costs with TPA only was largely driven by a substantially longer time spent hospitalized among the TPA-only patients, compared with those treated with thrombectomy plus TPA, said Dr. Campbell, a neurologist and head of hyperacute stroke at Royal Melbourne Hospital.
In addition, adding thrombectomy resulted in a projected average 4-year increase in life expectancy, and an average gain of about 3 QALYs per patient. Thrombectomy “is an incredibly powerful procedure, not just in terms of clinical response but also in terms of economics,” he concluded. Even when judged by the worst-case scenario of the analysis, “there is a 100% probability that the cost-effectiveness per QALY is less than $10,000 U.S., which is incredible value,” Dr. Campbell said.
SWIFT-PRIME was sponsored by Covidien/Medtronic. EXTEND-IA received partial funding through an unrestricted grant from Covidien/Medtronic. Dr. Shireman and Dr. Campbell had no personal disclosures.
On Twitter @mitchelzoler
LOS ANGELES – Endovascular thrombectomy is not only clinically the best option for many patients with acute, ischemic strokes involving a proximal occlusion in a large cerebral artery; it’s also highly cost effective, based on follow-up analyses of two of the five randomized trials published in 2015 that collectively established thrombectomy as standard of care for these patients.
Thrombectomy plus administration of intravenous tissue plasminogen activator (TPA), compared with TPA only, “is highly cost effective and economically dominant with lower long-term cost and better outcomes,” Theresa I. Shireman, Ph.D., said at the International Stroke Conference.
And in an independent analysis of data from a totally different trial, endovascular thrombectomy on average reduced patients’ acute length of hospitalization, improved their survival and quality of life, and was cost saving when compared with treatment with intravenous TPA only, which had previously been the standard of care, Dr. Bruce C.V. Campbell reported at the meeting.
The analysis presented by Dr. Shireman used data collected in the SWIFT-PRIME trial, which randomized 196 patients at centers in the United States and Europe to treatment with either intravenous TPA plus endovascular thrombectomy or TPA alone. Average total costs during the index hospitalization ran to roughly $46,000 in the combined-treatment arm and about $29,000 in the TPA-only arm, a difference largely driven by a roughly $15,000 average incremental cost for the thrombectomy procedure, said Dr. Shireman, professor of health services research at Brown University in Providence, R.I.
However, the cost-effectiveness of thrombectomy began to kick in soon after. During the 90 days following index hospitalization, patients who underwent thrombectomy had substantial average reductions in their need for inpatient rehabilitation, time spent in skilled nursing facilities, and in outpatient rehabilitation. Overall, total medical costs during the first 90 days post discharge ran on average close to $5,000 less per patient following thrombectomy. In addition, based on their health status after 90 days, patients treated with thrombectomy were projected to have a greater than 1.7-year average life expectancy than those randomized to TPA only, with a projected net gain of 1.74 quality-adjusted life years (QALY) per patient and with a projected average decrease of roughly $23,000 in total lifetime medical costs.
Based on this average increase in QALYs and decreased long-term cost, adding thrombectomy to TPA for routine treatment of the types of patients enrolled in SWIFT-PRIME was economically dominant, Dr. Shireman said at the meeting sponsored by the American Heart Association. She also projected that despite the higher upfront cost for adding thrombectomy to treatment, the eventual savings in long-term care meant that thrombectomy began producing a net saving once patients survived for more than 22 months following their index hospitalization.
Dr. Campbell reported very similar findings in his analysis of data collected from the EXTEND-IA trial, which randomized 70 patients at 10 centers in Australia and New Zealand. During the first 90 days of treatment, including the index hospitalization, treatment with thrombectomy plus TPA saved an average of roughly $4,000 U.S.per patient, compared with TPA only, even though the average incremental cost for adding thrombectomy was nearly $11,000 U.S. The overall increased total 90-day costs with TPA only was largely driven by a substantially longer time spent hospitalized among the TPA-only patients, compared with those treated with thrombectomy plus TPA, said Dr. Campbell, a neurologist and head of hyperacute stroke at Royal Melbourne Hospital.
In addition, adding thrombectomy resulted in a projected average 4-year increase in life expectancy, and an average gain of about 3 QALYs per patient. Thrombectomy “is an incredibly powerful procedure, not just in terms of clinical response but also in terms of economics,” he concluded. Even when judged by the worst-case scenario of the analysis, “there is a 100% probability that the cost-effectiveness per QALY is less than $10,000 U.S., which is incredible value,” Dr. Campbell said.
SWIFT-PRIME was sponsored by Covidien/Medtronic. EXTEND-IA received partial funding through an unrestricted grant from Covidien/Medtronic. Dr. Shireman and Dr. Campbell had no personal disclosures.
On Twitter @mitchelzoler
LOS ANGELES – Endovascular thrombectomy is not only clinically the best option for many patients with acute, ischemic strokes involving a proximal occlusion in a large cerebral artery; it’s also highly cost effective, based on follow-up analyses of two of the five randomized trials published in 2015 that collectively established thrombectomy as standard of care for these patients.
Thrombectomy plus administration of intravenous tissue plasminogen activator (TPA), compared with TPA only, “is highly cost effective and economically dominant with lower long-term cost and better outcomes,” Theresa I. Shireman, Ph.D., said at the International Stroke Conference.
And in an independent analysis of data from a totally different trial, endovascular thrombectomy on average reduced patients’ acute length of hospitalization, improved their survival and quality of life, and was cost saving when compared with treatment with intravenous TPA only, which had previously been the standard of care, Dr. Bruce C.V. Campbell reported at the meeting.
The analysis presented by Dr. Shireman used data collected in the SWIFT-PRIME trial, which randomized 196 patients at centers in the United States and Europe to treatment with either intravenous TPA plus endovascular thrombectomy or TPA alone. Average total costs during the index hospitalization ran to roughly $46,000 in the combined-treatment arm and about $29,000 in the TPA-only arm, a difference largely driven by a roughly $15,000 average incremental cost for the thrombectomy procedure, said Dr. Shireman, professor of health services research at Brown University in Providence, R.I.
However, the cost-effectiveness of thrombectomy began to kick in soon after. During the 90 days following index hospitalization, patients who underwent thrombectomy had substantial average reductions in their need for inpatient rehabilitation, time spent in skilled nursing facilities, and in outpatient rehabilitation. Overall, total medical costs during the first 90 days post discharge ran on average close to $5,000 less per patient following thrombectomy. In addition, based on their health status after 90 days, patients treated with thrombectomy were projected to have a greater than 1.7-year average life expectancy than those randomized to TPA only, with a projected net gain of 1.74 quality-adjusted life years (QALY) per patient and with a projected average decrease of roughly $23,000 in total lifetime medical costs.
Based on this average increase in QALYs and decreased long-term cost, adding thrombectomy to TPA for routine treatment of the types of patients enrolled in SWIFT-PRIME was economically dominant, Dr. Shireman said at the meeting sponsored by the American Heart Association. She also projected that despite the higher upfront cost for adding thrombectomy to treatment, the eventual savings in long-term care meant that thrombectomy began producing a net saving once patients survived for more than 22 months following their index hospitalization.
Dr. Campbell reported very similar findings in his analysis of data collected from the EXTEND-IA trial, which randomized 70 patients at 10 centers in Australia and New Zealand. During the first 90 days of treatment, including the index hospitalization, treatment with thrombectomy plus TPA saved an average of roughly $4,000 U.S.per patient, compared with TPA only, even though the average incremental cost for adding thrombectomy was nearly $11,000 U.S. The overall increased total 90-day costs with TPA only was largely driven by a substantially longer time spent hospitalized among the TPA-only patients, compared with those treated with thrombectomy plus TPA, said Dr. Campbell, a neurologist and head of hyperacute stroke at Royal Melbourne Hospital.
In addition, adding thrombectomy resulted in a projected average 4-year increase in life expectancy, and an average gain of about 3 QALYs per patient. Thrombectomy “is an incredibly powerful procedure, not just in terms of clinical response but also in terms of economics,” he concluded. Even when judged by the worst-case scenario of the analysis, “there is a 100% probability that the cost-effectiveness per QALY is less than $10,000 U.S., which is incredible value,” Dr. Campbell said.
SWIFT-PRIME was sponsored by Covidien/Medtronic. EXTEND-IA received partial funding through an unrestricted grant from Covidien/Medtronic. Dr. Shireman and Dr. Campbell had no personal disclosures.
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Adding endovascular thrombectomy to TPA treatment for selected patients with acute, ischemic stroke proved highly cost effective on the basis of data collected in two independent randomized trials.
Major finding: In SWIFT-PRIME, thrombectomy saved a projected average of $23,000 in lifetime health care costs and added 1.74 QALYs.
Data source: SWIFT-PRIME, an international, multicenter, randomized trial that enrolled 196 patients.
Disclosures: SWIFT-PRIME was sponsored by Covidien/Medtronic. EXTEND-IA received partial funding through an unrestricted grant from Covidien/Medtronic. Dr. Shireman and Dr. Campbell had no personal disclosures.
ISC: Carotid surgery, stenting offer patients balanced alternatives
LOS ANGELES – The equipoise between carotid stenting and endarterectomy received a further boost in 10-year results from the landmark Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) that compared the two options head-to-head.
Reported the day after results from another big trial that pitted carotid stenting against surgery, the Asymptomatic Carotid Trial (ACT I), the new long-term results from the CREST study mean that deciding among the options relies largely on patient preference although individual clinical characteristics might favor one approach or the other, experts said.
The big remaining unknown and wild card is whether doing no procedural intervention at all and relying entirely on optimal, contemporary medical treatment works just as well as endarterectomy or carotid stenting. The role for stand-alone medical therapy against carotid surgery or stenting (on top of medical therapy) is currently undergoing a formal, direct comparison in the randomized Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2).
Taking the 5-year outcome results from ACT I and the 10-year outcome results from CREST both into account, “we now have a lot of evidence that both carotid stenting and surgery are safe and durable. The results support both options” for either patients with symptomatic carotid artery stenosis or asymptomatic patients with carotid stenosis as extensive as in the patients enrolled in these trials, said Dr. Thomas G. Brott at the International Stroke Conference.
“In routine practice, we lay out the options of endarterectomy, carotid stenting, or no intervention with just medical treatment to patients and let them decide,” noted Dr. Brott, professor of neurology and director of research at the Mayo Clinic in Jacksonville, Fla.
CREST randomized 2,502 symptomatic or asymptomatic patients with significant carotid stenosis during 2000-2008 at 117 U.S. and Canadian centers. From this group, 1,607 consented and were available for long-term follow-up, done at a median of 7.4 years and as long as 10 years after follow-up.
The study’s primary, long-term endpoint was stroke, MI, or death during the periprocedural period (30 days after treatment or 36 days after enrollment depending on when the procedural intervention occurred) plus the rate of ipsilateral stroke during up to 10 years of follow-up. This combined endpoint occurred in 10% of the patients who underwent endarterectomy and in 12% of those who had stenting, a difference that was not statistically significant, Dr. Brott reported. Concurrent with his presentation at the meeting, sponsored by the American Heart Association, the results also were published online (N Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMoa1505215).
The results included a secondary endpoint that showed a significant benefit for endarterectomy. The tally of periprocedural strokes or deaths plus ipsilateral strokes during 10-year follow-up was 8% for the surgical group and 11% for those who received a stent, a 37% excess hazard with stenting.
Dr. Brott attributed this secondary difference between the two arms of the study to a statistically significant excess of stroke or death during the periprocedural period in the patients treated by stenting, and more specifically an excess of strokes. The rate of total periprocedural strokes was 4% with stenting and 2% with endarterectomy, a statistically significant difference. Although an embolic protection device was used “when feasible” during stenting, this protection can be fallible, Dr. Brott noted. In contrast, the results from the ACT I trial showed no statistically significant difference in the rate of periprocedural total strokes between the stented and endarterectomy patients.
Dr. Brott had no relevant disclosures. The CREST trial received partial funding from Abbott Vascular.
On Twitter @mitchelzoler
The 10-year CREST results are good news for patients with carotid disease because they show the durability of both interventions we can offer patients. Having these data and the results from ACT I allows physicians to have an informed discussion with patients about their treatment options. I also hope that with these results from both trials, reimbursement will cease to be a deciding factor and that both surgery and stenting will be on a level playing field for insurance coverage.
Although on a population level stenting and surgery appear to produce comparable results, individual patient characteristics can make one option more appropriate. These include the morphology of a patient’s carotid arteries and stenotic lesions that can make stenting a technical challenge, and a patient’s medical condition and comorbidities which could put them at higher risk for general anesthesia and surgery. Also, a big concern for many patients is how long they will require hospitalization.
 |
Dr. Mark J. Alberts |
A major unresolved question now about treating carotid disease is whether medical treatment alone is an equally good third alternative for asymptomatic patients. We are in a relatively new era of medical therapy, with more options for smoking cessation, better and more diverse drugs for blood pressure and hyperglycemia control, and wider use of high-dose statins. Some patients are eager to avoid any intervention and already opt for medical management only, but only after CREST-2 is completed will we know whether they will truly fare as well as patients who have a procedure performed.
Another issue that needs to be considered when extrapolating the results from CREST and ACT I to routine practice is that the surgeons and interventionalists who performed the procedures in these trials were highly selected and experienced. One cannot assume that the results in these trials will be replicated by any surgeon or interventionalist in the community. I suggest that patients investigate the track record of their community hospitals and operators by consulting the performance information that is increasingly posted on the Internet.
Dr. Mark J. Alberts is professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas. He had no disclosures. He made these comments in an interview.
The 10-year CREST results are good news for patients with carotid disease because they show the durability of both interventions we can offer patients. Having these data and the results from ACT I allows physicians to have an informed discussion with patients about their treatment options. I also hope that with these results from both trials, reimbursement will cease to be a deciding factor and that both surgery and stenting will be on a level playing field for insurance coverage.
Although on a population level stenting and surgery appear to produce comparable results, individual patient characteristics can make one option more appropriate. These include the morphology of a patient’s carotid arteries and stenotic lesions that can make stenting a technical challenge, and a patient’s medical condition and comorbidities which could put them at higher risk for general anesthesia and surgery. Also, a big concern for many patients is how long they will require hospitalization.
 |
Dr. Mark J. Alberts |
A major unresolved question now about treating carotid disease is whether medical treatment alone is an equally good third alternative for asymptomatic patients. We are in a relatively new era of medical therapy, with more options for smoking cessation, better and more diverse drugs for blood pressure and hyperglycemia control, and wider use of high-dose statins. Some patients are eager to avoid any intervention and already opt for medical management only, but only after CREST-2 is completed will we know whether they will truly fare as well as patients who have a procedure performed.
Another issue that needs to be considered when extrapolating the results from CREST and ACT I to routine practice is that the surgeons and interventionalists who performed the procedures in these trials were highly selected and experienced. One cannot assume that the results in these trials will be replicated by any surgeon or interventionalist in the community. I suggest that patients investigate the track record of their community hospitals and operators by consulting the performance information that is increasingly posted on the Internet.
Dr. Mark J. Alberts is professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas. He had no disclosures. He made these comments in an interview.
The 10-year CREST results are good news for patients with carotid disease because they show the durability of both interventions we can offer patients. Having these data and the results from ACT I allows physicians to have an informed discussion with patients about their treatment options. I also hope that with these results from both trials, reimbursement will cease to be a deciding factor and that both surgery and stenting will be on a level playing field for insurance coverage.
Although on a population level stenting and surgery appear to produce comparable results, individual patient characteristics can make one option more appropriate. These include the morphology of a patient’s carotid arteries and stenotic lesions that can make stenting a technical challenge, and a patient’s medical condition and comorbidities which could put them at higher risk for general anesthesia and surgery. Also, a big concern for many patients is how long they will require hospitalization.
 |
Dr. Mark J. Alberts |
A major unresolved question now about treating carotid disease is whether medical treatment alone is an equally good third alternative for asymptomatic patients. We are in a relatively new era of medical therapy, with more options for smoking cessation, better and more diverse drugs for blood pressure and hyperglycemia control, and wider use of high-dose statins. Some patients are eager to avoid any intervention and already opt for medical management only, but only after CREST-2 is completed will we know whether they will truly fare as well as patients who have a procedure performed.
Another issue that needs to be considered when extrapolating the results from CREST and ACT I to routine practice is that the surgeons and interventionalists who performed the procedures in these trials were highly selected and experienced. One cannot assume that the results in these trials will be replicated by any surgeon or interventionalist in the community. I suggest that patients investigate the track record of their community hospitals and operators by consulting the performance information that is increasingly posted on the Internet.
Dr. Mark J. Alberts is professor of neurology and medical director of the neurology service at the University of Texas Southwestern Medical Center in Dallas. He had no disclosures. He made these comments in an interview.
LOS ANGELES – The equipoise between carotid stenting and endarterectomy received a further boost in 10-year results from the landmark Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) that compared the two options head-to-head.
Reported the day after results from another big trial that pitted carotid stenting against surgery, the Asymptomatic Carotid Trial (ACT I), the new long-term results from the CREST study mean that deciding among the options relies largely on patient preference although individual clinical characteristics might favor one approach or the other, experts said.
The big remaining unknown and wild card is whether doing no procedural intervention at all and relying entirely on optimal, contemporary medical treatment works just as well as endarterectomy or carotid stenting. The role for stand-alone medical therapy against carotid surgery or stenting (on top of medical therapy) is currently undergoing a formal, direct comparison in the randomized Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2).
Taking the 5-year outcome results from ACT I and the 10-year outcome results from CREST both into account, “we now have a lot of evidence that both carotid stenting and surgery are safe and durable. The results support both options” for either patients with symptomatic carotid artery stenosis or asymptomatic patients with carotid stenosis as extensive as in the patients enrolled in these trials, said Dr. Thomas G. Brott at the International Stroke Conference.
“In routine practice, we lay out the options of endarterectomy, carotid stenting, or no intervention with just medical treatment to patients and let them decide,” noted Dr. Brott, professor of neurology and director of research at the Mayo Clinic in Jacksonville, Fla.
CREST randomized 2,502 symptomatic or asymptomatic patients with significant carotid stenosis during 2000-2008 at 117 U.S. and Canadian centers. From this group, 1,607 consented and were available for long-term follow-up, done at a median of 7.4 years and as long as 10 years after follow-up.
The study’s primary, long-term endpoint was stroke, MI, or death during the periprocedural period (30 days after treatment or 36 days after enrollment depending on when the procedural intervention occurred) plus the rate of ipsilateral stroke during up to 10 years of follow-up. This combined endpoint occurred in 10% of the patients who underwent endarterectomy and in 12% of those who had stenting, a difference that was not statistically significant, Dr. Brott reported. Concurrent with his presentation at the meeting, sponsored by the American Heart Association, the results also were published online (N Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMoa1505215).
The results included a secondary endpoint that showed a significant benefit for endarterectomy. The tally of periprocedural strokes or deaths plus ipsilateral strokes during 10-year follow-up was 8% for the surgical group and 11% for those who received a stent, a 37% excess hazard with stenting.
Dr. Brott attributed this secondary difference between the two arms of the study to a statistically significant excess of stroke or death during the periprocedural period in the patients treated by stenting, and more specifically an excess of strokes. The rate of total periprocedural strokes was 4% with stenting and 2% with endarterectomy, a statistically significant difference. Although an embolic protection device was used “when feasible” during stenting, this protection can be fallible, Dr. Brott noted. In contrast, the results from the ACT I trial showed no statistically significant difference in the rate of periprocedural total strokes between the stented and endarterectomy patients.
Dr. Brott had no relevant disclosures. The CREST trial received partial funding from Abbott Vascular.
On Twitter @mitchelzoler
LOS ANGELES – The equipoise between carotid stenting and endarterectomy received a further boost in 10-year results from the landmark Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) that compared the two options head-to-head.
Reported the day after results from another big trial that pitted carotid stenting against surgery, the Asymptomatic Carotid Trial (ACT I), the new long-term results from the CREST study mean that deciding among the options relies largely on patient preference although individual clinical characteristics might favor one approach or the other, experts said.
The big remaining unknown and wild card is whether doing no procedural intervention at all and relying entirely on optimal, contemporary medical treatment works just as well as endarterectomy or carotid stenting. The role for stand-alone medical therapy against carotid surgery or stenting (on top of medical therapy) is currently undergoing a formal, direct comparison in the randomized Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2).
Taking the 5-year outcome results from ACT I and the 10-year outcome results from CREST both into account, “we now have a lot of evidence that both carotid stenting and surgery are safe and durable. The results support both options” for either patients with symptomatic carotid artery stenosis or asymptomatic patients with carotid stenosis as extensive as in the patients enrolled in these trials, said Dr. Thomas G. Brott at the International Stroke Conference.
“In routine practice, we lay out the options of endarterectomy, carotid stenting, or no intervention with just medical treatment to patients and let them decide,” noted Dr. Brott, professor of neurology and director of research at the Mayo Clinic in Jacksonville, Fla.
CREST randomized 2,502 symptomatic or asymptomatic patients with significant carotid stenosis during 2000-2008 at 117 U.S. and Canadian centers. From this group, 1,607 consented and were available for long-term follow-up, done at a median of 7.4 years and as long as 10 years after follow-up.
The study’s primary, long-term endpoint was stroke, MI, or death during the periprocedural period (30 days after treatment or 36 days after enrollment depending on when the procedural intervention occurred) plus the rate of ipsilateral stroke during up to 10 years of follow-up. This combined endpoint occurred in 10% of the patients who underwent endarterectomy and in 12% of those who had stenting, a difference that was not statistically significant, Dr. Brott reported. Concurrent with his presentation at the meeting, sponsored by the American Heart Association, the results also were published online (N Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMoa1505215).
The results included a secondary endpoint that showed a significant benefit for endarterectomy. The tally of periprocedural strokes or deaths plus ipsilateral strokes during 10-year follow-up was 8% for the surgical group and 11% for those who received a stent, a 37% excess hazard with stenting.
Dr. Brott attributed this secondary difference between the two arms of the study to a statistically significant excess of stroke or death during the periprocedural period in the patients treated by stenting, and more specifically an excess of strokes. The rate of total periprocedural strokes was 4% with stenting and 2% with endarterectomy, a statistically significant difference. Although an embolic protection device was used “when feasible” during stenting, this protection can be fallible, Dr. Brott noted. In contrast, the results from the ACT I trial showed no statistically significant difference in the rate of periprocedural total strokes between the stented and endarterectomy patients.
Dr. Brott had no relevant disclosures. The CREST trial received partial funding from Abbott Vascular.
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Long-term follow-up of the CREST trial out to 10 years showed no statistically significant difference between endarterectomy or carotid stenting for patients with carotid artery stenosis.
Major finding: The primary, long-term endpoint occurred in 10% of endarterectomy patients and 12% of stented patients, a nonsignificant difference.
Data source: The CREST trial, which followed 1,607 patients for up to 10 years after their randomized intervention.
Disclosures: Dr. Brott had no relevant disclosures. The CREST trial received partial funding from Abbott Vascular.
Heightened emphasis on sex-specific cardiovascular risk factors
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Vascular: The Final Frontier - Pushing vascular science where no science has gone before
Space is truly a magical place, enchanting philosophers, scientists, artists and dreamers. From ancient civilizations that found pantheons of gods among the stars, to novelist Andy Weir’s visionary tale of human efforts to colonize Mars recently portrayed in the movie “The Martian,” to George Lucas’ epic drama between Jedis and Sith lords in “Star Wars,” it is clear that space draws humanity to push the frontiers of science and technology – or maybe just draws us to the box office.
Nonetheless, in this day and age there are astronauts and cosmonauts who have colonized lower earth orbit (LEO) on the International Space Station (ISS), in a situation quite similar to that of the station Arthur C. Clark envisioned in his 1968 science fiction novel, “2001: A Space Odyssey.”
Unfortunately, human physiology, which has evolved in and grown accustomed to Earth’s gravity, is completely altered in space where there is either no gravity effect or different gravitational pulls result from different planetary bodies. Because of this unique medical anomaly, the ISS is a platform for research of interest to a forward-thinking vascular specialist.
Dr. Richard Hughson, from the University of Waterloo in Waterloo, Ontario, is researching vascular aging in spaceflight crew members. His work is a part of the Schlegel-University of Waterloo Research Institute for Aging, where he is theme leader/chair of vascular aging and brain health and holds the Schlegel Research Chair in that discipline.
Dr. Hughson is supported by the Canadian Space Agency (CSA) and Canadian Institute for Health Research (CIHR) He discussed his research in a recent audio interview (http://cihr-irsc.gc.ca/e/49523.html).
Observations have demonstrated that short-duration and extended spaceflight missions may simulate accelerated vascular aging in some of these highly fit individuals traveling to space. Specifically, spaceflight crew members have been shown to have difficulty controlling a rise in their blood pressure, perhaps secondary to the loss of Earth’s gravity, but rather in the inherent cephalad fluid shift (as blood no longer pools in the legs). In addition, significant postflight postural hypotension and physical deconditioning with resultant sarcopenia and osteopenia are known to occur.
Dr. Hughson has shown through ultrasonography that the carotid arteries of spaceflight crew members are considerably stiffer compared to their preflight arteries and that they appear to have “aged the equivalent of 20-30 years in stiffness.” The ramifications of this type of research on the study of the normal earthbound vascular aging processes are under investigation.
To counteract the effects of vascular aging and physical deconditioning in space, physical activity is key; however, the 30 minutes per day allotted to busy astronauts amid their responsibilities is just not cutting it, according to Dr. Hughson. Missions are being extended for longer periods of time, leading to serious physical consequences, For example, American astronaut Scott Kelly’s year in space will certainly result in considerable accelerated aging in his arterial system. Thus, it becomes increasingly necessary to understand and prevent the vascular aging process in astronauts, future spaceflight crew members, and perhaps one day those seeking to colonize the Moon, Mars, and beyond.
When colonization time arrives, space agencies certainly should be in the market for well-qualified vascular specialists.
Perhaps great job opportunities await those in our profession who will be brave enough to leave Earth’s cradle.
Dr Drudi is a vascular surgery resident at McGill University, Montreal.
Space is truly a magical place, enchanting philosophers, scientists, artists and dreamers. From ancient civilizations that found pantheons of gods among the stars, to novelist Andy Weir’s visionary tale of human efforts to colonize Mars recently portrayed in the movie “The Martian,” to George Lucas’ epic drama between Jedis and Sith lords in “Star Wars,” it is clear that space draws humanity to push the frontiers of science and technology – or maybe just draws us to the box office.
Nonetheless, in this day and age there are astronauts and cosmonauts who have colonized lower earth orbit (LEO) on the International Space Station (ISS), in a situation quite similar to that of the station Arthur C. Clark envisioned in his 1968 science fiction novel, “2001: A Space Odyssey.”
Unfortunately, human physiology, which has evolved in and grown accustomed to Earth’s gravity, is completely altered in space where there is either no gravity effect or different gravitational pulls result from different planetary bodies. Because of this unique medical anomaly, the ISS is a platform for research of interest to a forward-thinking vascular specialist.
Dr. Richard Hughson, from the University of Waterloo in Waterloo, Ontario, is researching vascular aging in spaceflight crew members. His work is a part of the Schlegel-University of Waterloo Research Institute for Aging, where he is theme leader/chair of vascular aging and brain health and holds the Schlegel Research Chair in that discipline.
Dr. Hughson is supported by the Canadian Space Agency (CSA) and Canadian Institute for Health Research (CIHR) He discussed his research in a recent audio interview (http://cihr-irsc.gc.ca/e/49523.html).
Observations have demonstrated that short-duration and extended spaceflight missions may simulate accelerated vascular aging in some of these highly fit individuals traveling to space. Specifically, spaceflight crew members have been shown to have difficulty controlling a rise in their blood pressure, perhaps secondary to the loss of Earth’s gravity, but rather in the inherent cephalad fluid shift (as blood no longer pools in the legs). In addition, significant postflight postural hypotension and physical deconditioning with resultant sarcopenia and osteopenia are known to occur.
Dr. Hughson has shown through ultrasonography that the carotid arteries of spaceflight crew members are considerably stiffer compared to their preflight arteries and that they appear to have “aged the equivalent of 20-30 years in stiffness.” The ramifications of this type of research on the study of the normal earthbound vascular aging processes are under investigation.
To counteract the effects of vascular aging and physical deconditioning in space, physical activity is key; however, the 30 minutes per day allotted to busy astronauts amid their responsibilities is just not cutting it, according to Dr. Hughson. Missions are being extended for longer periods of time, leading to serious physical consequences, For example, American astronaut Scott Kelly’s year in space will certainly result in considerable accelerated aging in his arterial system. Thus, it becomes increasingly necessary to understand and prevent the vascular aging process in astronauts, future spaceflight crew members, and perhaps one day those seeking to colonize the Moon, Mars, and beyond.
When colonization time arrives, space agencies certainly should be in the market for well-qualified vascular specialists.
Perhaps great job opportunities await those in our profession who will be brave enough to leave Earth’s cradle.
Dr Drudi is a vascular surgery resident at McGill University, Montreal.
Space is truly a magical place, enchanting philosophers, scientists, artists and dreamers. From ancient civilizations that found pantheons of gods among the stars, to novelist Andy Weir’s visionary tale of human efforts to colonize Mars recently portrayed in the movie “The Martian,” to George Lucas’ epic drama between Jedis and Sith lords in “Star Wars,” it is clear that space draws humanity to push the frontiers of science and technology – or maybe just draws us to the box office.
Nonetheless, in this day and age there are astronauts and cosmonauts who have colonized lower earth orbit (LEO) on the International Space Station (ISS), in a situation quite similar to that of the station Arthur C. Clark envisioned in his 1968 science fiction novel, “2001: A Space Odyssey.”
Unfortunately, human physiology, which has evolved in and grown accustomed to Earth’s gravity, is completely altered in space where there is either no gravity effect or different gravitational pulls result from different planetary bodies. Because of this unique medical anomaly, the ISS is a platform for research of interest to a forward-thinking vascular specialist.
Dr. Richard Hughson, from the University of Waterloo in Waterloo, Ontario, is researching vascular aging in spaceflight crew members. His work is a part of the Schlegel-University of Waterloo Research Institute for Aging, where he is theme leader/chair of vascular aging and brain health and holds the Schlegel Research Chair in that discipline.
Dr. Hughson is supported by the Canadian Space Agency (CSA) and Canadian Institute for Health Research (CIHR) He discussed his research in a recent audio interview (http://cihr-irsc.gc.ca/e/49523.html).
Observations have demonstrated that short-duration and extended spaceflight missions may simulate accelerated vascular aging in some of these highly fit individuals traveling to space. Specifically, spaceflight crew members have been shown to have difficulty controlling a rise in their blood pressure, perhaps secondary to the loss of Earth’s gravity, but rather in the inherent cephalad fluid shift (as blood no longer pools in the legs). In addition, significant postflight postural hypotension and physical deconditioning with resultant sarcopenia and osteopenia are known to occur.
Dr. Hughson has shown through ultrasonography that the carotid arteries of spaceflight crew members are considerably stiffer compared to their preflight arteries and that they appear to have “aged the equivalent of 20-30 years in stiffness.” The ramifications of this type of research on the study of the normal earthbound vascular aging processes are under investigation.
To counteract the effects of vascular aging and physical deconditioning in space, physical activity is key; however, the 30 minutes per day allotted to busy astronauts amid their responsibilities is just not cutting it, according to Dr. Hughson. Missions are being extended for longer periods of time, leading to serious physical consequences, For example, American astronaut Scott Kelly’s year in space will certainly result in considerable accelerated aging in his arterial system. Thus, it becomes increasingly necessary to understand and prevent the vascular aging process in astronauts, future spaceflight crew members, and perhaps one day those seeking to colonize the Moon, Mars, and beyond.
When colonization time arrives, space agencies certainly should be in the market for well-qualified vascular specialists.
Perhaps great job opportunities await those in our profession who will be brave enough to leave Earth’s cradle.
Dr Drudi is a vascular surgery resident at McGill University, Montreal.
Meta-analysis backs SPRINT findings, argues for lower BP targets
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
FROM THE LANCET
Key clinical point: Blood pressure lowering is associated with significant reductions in vascular events in patients with a range of comorbidities and baseline blood pressures.
Major finding: Each 10–mm Hg reduction in systolic blood pressure is associated with a 20% reduction in major cardiovascular disease events.
Data source: A meta-analysis of 123 randomized controlled trials of blood pressure lowering treatment, involving a total of 613,815 participants.
Disclosures: Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
AHA: New emphasis on percent LDL reduction on-treatment
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: An individual’s percent reduction in LDL cholesterol achieved on statin therapy is a clinically important measurement.
Major finding: The percent LDL cholesterol lowering achieved with high-intensity statin therapy varies widely between individuals and tracks closely with the magnitude of cardiovascular risk reduction.
Data source: A secondary analysis of the JUPITER trial, in which more than 17,000 apparently healthy subjects were randomized to 20 mg/day of rosuvastatin or placebo.
Disclosures: The presenter reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
Endovascular thrombectomy vs tPA: better function, same mortality
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Endovascular mechanical thrombectomy yielded better function and angiographic revascularization but similar mortality and rate of intracranial hemorrhage compared with standard medical therapy (tPA) for acute ischemic stroke.
Major finding: Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8.
Data source: A meta-analysis of eight high-quality multicenter randomized clinical trials published during 2013-2015 involving 2,423 adults with acute ischemic stroke.
Disclosures: No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
Beta-blockers cut CAS stroke, deaths
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to nonusers, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005].
“Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with postprocedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think [the benefits are due to] up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days.
There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short-term users were more symptomatic; those and other differences were controlled for on multivariate analysis.
Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average patientage in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators reported that they had no disclosures.
This retrospective study by Malas et al. showed a 34% significant reduction of stroke and death in patients undergoing carotid artery stenting (CAS) who had been on beta-blockade (BB) for at least 1 month beforehand. Presumably, most of these patients were already on longstanding BB. Current cardiology guidelines recommend the continuation of established BB for surgical patients, and this may also mitigate the cardiac risk in CAS patients. The short-term use of BB has been shown to have risk during and after noncardiac surgery and, intuitively, could lead to severe hypotension during CAS. The reason for the stroke reduction seen with well-established BB is not clearly understood.
This study begs the question: Should every patient being considered for CAS be on BB at least 1 month before the intervention, even those with few or no cardiac risk factors? If so, then it would be difficult to advocate for CAS in acutely symptomatic patients not already on a BB, thus further limiting the usefulness of this procedure.
Dr. Mark L. Friedell is chairman of the department of Surgery, University of Missouri Kansas City School of Medicine.
This retrospective study by Malas et al. showed a 34% significant reduction of stroke and death in patients undergoing carotid artery stenting (CAS) who had been on beta-blockade (BB) for at least 1 month beforehand. Presumably, most of these patients were already on longstanding BB. Current cardiology guidelines recommend the continuation of established BB for surgical patients, and this may also mitigate the cardiac risk in CAS patients. The short-term use of BB has been shown to have risk during and after noncardiac surgery and, intuitively, could lead to severe hypotension during CAS. The reason for the stroke reduction seen with well-established BB is not clearly understood.
This study begs the question: Should every patient being considered for CAS be on BB at least 1 month before the intervention, even those with few or no cardiac risk factors? If so, then it would be difficult to advocate for CAS in acutely symptomatic patients not already on a BB, thus further limiting the usefulness of this procedure.
Dr. Mark L. Friedell is chairman of the department of Surgery, University of Missouri Kansas City School of Medicine.
This retrospective study by Malas et al. showed a 34% significant reduction of stroke and death in patients undergoing carotid artery stenting (CAS) who had been on beta-blockade (BB) for at least 1 month beforehand. Presumably, most of these patients were already on longstanding BB. Current cardiology guidelines recommend the continuation of established BB for surgical patients, and this may also mitigate the cardiac risk in CAS patients. The short-term use of BB has been shown to have risk during and after noncardiac surgery and, intuitively, could lead to severe hypotension during CAS. The reason for the stroke reduction seen with well-established BB is not clearly understood.
This study begs the question: Should every patient being considered for CAS be on BB at least 1 month before the intervention, even those with few or no cardiac risk factors? If so, then it would be difficult to advocate for CAS in acutely symptomatic patients not already on a BB, thus further limiting the usefulness of this procedure.
Dr. Mark L. Friedell is chairman of the department of Surgery, University of Missouri Kansas City School of Medicine.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to nonusers, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005].
“Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with postprocedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think [the benefits are due to] up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days.
There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short-term users were more symptomatic; those and other differences were controlled for on multivariate analysis.
Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average patientage in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators reported that they had no disclosures.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to nonusers, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005].
“Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with postprocedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think [the benefits are due to] up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days.
There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short-term users were more symptomatic; those and other differences were controlled for on multivariate analysis.
Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average patientage in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators reported that they had no disclosures.
Lives saved with lower systolic BP: SPRINT trial
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
FROM AN NHLBI TELEBRIEFING