User login
Role of Prophylactic Cranial Irradiation in Small Cell Carcinoma of Urinary Bladder: Case Report and Literature Review
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.
Metastatic Urothelial Carcinoma Presenting as Mediastinal Lymphadenopathy Without Appreciable Bladder Mass in a Patient With Chronic Lymphocytic Leukemia
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
Survival of Follicular Thyroid Cancer Between Surgical Subtypes: A SEER Database Analysis
INTRODUCTION
Follicular thyroid cancer (FTC) is a common endocrine malignancy that is mainly treated with surgical resection. Few prior studies have investigated the optimal type of surgery for this FTC, particularly at a national registry level. The aim of this study is to examine the differences between surgical subtypes in the management of FTC.
METHODS
Patients from the Surveillance, Epidemiology, and End Results (SEER) database who were diagnosed with FTC between 2000-2020 were selected. The surgeries were categorized into sublobectomy, lobectomy, subtotal thyroidectomy, or thyroidectomy groups based on the surgical procedure performed. Additional variables were collected including age, sex, race, stage, radiation status, time to treatment, household income, and population size. Kaplan-Meier, Chi-square and logistic regression analyses were performed.
RESULTS
A total of 9,983 patients were included. Using Kaplan-Meier, there was improved survival for patients that received surgery (p<0.001). Patients who underwent lobectomy had greater survival than all groups (p<0.001) while thyroidectomy had greater survival compared to sub-lobectomy (p=0.015). On Chi-square, differences at one- and five-year survival were present between surgical groups (p=0.022 and p<0.001, respectively). However, logistic regression showed no survival difference between surgery type at one- and five-years. Additional findings include regional and distal staging having worse survival at one- and five-years (p’s<0.001) while median household income >$75,000 and receipt of radiation improved survival at one-year (p’s<0.05). Household income >$75,000 and radiation status no longer improved survival at five-years. Patients living outside metropolitan areas showed an improved survival at fiveyears (p=0.036).
CONCLUSIONS
The results of the preliminary Kaplan- Meier and Chi-square analysis showed that there are significant differences in survival between different surgery subtypes. However, after controlling for multiple variables, no survival differences were observed between surgical types. Despite minimal differences in FTC survival based on the type of surgical intervention, clinical factors like stage and radiation and socioeconomic factors like household income and population size may influence FTC survival. Identifying and controlling for these variables should be considered in future research on FTC.
INTRODUCTION
Follicular thyroid cancer (FTC) is a common endocrine malignancy that is mainly treated with surgical resection. Few prior studies have investigated the optimal type of surgery for this FTC, particularly at a national registry level. The aim of this study is to examine the differences between surgical subtypes in the management of FTC.
METHODS
Patients from the Surveillance, Epidemiology, and End Results (SEER) database who were diagnosed with FTC between 2000-2020 were selected. The surgeries were categorized into sublobectomy, lobectomy, subtotal thyroidectomy, or thyroidectomy groups based on the surgical procedure performed. Additional variables were collected including age, sex, race, stage, radiation status, time to treatment, household income, and population size. Kaplan-Meier, Chi-square and logistic regression analyses were performed.
RESULTS
A total of 9,983 patients were included. Using Kaplan-Meier, there was improved survival for patients that received surgery (p<0.001). Patients who underwent lobectomy had greater survival than all groups (p<0.001) while thyroidectomy had greater survival compared to sub-lobectomy (p=0.015). On Chi-square, differences at one- and five-year survival were present between surgical groups (p=0.022 and p<0.001, respectively). However, logistic regression showed no survival difference between surgery type at one- and five-years. Additional findings include regional and distal staging having worse survival at one- and five-years (p’s<0.001) while median household income >$75,000 and receipt of radiation improved survival at one-year (p’s<0.05). Household income >$75,000 and radiation status no longer improved survival at five-years. Patients living outside metropolitan areas showed an improved survival at fiveyears (p=0.036).
CONCLUSIONS
The results of the preliminary Kaplan- Meier and Chi-square analysis showed that there are significant differences in survival between different surgery subtypes. However, after controlling for multiple variables, no survival differences were observed between surgical types. Despite minimal differences in FTC survival based on the type of surgical intervention, clinical factors like stage and radiation and socioeconomic factors like household income and population size may influence FTC survival. Identifying and controlling for these variables should be considered in future research on FTC.
INTRODUCTION
Follicular thyroid cancer (FTC) is a common endocrine malignancy that is mainly treated with surgical resection. Few prior studies have investigated the optimal type of surgery for this FTC, particularly at a national registry level. The aim of this study is to examine the differences between surgical subtypes in the management of FTC.
METHODS
Patients from the Surveillance, Epidemiology, and End Results (SEER) database who were diagnosed with FTC between 2000-2020 were selected. The surgeries were categorized into sublobectomy, lobectomy, subtotal thyroidectomy, or thyroidectomy groups based on the surgical procedure performed. Additional variables were collected including age, sex, race, stage, radiation status, time to treatment, household income, and population size. Kaplan-Meier, Chi-square and logistic regression analyses were performed.
RESULTS
A total of 9,983 patients were included. Using Kaplan-Meier, there was improved survival for patients that received surgery (p<0.001). Patients who underwent lobectomy had greater survival than all groups (p<0.001) while thyroidectomy had greater survival compared to sub-lobectomy (p=0.015). On Chi-square, differences at one- and five-year survival were present between surgical groups (p=0.022 and p<0.001, respectively). However, logistic regression showed no survival difference between surgery type at one- and five-years. Additional findings include regional and distal staging having worse survival at one- and five-years (p’s<0.001) while median household income >$75,000 and receipt of radiation improved survival at one-year (p’s<0.05). Household income >$75,000 and radiation status no longer improved survival at five-years. Patients living outside metropolitan areas showed an improved survival at fiveyears (p=0.036).
CONCLUSIONS
The results of the preliminary Kaplan- Meier and Chi-square analysis showed that there are significant differences in survival between different surgery subtypes. However, after controlling for multiple variables, no survival differences were observed between surgical types. Despite minimal differences in FTC survival based on the type of surgical intervention, clinical factors like stage and radiation and socioeconomic factors like household income and population size may influence FTC survival. Identifying and controlling for these variables should be considered in future research on FTC.
Survival and Treatment in Older Patients With Ewing Sarcoma
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
Recurrence of Adult Cerebellar Medulloblastoma With Bone Marrow Metastasis: A Case Report and Review of the Literature
INTRODUCTION
Medulloblastoma (MB) is rarely seen in adulthood. Treatment guidelines are derived from studies of the pediatric population, results favoring the Packer regimen (cisplatin plus cyclophosphamide or lomustine plus vincristine). MB rarely has extraneural metastases, especially the bone marrow.
CASE PRESENTATION
A 32-year-old female with a past medical history of cerebellar MB confirmed on surgical pathology status post resection, weekly radiation and vincristine treatment presented to us in clinic to re-establish care. She was lost to follow-up 9 months after initial diagnosis and wished to continue treatment. She was started on Lomustine, Cisplatin and Vincristine after discussion with our colleagues at MSKCC, where she had received her initial treatment. After cycle three, she developed intractable bone pain and pancytopenia. Bone marrow biopsy revealed metastasis of Sonic Hedgehog Desmoplastic/nodular variant MB. PET and CT imaging confirmed metastatic disease in the bone marrow and repeat MRI brain showed abnormal nodular enhancement. CSF analysis to assess for spinal metastasis was negative. The patient was started on Temozolomide, Irinotecan and Bevacizumab with significant improvement in bone pain and radiological improvement noted on PET and CT scans. After cycle six, the patient had increased bone pain and repeat FDG-PET showed increased uptake, however, she continued to receive treatment and her pain has improved off narcotics.
DISCUSSION
We highlight a case of adult MB in the bone marrow responsive to temozolomide, irinotecan and bevacizumab. We conducted a literature search using PubMed, Medline and Web of Science between 1990 to 2022. In 2021, COG Phase 2 screening trial showed bevacizumab, temozolamide/irinotecan therapy significantly reduced the risk of death with recurrent MBs, two studies included patients up to 21 and 23 years of age. Other modalities showing some response include Vincristine plus cyclophosphamide as well as high dose carboplatin, thiotepa and etoposide alongside autologous SCT. Vismodegib has also shown varied response of 15 months in two adults with extraneural MB metastasis. Given the unique entity of adult MB and extraneural metastasis, limitations include small sample and lack of generalizability.
CONCLUSIONS
Extraneural metastasis of MB yields a poor prognosis. Future considerations include randomized trials to establish efficacy of Temozolomide, Irinotecan plus Bevacizumab in this population.
INTRODUCTION
Medulloblastoma (MB) is rarely seen in adulthood. Treatment guidelines are derived from studies of the pediatric population, results favoring the Packer regimen (cisplatin plus cyclophosphamide or lomustine plus vincristine). MB rarely has extraneural metastases, especially the bone marrow.
CASE PRESENTATION
A 32-year-old female with a past medical history of cerebellar MB confirmed on surgical pathology status post resection, weekly radiation and vincristine treatment presented to us in clinic to re-establish care. She was lost to follow-up 9 months after initial diagnosis and wished to continue treatment. She was started on Lomustine, Cisplatin and Vincristine after discussion with our colleagues at MSKCC, where she had received her initial treatment. After cycle three, she developed intractable bone pain and pancytopenia. Bone marrow biopsy revealed metastasis of Sonic Hedgehog Desmoplastic/nodular variant MB. PET and CT imaging confirmed metastatic disease in the bone marrow and repeat MRI brain showed abnormal nodular enhancement. CSF analysis to assess for spinal metastasis was negative. The patient was started on Temozolomide, Irinotecan and Bevacizumab with significant improvement in bone pain and radiological improvement noted on PET and CT scans. After cycle six, the patient had increased bone pain and repeat FDG-PET showed increased uptake, however, she continued to receive treatment and her pain has improved off narcotics.
DISCUSSION
We highlight a case of adult MB in the bone marrow responsive to temozolomide, irinotecan and bevacizumab. We conducted a literature search using PubMed, Medline and Web of Science between 1990 to 2022. In 2021, COG Phase 2 screening trial showed bevacizumab, temozolamide/irinotecan therapy significantly reduced the risk of death with recurrent MBs, two studies included patients up to 21 and 23 years of age. Other modalities showing some response include Vincristine plus cyclophosphamide as well as high dose carboplatin, thiotepa and etoposide alongside autologous SCT. Vismodegib has also shown varied response of 15 months in two adults with extraneural MB metastasis. Given the unique entity of adult MB and extraneural metastasis, limitations include small sample and lack of generalizability.
CONCLUSIONS
Extraneural metastasis of MB yields a poor prognosis. Future considerations include randomized trials to establish efficacy of Temozolomide, Irinotecan plus Bevacizumab in this population.
INTRODUCTION
Medulloblastoma (MB) is rarely seen in adulthood. Treatment guidelines are derived from studies of the pediatric population, results favoring the Packer regimen (cisplatin plus cyclophosphamide or lomustine plus vincristine). MB rarely has extraneural metastases, especially the bone marrow.
CASE PRESENTATION
A 32-year-old female with a past medical history of cerebellar MB confirmed on surgical pathology status post resection, weekly radiation and vincristine treatment presented to us in clinic to re-establish care. She was lost to follow-up 9 months after initial diagnosis and wished to continue treatment. She was started on Lomustine, Cisplatin and Vincristine after discussion with our colleagues at MSKCC, where she had received her initial treatment. After cycle three, she developed intractable bone pain and pancytopenia. Bone marrow biopsy revealed metastasis of Sonic Hedgehog Desmoplastic/nodular variant MB. PET and CT imaging confirmed metastatic disease in the bone marrow and repeat MRI brain showed abnormal nodular enhancement. CSF analysis to assess for spinal metastasis was negative. The patient was started on Temozolomide, Irinotecan and Bevacizumab with significant improvement in bone pain and radiological improvement noted on PET and CT scans. After cycle six, the patient had increased bone pain and repeat FDG-PET showed increased uptake, however, she continued to receive treatment and her pain has improved off narcotics.
DISCUSSION
We highlight a case of adult MB in the bone marrow responsive to temozolomide, irinotecan and bevacizumab. We conducted a literature search using PubMed, Medline and Web of Science between 1990 to 2022. In 2021, COG Phase 2 screening trial showed bevacizumab, temozolamide/irinotecan therapy significantly reduced the risk of death with recurrent MBs, two studies included patients up to 21 and 23 years of age. Other modalities showing some response include Vincristine plus cyclophosphamide as well as high dose carboplatin, thiotepa and etoposide alongside autologous SCT. Vismodegib has also shown varied response of 15 months in two adults with extraneural MB metastasis. Given the unique entity of adult MB and extraneural metastasis, limitations include small sample and lack of generalizability.
CONCLUSIONS
Extraneural metastasis of MB yields a poor prognosis. Future considerations include randomized trials to establish efficacy of Temozolomide, Irinotecan plus Bevacizumab in this population.
Rasburicase Use and Glucose-6-Phosphate Dehydrogenase Testing
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
Implementation of an Interfacility Telehealth Cancer Genetics Clinic
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
Unlocking the secrets of brown fat
Brown fat, or thermogenic adipose tissue, appears to act as a “nutrient sink,” consuming glucose and lactate, among other metabolites, say U.S. researchers in a mouse study that supports its potential role in tackling obesity and even cancer.
The research, published recently in Nature Metabolism, was led by David A. Guertin, PhD, of the program in molecular medicine, University of Massachusetts, Worcester.
What is adaptive thermogenesis, and why is it important in temperature regulation?
Adaptive thermogenesis is a physiologic process that occurs in a special type of fat cell, called a brown adipocyte, in which intracellular stored lipids and nutrients taken up from the blood are catabolized to generate heat.
The heat generated by these thermogenic adipocytes is critical for warming the blood and maintaining body temperature in cold environments, and is especially critical in human infants and small mammals, which are more sensitive to low temperatures.
The process is stimulated by the sympathetic nervous system, especially in response to feeling cold, but it can be activated by other stresses as well.
While adaptative thermogenesis is also called nonshivering thermogenesis to distinguish it from muscle shivering, both means of generating heat can work together to maintain body temperature.
Why is it considered a potential target for obesity?
Adult humans have brown adipocytes in specific locations in the body called brown adipose tissues (BAT) or, more simply, “brown fat.”
Intriguingly, clinical data show that the more BAT you have, the more likely you are to be protected against cardiometabolic disorders associated with obesity.
Since obesity results from an imbalance between energy intake and energy expenditure, one model proposes that brown adipocytes rebalance this formula by expending the excess energy (calories) as heat rather than storing it.
This has been referred to as the “nutrient sink” model, and the ability to activate this process therapeutically is a very attractive antiobesity strategy.
Why was it important to understand which circulating metabolites BAT uses for thermogenesis?
It is still not clear why brown fat is so beneficial for human health, and thus there is strong rationale for understanding its metabolism and how it cooperates with other tissues in the body.
For example, prior to our work, the field lacked a broad quantitative picture of how much any individual nutrient from the blood was used by brown fat, or which specific nutrients brown fat prefers to use to make heat – such as lipids, glucose, amino acids, etc. Knowing this information helps us identify more precise strategies to activate brown fat.
In addition, circulating metabolites sometimes also have messenger functions, similar to those of hormones, that stimulate physiologic processes such as adaptative thermogenesis. Highly metabolic tissues also put metabolites back into the blood, which can send messages to the brain and other tissues.
We don’t have a lot of information yet on how brown fat might engage in these processes, and so our study also aimed at finding these special metabolite messengers.
You found that glucose and lactate predominate as BAT fuel sources. What does that tell us?
The major fuels used by brown fat have been debated for a long time.
Our study suggests that BAT in mice mainly prefers glucose and lactate, which is generated from glucose. On one hand, this shows us that thermogenic adipocytes may be especially useful in treating hyperglycemia, or even tumors, by reducing the amount of circulating glucose.
It also tells us that we need to focus more on why brown fat needs so much glucose. Other studies suggest that glucose is not just used as a fuel to generate heat but also may have other important functions in keeping brown adipocytes active and healthy.
We need to know that information so that therapeutic strategies targeting brown adipocytes can be optimized to have the best chance of success.
It’s worth noting that we did our study in mice that had free access to food. If the mice were fasting, they would use more lipids from the blood to supplement for the lack of available glucose, but we think that a baseline amount of glucose is still necessary.
What could be the clinical implications of your results if replicated in humans?
They suggest that glucose is an important resource that thermogenic adipocytes cannot do without, and moreover, that glucose is more than just a carbon source.
Resolving those other functions of glucose may provide insight into mechanisms to stimulate these cells or help explain why overweight or obese people who are insulin resistant have less brown fat activity, as insulin stimulates glucose uptake.
Beyond glucose, if any of these other metabolites made or released by brown fat have beneficial messenger functions, there may be ways to pharmacologically mimic them.
How easily do you think your findings could be applied to humans?
On a fundamental level, the basic cellular mechanisms that drive adaptative thermogenesis are likely the same between mice and humans, but the wiring to the sympathetic nervous system is a bit different.
This is why it’s important to look deeply at brown fat metabolism in mouse models to find pathways fundamental to the basic mechanisms of adaptative thermogenesis in both mice and humans, which could reveal unique therapeutic opportunities.
Another big challenge with comparing humans and mice is that humans typically keep their environment warm, so their brown fat is not that active.
In contrast, mice are often raised their entire lives in a facility kept at room temperature, around 22° C (72° F). While comfortable for the humans working with them, it’s cold for a small mouse, and so mice live with constantly active brown fat.
We can change the mouse environment to alter mouse brown fat activity, but that can’t be done with people. This makes comparative studies difficult.
Nevertheless, studies have shown that people who live in cold climates often have more brown fat, and, conversely, mice raised in warmer environments have brown fat that looks a lot more like human brown fat.
What further research do you have planned, or are looking forward to, in this area?
This is the most fun part of what we do, and I’ve been fortunate to have an amazing team passionately working on these questions.
One is to figure out why glucose is so important for these fascinating cells, which will keep us busy for years. We also need to modify the dietary conditions to determine whether the body prioritizes the use of glucose for adaptive thermogenesis even when there isn’t much available.
Another goal is to test whether any of the other metabolites we identified have bioactive functions. We also discovered a unique role for glutamine metabolism in brown fat, through the consumption of amino acids, that we haven’t yet resolved.
Finally, we want to understand how and why brown fat protects other organs from metabolic diseases, and we are just at the tip of the iceberg here.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Alcohol Abuse and Alcoholism; the National Heart, Lung, & Blood Institute; the National Institutes of Health; the AASLD Foundation Pinnacle Research Award in Liver Disease; the Edward Mallinckrodt Jr. Foundation Award; and the Basic Science Research Program of the Ministry of Education (South Korea). No relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
Brown fat, or thermogenic adipose tissue, appears to act as a “nutrient sink,” consuming glucose and lactate, among other metabolites, say U.S. researchers in a mouse study that supports its potential role in tackling obesity and even cancer.
The research, published recently in Nature Metabolism, was led by David A. Guertin, PhD, of the program in molecular medicine, University of Massachusetts, Worcester.
What is adaptive thermogenesis, and why is it important in temperature regulation?
Adaptive thermogenesis is a physiologic process that occurs in a special type of fat cell, called a brown adipocyte, in which intracellular stored lipids and nutrients taken up from the blood are catabolized to generate heat.
The heat generated by these thermogenic adipocytes is critical for warming the blood and maintaining body temperature in cold environments, and is especially critical in human infants and small mammals, which are more sensitive to low temperatures.
The process is stimulated by the sympathetic nervous system, especially in response to feeling cold, but it can be activated by other stresses as well.
While adaptative thermogenesis is also called nonshivering thermogenesis to distinguish it from muscle shivering, both means of generating heat can work together to maintain body temperature.
Why is it considered a potential target for obesity?
Adult humans have brown adipocytes in specific locations in the body called brown adipose tissues (BAT) or, more simply, “brown fat.”
Intriguingly, clinical data show that the more BAT you have, the more likely you are to be protected against cardiometabolic disorders associated with obesity.
Since obesity results from an imbalance between energy intake and energy expenditure, one model proposes that brown adipocytes rebalance this formula by expending the excess energy (calories) as heat rather than storing it.
This has been referred to as the “nutrient sink” model, and the ability to activate this process therapeutically is a very attractive antiobesity strategy.
Why was it important to understand which circulating metabolites BAT uses for thermogenesis?
It is still not clear why brown fat is so beneficial for human health, and thus there is strong rationale for understanding its metabolism and how it cooperates with other tissues in the body.
For example, prior to our work, the field lacked a broad quantitative picture of how much any individual nutrient from the blood was used by brown fat, or which specific nutrients brown fat prefers to use to make heat – such as lipids, glucose, amino acids, etc. Knowing this information helps us identify more precise strategies to activate brown fat.
In addition, circulating metabolites sometimes also have messenger functions, similar to those of hormones, that stimulate physiologic processes such as adaptative thermogenesis. Highly metabolic tissues also put metabolites back into the blood, which can send messages to the brain and other tissues.
We don’t have a lot of information yet on how brown fat might engage in these processes, and so our study also aimed at finding these special metabolite messengers.
You found that glucose and lactate predominate as BAT fuel sources. What does that tell us?
The major fuels used by brown fat have been debated for a long time.
Our study suggests that BAT in mice mainly prefers glucose and lactate, which is generated from glucose. On one hand, this shows us that thermogenic adipocytes may be especially useful in treating hyperglycemia, or even tumors, by reducing the amount of circulating glucose.
It also tells us that we need to focus more on why brown fat needs so much glucose. Other studies suggest that glucose is not just used as a fuel to generate heat but also may have other important functions in keeping brown adipocytes active and healthy.
We need to know that information so that therapeutic strategies targeting brown adipocytes can be optimized to have the best chance of success.
It’s worth noting that we did our study in mice that had free access to food. If the mice were fasting, they would use more lipids from the blood to supplement for the lack of available glucose, but we think that a baseline amount of glucose is still necessary.
What could be the clinical implications of your results if replicated in humans?
They suggest that glucose is an important resource that thermogenic adipocytes cannot do without, and moreover, that glucose is more than just a carbon source.
Resolving those other functions of glucose may provide insight into mechanisms to stimulate these cells or help explain why overweight or obese people who are insulin resistant have less brown fat activity, as insulin stimulates glucose uptake.
Beyond glucose, if any of these other metabolites made or released by brown fat have beneficial messenger functions, there may be ways to pharmacologically mimic them.
How easily do you think your findings could be applied to humans?
On a fundamental level, the basic cellular mechanisms that drive adaptative thermogenesis are likely the same between mice and humans, but the wiring to the sympathetic nervous system is a bit different.
This is why it’s important to look deeply at brown fat metabolism in mouse models to find pathways fundamental to the basic mechanisms of adaptative thermogenesis in both mice and humans, which could reveal unique therapeutic opportunities.
Another big challenge with comparing humans and mice is that humans typically keep their environment warm, so their brown fat is not that active.
In contrast, mice are often raised their entire lives in a facility kept at room temperature, around 22° C (72° F). While comfortable for the humans working with them, it’s cold for a small mouse, and so mice live with constantly active brown fat.
We can change the mouse environment to alter mouse brown fat activity, but that can’t be done with people. This makes comparative studies difficult.
Nevertheless, studies have shown that people who live in cold climates often have more brown fat, and, conversely, mice raised in warmer environments have brown fat that looks a lot more like human brown fat.
What further research do you have planned, or are looking forward to, in this area?
This is the most fun part of what we do, and I’ve been fortunate to have an amazing team passionately working on these questions.
One is to figure out why glucose is so important for these fascinating cells, which will keep us busy for years. We also need to modify the dietary conditions to determine whether the body prioritizes the use of glucose for adaptive thermogenesis even when there isn’t much available.
Another goal is to test whether any of the other metabolites we identified have bioactive functions. We also discovered a unique role for glutamine metabolism in brown fat, through the consumption of amino acids, that we haven’t yet resolved.
Finally, we want to understand how and why brown fat protects other organs from metabolic diseases, and we are just at the tip of the iceberg here.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Alcohol Abuse and Alcoholism; the National Heart, Lung, & Blood Institute; the National Institutes of Health; the AASLD Foundation Pinnacle Research Award in Liver Disease; the Edward Mallinckrodt Jr. Foundation Award; and the Basic Science Research Program of the Ministry of Education (South Korea). No relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
Brown fat, or thermogenic adipose tissue, appears to act as a “nutrient sink,” consuming glucose and lactate, among other metabolites, say U.S. researchers in a mouse study that supports its potential role in tackling obesity and even cancer.
The research, published recently in Nature Metabolism, was led by David A. Guertin, PhD, of the program in molecular medicine, University of Massachusetts, Worcester.
What is adaptive thermogenesis, and why is it important in temperature regulation?
Adaptive thermogenesis is a physiologic process that occurs in a special type of fat cell, called a brown adipocyte, in which intracellular stored lipids and nutrients taken up from the blood are catabolized to generate heat.
The heat generated by these thermogenic adipocytes is critical for warming the blood and maintaining body temperature in cold environments, and is especially critical in human infants and small mammals, which are more sensitive to low temperatures.
The process is stimulated by the sympathetic nervous system, especially in response to feeling cold, but it can be activated by other stresses as well.
While adaptative thermogenesis is also called nonshivering thermogenesis to distinguish it from muscle shivering, both means of generating heat can work together to maintain body temperature.
Why is it considered a potential target for obesity?
Adult humans have brown adipocytes in specific locations in the body called brown adipose tissues (BAT) or, more simply, “brown fat.”
Intriguingly, clinical data show that the more BAT you have, the more likely you are to be protected against cardiometabolic disorders associated with obesity.
Since obesity results from an imbalance between energy intake and energy expenditure, one model proposes that brown adipocytes rebalance this formula by expending the excess energy (calories) as heat rather than storing it.
This has been referred to as the “nutrient sink” model, and the ability to activate this process therapeutically is a very attractive antiobesity strategy.
Why was it important to understand which circulating metabolites BAT uses for thermogenesis?
It is still not clear why brown fat is so beneficial for human health, and thus there is strong rationale for understanding its metabolism and how it cooperates with other tissues in the body.
For example, prior to our work, the field lacked a broad quantitative picture of how much any individual nutrient from the blood was used by brown fat, or which specific nutrients brown fat prefers to use to make heat – such as lipids, glucose, amino acids, etc. Knowing this information helps us identify more precise strategies to activate brown fat.
In addition, circulating metabolites sometimes also have messenger functions, similar to those of hormones, that stimulate physiologic processes such as adaptative thermogenesis. Highly metabolic tissues also put metabolites back into the blood, which can send messages to the brain and other tissues.
We don’t have a lot of information yet on how brown fat might engage in these processes, and so our study also aimed at finding these special metabolite messengers.
You found that glucose and lactate predominate as BAT fuel sources. What does that tell us?
The major fuels used by brown fat have been debated for a long time.
Our study suggests that BAT in mice mainly prefers glucose and lactate, which is generated from glucose. On one hand, this shows us that thermogenic adipocytes may be especially useful in treating hyperglycemia, or even tumors, by reducing the amount of circulating glucose.
It also tells us that we need to focus more on why brown fat needs so much glucose. Other studies suggest that glucose is not just used as a fuel to generate heat but also may have other important functions in keeping brown adipocytes active and healthy.
We need to know that information so that therapeutic strategies targeting brown adipocytes can be optimized to have the best chance of success.
It’s worth noting that we did our study in mice that had free access to food. If the mice were fasting, they would use more lipids from the blood to supplement for the lack of available glucose, but we think that a baseline amount of glucose is still necessary.
What could be the clinical implications of your results if replicated in humans?
They suggest that glucose is an important resource that thermogenic adipocytes cannot do without, and moreover, that glucose is more than just a carbon source.
Resolving those other functions of glucose may provide insight into mechanisms to stimulate these cells or help explain why overweight or obese people who are insulin resistant have less brown fat activity, as insulin stimulates glucose uptake.
Beyond glucose, if any of these other metabolites made or released by brown fat have beneficial messenger functions, there may be ways to pharmacologically mimic them.
How easily do you think your findings could be applied to humans?
On a fundamental level, the basic cellular mechanisms that drive adaptative thermogenesis are likely the same between mice and humans, but the wiring to the sympathetic nervous system is a bit different.
This is why it’s important to look deeply at brown fat metabolism in mouse models to find pathways fundamental to the basic mechanisms of adaptative thermogenesis in both mice and humans, which could reveal unique therapeutic opportunities.
Another big challenge with comparing humans and mice is that humans typically keep their environment warm, so their brown fat is not that active.
In contrast, mice are often raised their entire lives in a facility kept at room temperature, around 22° C (72° F). While comfortable for the humans working with them, it’s cold for a small mouse, and so mice live with constantly active brown fat.
We can change the mouse environment to alter mouse brown fat activity, but that can’t be done with people. This makes comparative studies difficult.
Nevertheless, studies have shown that people who live in cold climates often have more brown fat, and, conversely, mice raised in warmer environments have brown fat that looks a lot more like human brown fat.
What further research do you have planned, or are looking forward to, in this area?
This is the most fun part of what we do, and I’ve been fortunate to have an amazing team passionately working on these questions.
One is to figure out why glucose is so important for these fascinating cells, which will keep us busy for years. We also need to modify the dietary conditions to determine whether the body prioritizes the use of glucose for adaptive thermogenesis even when there isn’t much available.
Another goal is to test whether any of the other metabolites we identified have bioactive functions. We also discovered a unique role for glutamine metabolism in brown fat, through the consumption of amino acids, that we haven’t yet resolved.
Finally, we want to understand how and why brown fat protects other organs from metabolic diseases, and we are just at the tip of the iceberg here.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Alcohol Abuse and Alcoholism; the National Heart, Lung, & Blood Institute; the National Institutes of Health; the AASLD Foundation Pinnacle Research Award in Liver Disease; the Edward Mallinckrodt Jr. Foundation Award; and the Basic Science Research Program of the Ministry of Education (South Korea). No relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
FROM NATURE METABOLISM
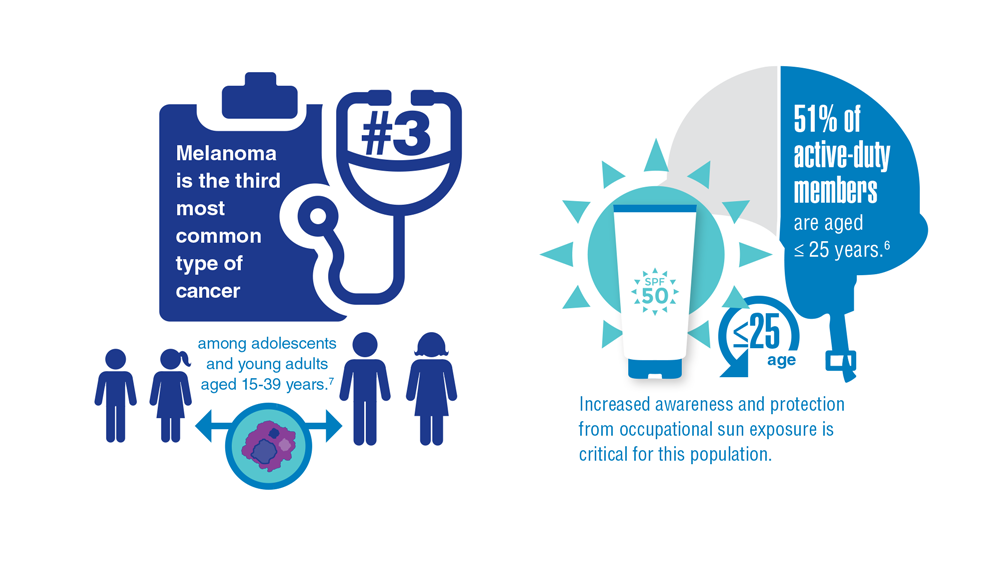
Necessary Updates to Skin Cancer Risk Stratification
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
Innovation in Cancer Treatment
- US Department of Veterans Affairs. National Precision Oncology Program (NPOP). June 10, 2019. Accessed December 8, 2022. https://www.cancer.va.gov/CANCER/NPOP.asp
- US Department of Veterans Affairs, Office of Research and Development. VA National Precision Oncology Program brings tailored cancer treatment to veterans. October 3, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/1019-VA-National-Precision-Oncology-Program-brings-tailored-cancer-treatment-to-Veterans.cfm
- Kelley M, Ahmed S. National Precision Oncology Program (NPOP): right treatment for the right patient at the right time. 2022. Unpublished data.
- Vashistha V et al. PLoS One. 2020;15(7):e0235861. doi:10.1371/journal.pone.0235861
- Dong OM et al. Value Health. 2022;25(4):582-594. doi:10.1016/j.jval.2021.09.017
- Sadik H et al. JCO Precis Oncol. 2022;6:e2200246. doi:10.1200/PO.22.00246
- Petrillo LA et al. J Pain Symptom Manage. 2021;62(3):e65-e74. doi:10.1016/j.jpainsymman.2021.02.010
- Waks AG, Winer EP. JAMA. 2019;321(3):288-300. doi:10.1001/jama.2018.19323
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Debela DT et al. SAGE Open Med. 2021;9:20503121211034366. doi:10.1177/20503121211034366
- Gambardella V et al. Cancers (Basel). 2020;12(4):1009. doi:10.3390/cancers12041009
- US Department of Veterans Affairs, Office of Research and Development. VA Lung Precision Oncology Program (LPOP). Updated January 27, 2022. Accessed January 23, 2023. https://www.research.va.gov/programs/pop/lpop.cfm
- Montgomery B et al. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
- Kelley MJ. Fed Pract. 2020;37(suppl 4):S22-S27. doi:10.12788/fp.0037
- Poonnen PJ et al. JCO Precis Oncol. 2019;3:PO.19.00075. doi:10.1200/PO.19.00075
- Natera awarded national MRD testing contract by the U.S. Department of Veterans Affairs [press release]. Natera. November 2, 2022. Accessed January 23, 2023. https://www.natera.com/company/news/natera-awarded-national-mrd-testing-contract-by-the-u-s-department-of-veterans-affairs/
- Katsoulakis E et al. JCO Precis Oncol. 2020;4:PO.19.00118. doi:10.1200/PO.19.00118
- Skoulidis F et al. N Engl J Med. 2021;384(25):2371-2381. doi:10.1056/NEJMoa2103695
- To KKW et al. Front Oncol. 2021;11:635007. doi:10.3389/fonc.2021.635007
- Price MJ et al. JCO Precis Oncol. 2022;6(1):e2100461. doi:10.1200/PO.21.00461
- André T et al; KEYNOTE-177 Investigators. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
- Stivala S, Meyer SC. Cancers (Basel). 2021;13(20):5035. doi:10.3390/cancers13205035
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
- OncoKB™ - MSK's precision oncology knowledge base. OncoKB. Accessed December 22, 2022. https://www.oncokb.org/actionableGenes
- National Library of Medicine, National Center for Biotechnology Information. PubChem compound database. Accessed December 22, 2022. https://pubchem.ncbi.nlm.nih.gov/
- US Department of Veterans Affairs. National Precision Oncology Program (NPOP). June 10, 2019. Accessed December 8, 2022. https://www.cancer.va.gov/CANCER/NPOP.asp
- US Department of Veterans Affairs, Office of Research and Development. VA National Precision Oncology Program brings tailored cancer treatment to veterans. October 3, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/1019-VA-National-Precision-Oncology-Program-brings-tailored-cancer-treatment-to-Veterans.cfm
- Kelley M, Ahmed S. National Precision Oncology Program (NPOP): right treatment for the right patient at the right time. 2022. Unpublished data.
- Vashistha V et al. PLoS One. 2020;15(7):e0235861. doi:10.1371/journal.pone.0235861
- Dong OM et al. Value Health. 2022;25(4):582-594. doi:10.1016/j.jval.2021.09.017
- Sadik H et al. JCO Precis Oncol. 2022;6:e2200246. doi:10.1200/PO.22.00246
- Petrillo LA et al. J Pain Symptom Manage. 2021;62(3):e65-e74. doi:10.1016/j.jpainsymman.2021.02.010
- Waks AG, Winer EP. JAMA. 2019;321(3):288-300. doi:10.1001/jama.2018.19323
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Debela DT et al. SAGE Open Med. 2021;9:20503121211034366. doi:10.1177/20503121211034366
- Gambardella V et al. Cancers (Basel). 2020;12(4):1009. doi:10.3390/cancers12041009
- US Department of Veterans Affairs, Office of Research and Development. VA Lung Precision Oncology Program (LPOP). Updated January 27, 2022. Accessed January 23, 2023. https://www.research.va.gov/programs/pop/lpop.cfm
- Montgomery B et al. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
- Kelley MJ. Fed Pract. 2020;37(suppl 4):S22-S27. doi:10.12788/fp.0037
- Poonnen PJ et al. JCO Precis Oncol. 2019;3:PO.19.00075. doi:10.1200/PO.19.00075
- Natera awarded national MRD testing contract by the U.S. Department of Veterans Affairs [press release]. Natera. November 2, 2022. Accessed January 23, 2023. https://www.natera.com/company/news/natera-awarded-national-mrd-testing-contract-by-the-u-s-department-of-veterans-affairs/
- Katsoulakis E et al. JCO Precis Oncol. 2020;4:PO.19.00118. doi:10.1200/PO.19.00118
- Skoulidis F et al. N Engl J Med. 2021;384(25):2371-2381. doi:10.1056/NEJMoa2103695
- To KKW et al. Front Oncol. 2021;11:635007. doi:10.3389/fonc.2021.635007
- Price MJ et al. JCO Precis Oncol. 2022;6(1):e2100461. doi:10.1200/PO.21.00461
- André T et al; KEYNOTE-177 Investigators. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
- Stivala S, Meyer SC. Cancers (Basel). 2021;13(20):5035. doi:10.3390/cancers13205035
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
- OncoKB™ - MSK's precision oncology knowledge base. OncoKB. Accessed December 22, 2022. https://www.oncokb.org/actionableGenes
- National Library of Medicine, National Center for Biotechnology Information. PubChem compound database. Accessed December 22, 2022. https://pubchem.ncbi.nlm.nih.gov/
- US Department of Veterans Affairs. National Precision Oncology Program (NPOP). June 10, 2019. Accessed December 8, 2022. https://www.cancer.va.gov/CANCER/NPOP.asp
- US Department of Veterans Affairs, Office of Research and Development. VA National Precision Oncology Program brings tailored cancer treatment to veterans. October 3, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/1019-VA-National-Precision-Oncology-Program-brings-tailored-cancer-treatment-to-Veterans.cfm
- Kelley M, Ahmed S. National Precision Oncology Program (NPOP): right treatment for the right patient at the right time. 2022. Unpublished data.
- Vashistha V et al. PLoS One. 2020;15(7):e0235861. doi:10.1371/journal.pone.0235861
- Dong OM et al. Value Health. 2022;25(4):582-594. doi:10.1016/j.jval.2021.09.017
- Sadik H et al. JCO Precis Oncol. 2022;6:e2200246. doi:10.1200/PO.22.00246
- Petrillo LA et al. J Pain Symptom Manage. 2021;62(3):e65-e74. doi:10.1016/j.jpainsymman.2021.02.010
- Waks AG, Winer EP. JAMA. 2019;321(3):288-300. doi:10.1001/jama.2018.19323
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Debela DT et al. SAGE Open Med. 2021;9:20503121211034366. doi:10.1177/20503121211034366
- Gambardella V et al. Cancers (Basel). 2020;12(4):1009. doi:10.3390/cancers12041009
- US Department of Veterans Affairs, Office of Research and Development. VA Lung Precision Oncology Program (LPOP). Updated January 27, 2022. Accessed January 23, 2023. https://www.research.va.gov/programs/pop/lpop.cfm
- Montgomery B et al. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
- Kelley MJ. Fed Pract. 2020;37(suppl 4):S22-S27. doi:10.12788/fp.0037
- Poonnen PJ et al. JCO Precis Oncol. 2019;3:PO.19.00075. doi:10.1200/PO.19.00075
- Natera awarded national MRD testing contract by the U.S. Department of Veterans Affairs [press release]. Natera. November 2, 2022. Accessed January 23, 2023. https://www.natera.com/company/news/natera-awarded-national-mrd-testing-contract-by-the-u-s-department-of-veterans-affairs/
- Katsoulakis E et al. JCO Precis Oncol. 2020;4:PO.19.00118. doi:10.1200/PO.19.00118
- Skoulidis F et al. N Engl J Med. 2021;384(25):2371-2381. doi:10.1056/NEJMoa2103695
- To KKW et al. Front Oncol. 2021;11:635007. doi:10.3389/fonc.2021.635007
- Price MJ et al. JCO Precis Oncol. 2022;6(1):e2100461. doi:10.1200/PO.21.00461
- André T et al; KEYNOTE-177 Investigators. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
- Stivala S, Meyer SC. Cancers (Basel). 2021;13(20):5035. doi:10.3390/cancers13205035
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
- OncoKB™ - MSK's precision oncology knowledge base. OncoKB. Accessed December 22, 2022. https://www.oncokb.org/actionableGenes
- National Library of Medicine, National Center for Biotechnology Information. PubChem compound database. Accessed December 22, 2022. https://pubchem.ncbi.nlm.nih.gov/