User login
Forced hospitalization for mental illness not a permanent solution
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Bright light therapy boosts therapeutic response
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Lipid signature may flag schizophrenia
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Medication-induced rhabdomyolysis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Mood disorder? Assessment in primary care
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The pediatrician’s office may be the first setting for a child to present with mood symptoms.
Self-management app may boost quality of life
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Depression: Think outside of the box for diagnosis, treatment
In the treatment of depression, clinicians are commonly dealing with a mix of comorbidities that are more complex than just depression, and as such, effective treatment options may likewise require thinking outside of the box – and beyond the definitions of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision).
“The DSM-5 isn’t handed to us on tablets from Mount Sinai,” said Charles B. Nemeroff, MD, PhD, professor and chair in the department of psychiatry and behavioral sciences at the Mulva Clinic for the Neurosciences at the University of Texas at Austin. He spoke at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Our patients don’t fall into these very convenient buckets,” Dr. Nemeroff said. “The problem with depression is patients have very high rates of morbidity and comorbidity.”
The array of potential psychiatric comorbidities that are common in depression is somewhat staggering: As many as 70% of patients also have social anxiety disorder; 67% of patients have obsessive-compulsive disorder (OCD); up to 65% of patients have panic disorder; 48% of patients have posttraumatic stress disorder (PTSD); and 42% have generalized anxiety disorder, Dr. Nemeroff said.
And while the DSM-5 may have all those bases covered, in real world clinical practice, cracking the code of each patient’s unique and often more complicated psychiatric profile – and how to best manage it – can be a challenge. But Dr. Nemeroff said important clues can guide the clinician’s path.
A key starting point is making sure to gauge the severity of the patient’s core depression with one of the validated depression scales – whether it’s the self-reported Beck Depression Inventory, the clinician-rated Hamilton Rating Scale for Depression, the clinician-rated Montgomery Asberg Depression Rating Scale, or the Inventory of Depressive Symptoms, clinicians should pick one and track the score with each visit, Dr. Nemeroff advised.
“It doesn’t matter which tool you prefer – most tend to like the Beck Depression Scale, but the bottom line is that you have to get a measure of severity at every visit,” he said.
Among the most important comorbidities to identify as soon as possible is bipolar disorder, due to the potential worsening of the condition that can occur among those patients if treated with antidepressants, Dr. Nemeroff said.
“The question of whether the patient is bipolar should always be in the back of your mind,” he cautioned. “And if patients have been started on antidepressants, the clues may become evident very quickly.”
The most important indicator that the patient has bipolar disorder “is if they tell you that they were prescribed an antidepressant and it resulted in an increase in what we know to be hypomania – they may describe it as agitation or an inability to sleep,” Dr. Nemeroff said.
Of note, the effect is much more common with SNRIs [serotonin norepinephrine reuptake inhibitors] than SSRIs [selective serotonin reuptake inhibitors], he said.
“The effect is particularly notable with venlafaxine,” he said. “But SNRIs all have the propensity to switch people with depression into hypomania, but only patients who have bipolar disorder.”
“If you give a patient 150 mg of venlafaxine and they switch to developing hypomania, you now have the diagnosis of bipolar disorder, and you can treat them appropriately.”
Other important clues of bipolarity in depressed patients include:
- Family history: Most cases are genetically driven.
- Earlier age of onset (younger than age 25): “If the patient tells you they were depressed prepuberty, you should be thinking about the possibility of bipolar disorder, as it often presents as depression in childhood.”
- Psychotic features: As many as 80% of patients with psychotic depression end up being bipolar, Dr. Nemeroff said.
- Atypical depression: For example, depression with hypersomnia, or having an increased appetite instead of decreased, or a high amount of anxiety.
Remission should be the goal of treatment, and Dr. Nemeroff said that in efforts to accomplish that with the help of medications, psychiatrists may need to think “outside of the box” – or beyond the label.
“Many practitioners become slaves to the PDR [Physicians’ Desk Reference],” he said. “It is only a guide to what the clinical trials show, and not a mandate in terms of dosing.”
“There’s often strong data in the literature that supports going to a higher dose, if necessary, and I have [plenty] of patients, for instance, on 450 or 600 mg of venlafaxine who had not responded to 150 or even 300 mg.”
Treatment resistance
When patients continue to fail to respond, regardless of dosing or medication adjustments, Dr. Nemeroff suggested that clinicians should consider the potential important reasons. For instance, in addition to comorbid psychiatric conditions, practitioners should determine if there are medical conditions that they are not aware of.
“Does the patient have an underlying medical condition, such as thyroid dysfunction, early Parkinson’s disease, or even something like cancer?” he said.
There is also the inevitable question of whether the patient is indeed taking the medication. “We know that 30% of our patients do not follow their prescriptions, so of course that’s an important question to ask,” Dr. Nemeroff said.
Finally, while some pharmacogenomic tests are emerging with the suggestion of identifying which patients may or may not respond to certain drugs, Dr. Nemeroff says he’s seen little convincing evidence of their benefits.
“We have a problem in this field in that we don’t have the kinds of markers that they do in oncology, so we’re left with having to generally play trial and error,” he said.
“But when it comes to these pharmacogenomic tests, there’s just no ‘there there’,” he asserted. “From what I’ve seen so far, it’s frankly neuro-mythology.”
Dr. Nemeroff disclosed that he receives grant/research support from the National Institutes of Health and serves as a consultant for and/or on the advisory boards of multiple pharmaceutical companies.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
In the treatment of depression, clinicians are commonly dealing with a mix of comorbidities that are more complex than just depression, and as such, effective treatment options may likewise require thinking outside of the box – and beyond the definitions of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision).
“The DSM-5 isn’t handed to us on tablets from Mount Sinai,” said Charles B. Nemeroff, MD, PhD, professor and chair in the department of psychiatry and behavioral sciences at the Mulva Clinic for the Neurosciences at the University of Texas at Austin. He spoke at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Our patients don’t fall into these very convenient buckets,” Dr. Nemeroff said. “The problem with depression is patients have very high rates of morbidity and comorbidity.”
The array of potential psychiatric comorbidities that are common in depression is somewhat staggering: As many as 70% of patients also have social anxiety disorder; 67% of patients have obsessive-compulsive disorder (OCD); up to 65% of patients have panic disorder; 48% of patients have posttraumatic stress disorder (PTSD); and 42% have generalized anxiety disorder, Dr. Nemeroff said.
And while the DSM-5 may have all those bases covered, in real world clinical practice, cracking the code of each patient’s unique and often more complicated psychiatric profile – and how to best manage it – can be a challenge. But Dr. Nemeroff said important clues can guide the clinician’s path.
A key starting point is making sure to gauge the severity of the patient’s core depression with one of the validated depression scales – whether it’s the self-reported Beck Depression Inventory, the clinician-rated Hamilton Rating Scale for Depression, the clinician-rated Montgomery Asberg Depression Rating Scale, or the Inventory of Depressive Symptoms, clinicians should pick one and track the score with each visit, Dr. Nemeroff advised.
“It doesn’t matter which tool you prefer – most tend to like the Beck Depression Scale, but the bottom line is that you have to get a measure of severity at every visit,” he said.
Among the most important comorbidities to identify as soon as possible is bipolar disorder, due to the potential worsening of the condition that can occur among those patients if treated with antidepressants, Dr. Nemeroff said.
“The question of whether the patient is bipolar should always be in the back of your mind,” he cautioned. “And if patients have been started on antidepressants, the clues may become evident very quickly.”
The most important indicator that the patient has bipolar disorder “is if they tell you that they were prescribed an antidepressant and it resulted in an increase in what we know to be hypomania – they may describe it as agitation or an inability to sleep,” Dr. Nemeroff said.
Of note, the effect is much more common with SNRIs [serotonin norepinephrine reuptake inhibitors] than SSRIs [selective serotonin reuptake inhibitors], he said.
“The effect is particularly notable with venlafaxine,” he said. “But SNRIs all have the propensity to switch people with depression into hypomania, but only patients who have bipolar disorder.”
“If you give a patient 150 mg of venlafaxine and they switch to developing hypomania, you now have the diagnosis of bipolar disorder, and you can treat them appropriately.”
Other important clues of bipolarity in depressed patients include:
- Family history: Most cases are genetically driven.
- Earlier age of onset (younger than age 25): “If the patient tells you they were depressed prepuberty, you should be thinking about the possibility of bipolar disorder, as it often presents as depression in childhood.”
- Psychotic features: As many as 80% of patients with psychotic depression end up being bipolar, Dr. Nemeroff said.
- Atypical depression: For example, depression with hypersomnia, or having an increased appetite instead of decreased, or a high amount of anxiety.
Remission should be the goal of treatment, and Dr. Nemeroff said that in efforts to accomplish that with the help of medications, psychiatrists may need to think “outside of the box” – or beyond the label.
“Many practitioners become slaves to the PDR [Physicians’ Desk Reference],” he said. “It is only a guide to what the clinical trials show, and not a mandate in terms of dosing.”
“There’s often strong data in the literature that supports going to a higher dose, if necessary, and I have [plenty] of patients, for instance, on 450 or 600 mg of venlafaxine who had not responded to 150 or even 300 mg.”
Treatment resistance
When patients continue to fail to respond, regardless of dosing or medication adjustments, Dr. Nemeroff suggested that clinicians should consider the potential important reasons. For instance, in addition to comorbid psychiatric conditions, practitioners should determine if there are medical conditions that they are not aware of.
“Does the patient have an underlying medical condition, such as thyroid dysfunction, early Parkinson’s disease, or even something like cancer?” he said.
There is also the inevitable question of whether the patient is indeed taking the medication. “We know that 30% of our patients do not follow their prescriptions, so of course that’s an important question to ask,” Dr. Nemeroff said.
Finally, while some pharmacogenomic tests are emerging with the suggestion of identifying which patients may or may not respond to certain drugs, Dr. Nemeroff says he’s seen little convincing evidence of their benefits.
“We have a problem in this field in that we don’t have the kinds of markers that they do in oncology, so we’re left with having to generally play trial and error,” he said.
“But when it comes to these pharmacogenomic tests, there’s just no ‘there there’,” he asserted. “From what I’ve seen so far, it’s frankly neuro-mythology.”
Dr. Nemeroff disclosed that he receives grant/research support from the National Institutes of Health and serves as a consultant for and/or on the advisory boards of multiple pharmaceutical companies.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
In the treatment of depression, clinicians are commonly dealing with a mix of comorbidities that are more complex than just depression, and as such, effective treatment options may likewise require thinking outside of the box – and beyond the definitions of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision).
“The DSM-5 isn’t handed to us on tablets from Mount Sinai,” said Charles B. Nemeroff, MD, PhD, professor and chair in the department of psychiatry and behavioral sciences at the Mulva Clinic for the Neurosciences at the University of Texas at Austin. He spoke at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Our patients don’t fall into these very convenient buckets,” Dr. Nemeroff said. “The problem with depression is patients have very high rates of morbidity and comorbidity.”
The array of potential psychiatric comorbidities that are common in depression is somewhat staggering: As many as 70% of patients also have social anxiety disorder; 67% of patients have obsessive-compulsive disorder (OCD); up to 65% of patients have panic disorder; 48% of patients have posttraumatic stress disorder (PTSD); and 42% have generalized anxiety disorder, Dr. Nemeroff said.
And while the DSM-5 may have all those bases covered, in real world clinical practice, cracking the code of each patient’s unique and often more complicated psychiatric profile – and how to best manage it – can be a challenge. But Dr. Nemeroff said important clues can guide the clinician’s path.
A key starting point is making sure to gauge the severity of the patient’s core depression with one of the validated depression scales – whether it’s the self-reported Beck Depression Inventory, the clinician-rated Hamilton Rating Scale for Depression, the clinician-rated Montgomery Asberg Depression Rating Scale, or the Inventory of Depressive Symptoms, clinicians should pick one and track the score with each visit, Dr. Nemeroff advised.
“It doesn’t matter which tool you prefer – most tend to like the Beck Depression Scale, but the bottom line is that you have to get a measure of severity at every visit,” he said.
Among the most important comorbidities to identify as soon as possible is bipolar disorder, due to the potential worsening of the condition that can occur among those patients if treated with antidepressants, Dr. Nemeroff said.
“The question of whether the patient is bipolar should always be in the back of your mind,” he cautioned. “And if patients have been started on antidepressants, the clues may become evident very quickly.”
The most important indicator that the patient has bipolar disorder “is if they tell you that they were prescribed an antidepressant and it resulted in an increase in what we know to be hypomania – they may describe it as agitation or an inability to sleep,” Dr. Nemeroff said.
Of note, the effect is much more common with SNRIs [serotonin norepinephrine reuptake inhibitors] than SSRIs [selective serotonin reuptake inhibitors], he said.
“The effect is particularly notable with venlafaxine,” he said. “But SNRIs all have the propensity to switch people with depression into hypomania, but only patients who have bipolar disorder.”
“If you give a patient 150 mg of venlafaxine and they switch to developing hypomania, you now have the diagnosis of bipolar disorder, and you can treat them appropriately.”
Other important clues of bipolarity in depressed patients include:
- Family history: Most cases are genetically driven.
- Earlier age of onset (younger than age 25): “If the patient tells you they were depressed prepuberty, you should be thinking about the possibility of bipolar disorder, as it often presents as depression in childhood.”
- Psychotic features: As many as 80% of patients with psychotic depression end up being bipolar, Dr. Nemeroff said.
- Atypical depression: For example, depression with hypersomnia, or having an increased appetite instead of decreased, or a high amount of anxiety.
Remission should be the goal of treatment, and Dr. Nemeroff said that in efforts to accomplish that with the help of medications, psychiatrists may need to think “outside of the box” – or beyond the label.
“Many practitioners become slaves to the PDR [Physicians’ Desk Reference],” he said. “It is only a guide to what the clinical trials show, and not a mandate in terms of dosing.”
“There’s often strong data in the literature that supports going to a higher dose, if necessary, and I have [plenty] of patients, for instance, on 450 or 600 mg of venlafaxine who had not responded to 150 or even 300 mg.”
Treatment resistance
When patients continue to fail to respond, regardless of dosing or medication adjustments, Dr. Nemeroff suggested that clinicians should consider the potential important reasons. For instance, in addition to comorbid psychiatric conditions, practitioners should determine if there are medical conditions that they are not aware of.
“Does the patient have an underlying medical condition, such as thyroid dysfunction, early Parkinson’s disease, or even something like cancer?” he said.
There is also the inevitable question of whether the patient is indeed taking the medication. “We know that 30% of our patients do not follow their prescriptions, so of course that’s an important question to ask,” Dr. Nemeroff said.
Finally, while some pharmacogenomic tests are emerging with the suggestion of identifying which patients may or may not respond to certain drugs, Dr. Nemeroff says he’s seen little convincing evidence of their benefits.
“We have a problem in this field in that we don’t have the kinds of markers that they do in oncology, so we’re left with having to generally play trial and error,” he said.
“But when it comes to these pharmacogenomic tests, there’s just no ‘there there’,” he asserted. “From what I’ve seen so far, it’s frankly neuro-mythology.”
Dr. Nemeroff disclosed that he receives grant/research support from the National Institutes of Health and serves as a consultant for and/or on the advisory boards of multiple pharmaceutical companies.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Lithium toxicity: Lessons learned
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).
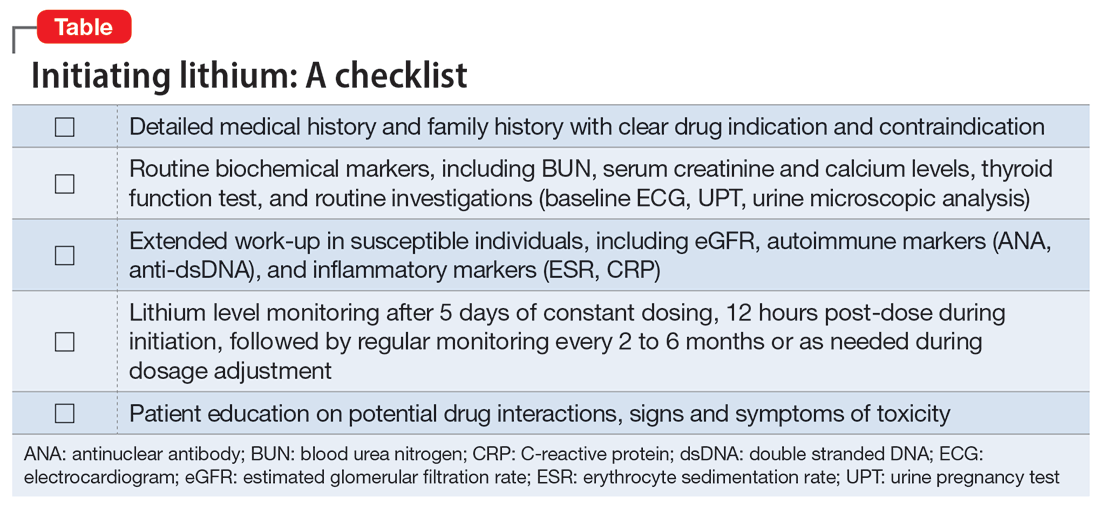
Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
Managing excited catatonia: A suggested approach
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3
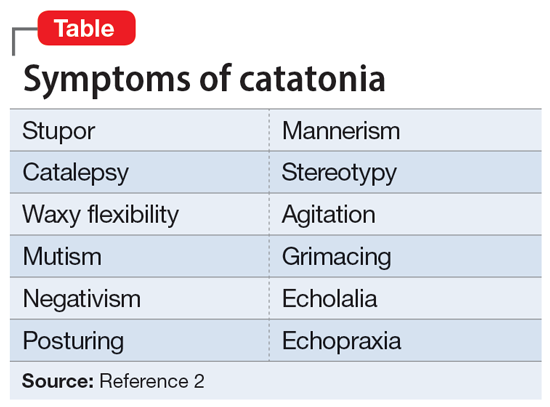
A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).
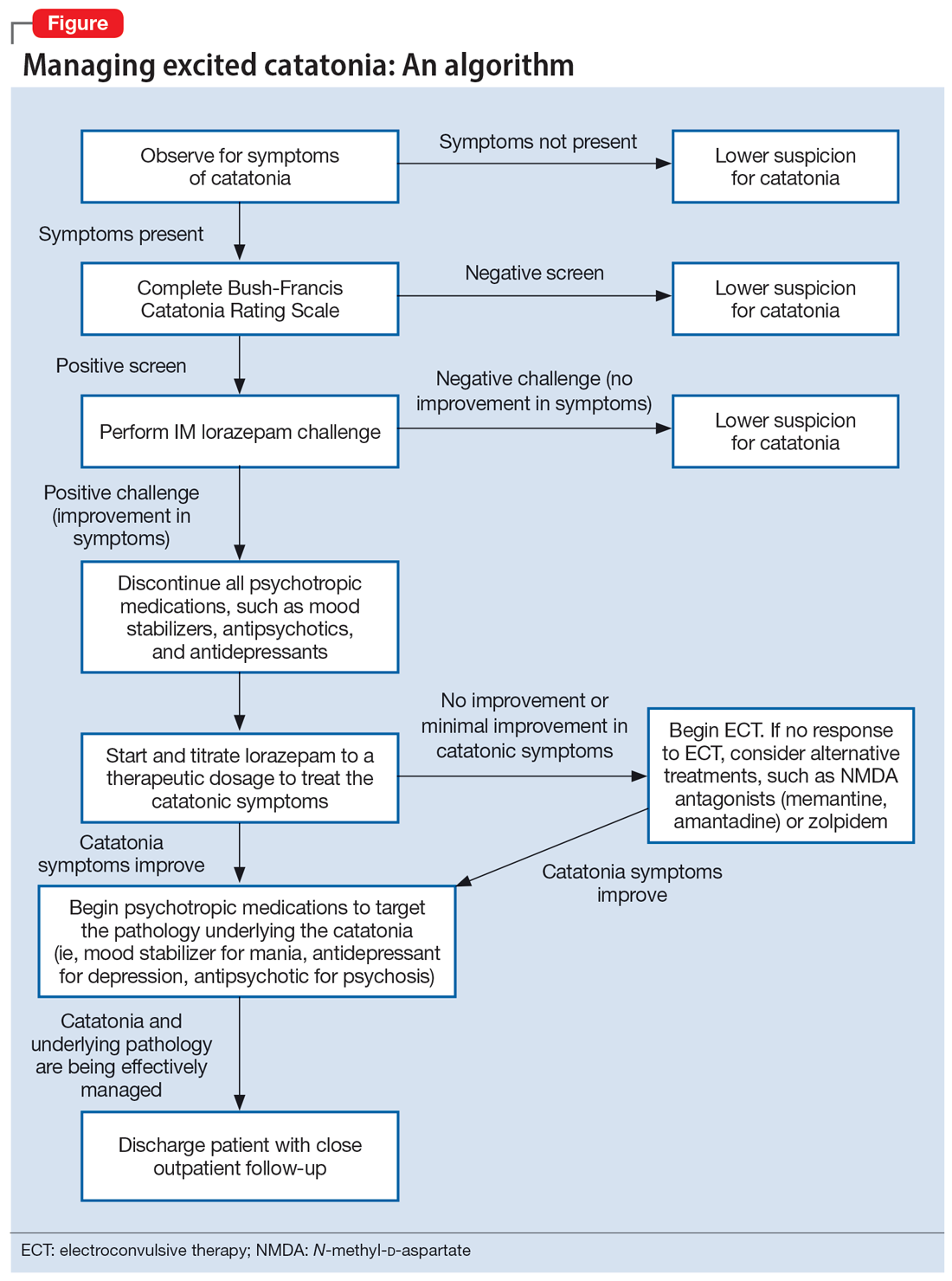
Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Behavioral treatment tied to lower medical, pharmacy costs
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN















