User login
Review finds no CV or VTE risk signal with use of JAK inhibitors for skin indications
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
FROM JAMA DERMATOLOGY
Phase 3 trial supports topical JAK inhibitor for AD in young children
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
AT THE EADV CONGRESS
Commentary: New and old treatments for AD, November 2023
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Adolescents with atopic dermatitis more likely to have experienced bullying, study finds
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Adolescents with AD have reported appearance-based bullying.
- To evaluate the association between AD and the prevalence and frequency of bullying, researchers analyzed cross-sectional data from adult caregivers of U.S. adolescents aged 12-17 years who participated in the 2021 National Health Interview Survey.
- Logistic regression and ordinal logistic regression were used to compare the prevalence of experiencing one or more bullying encounters during the previous 12 months and the frequency of bullying between adolescents with and those without AD.
TAKEAWAY:
- A total of 3,207 adolescents were included in the analysis. The mean age of the participants was 14.5 years, and 11.9% currently had AD. The prevalence of experiencing bullying was significantly higher among adolescents with AD, compared with those without AD (33.2% vs. 19%; P < .001), as was the prevalence of cyberbullying (9.1% vs. 5.8%; P = .04).
- Following adjustment for demographics and atopic comorbidities, adolescents with AD were at increased odds of bullying, compared with their peers without AD (adjusted odds ratio, 1.99; 95% confidence interval, 1.45-2.73).
- Following adjustment for demographics, adolescents with AD were also at increased odds of cyberbullying, compared with their peers without AD (AOR, 1.65; 95% CI, 1.04-2.62), but no association was observed following adjustment for atopic comorbidities (AOR, 1.27; 95% CI, 0.82-1.96).
- Following ordinal logistic regression that was adjusted for demographics and atopic comorbidities, adolescents with AD were at greater odds of being bullied at a higher frequency, compared with their peers without AD (AOR, 1.97; 95% CI, 1.44-2.68).
IN PRACTICE:
“Larger, future studies using clinical AD diagnoses and adolescent self-report can advance understanding of bullying and AD,” the researchers wrote. “Clinicians, families, and schools should address and monitor bullying among adolescents.”
SOURCE:
Howa Yeung, MD, of the department of dermatology at Emory University School of Medicine, Atlanta, led the research. The study was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include the study’s cross-sectional design. In addition, the investigators could not directly attribute bullying to skin-specific findings, and it was a caregiver report.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One of the authors, Joy Wan, MD, received grants from Pfizer and personal fees from Janssen and Sun Pharmaceuticals outside of the submitted work.
A version of this article first appeared on Medscape.com.
The Clinical Diversity of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.
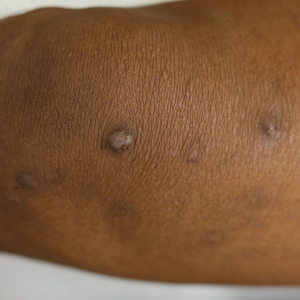
Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21
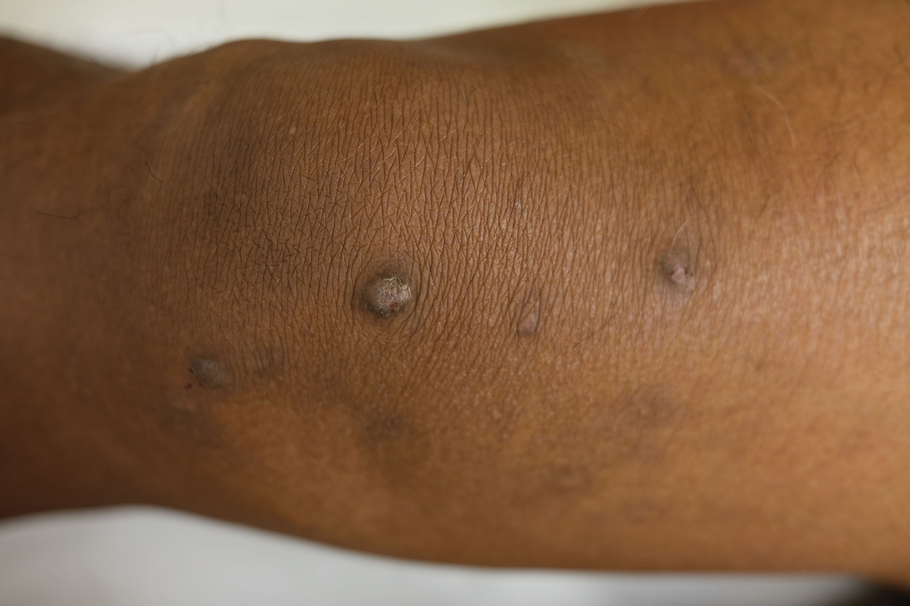
Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
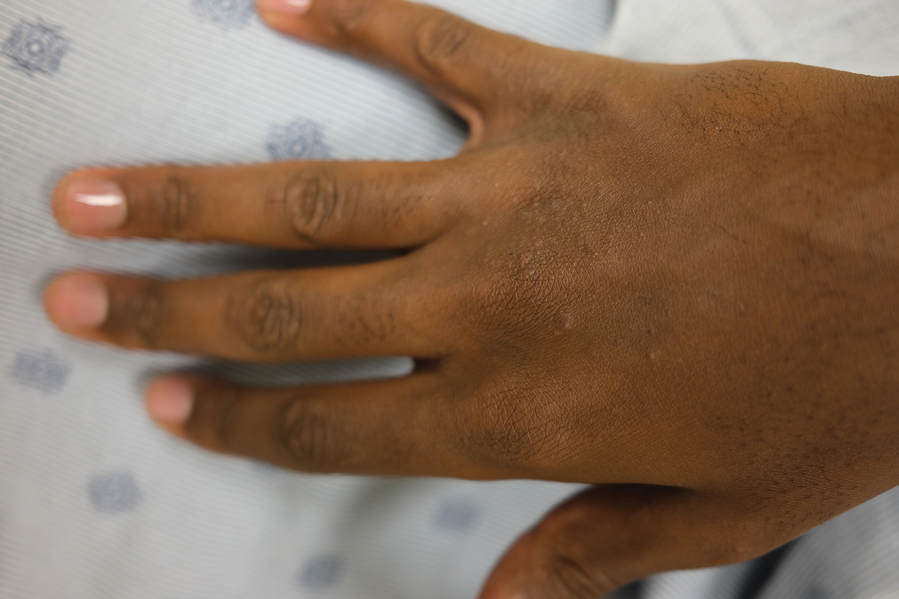
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.
Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
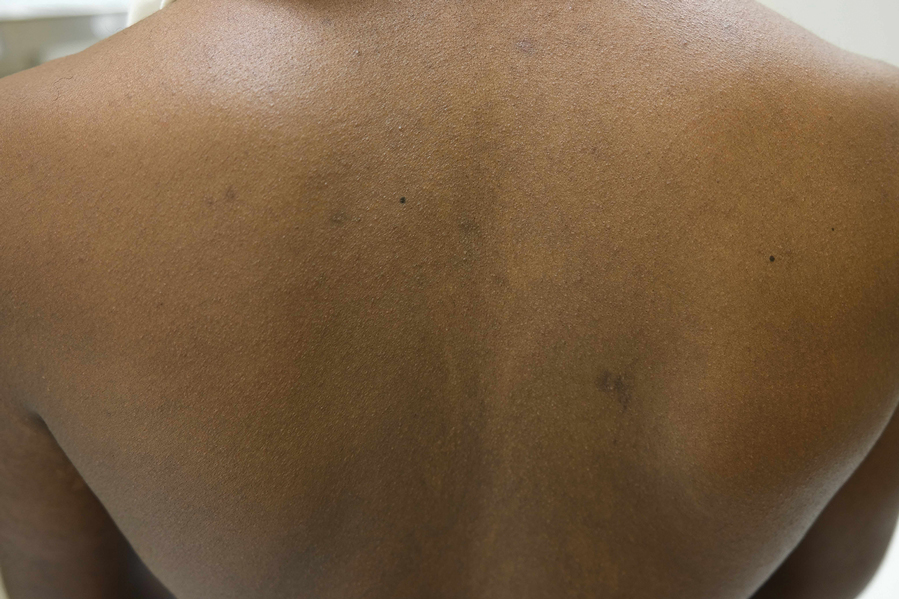
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32
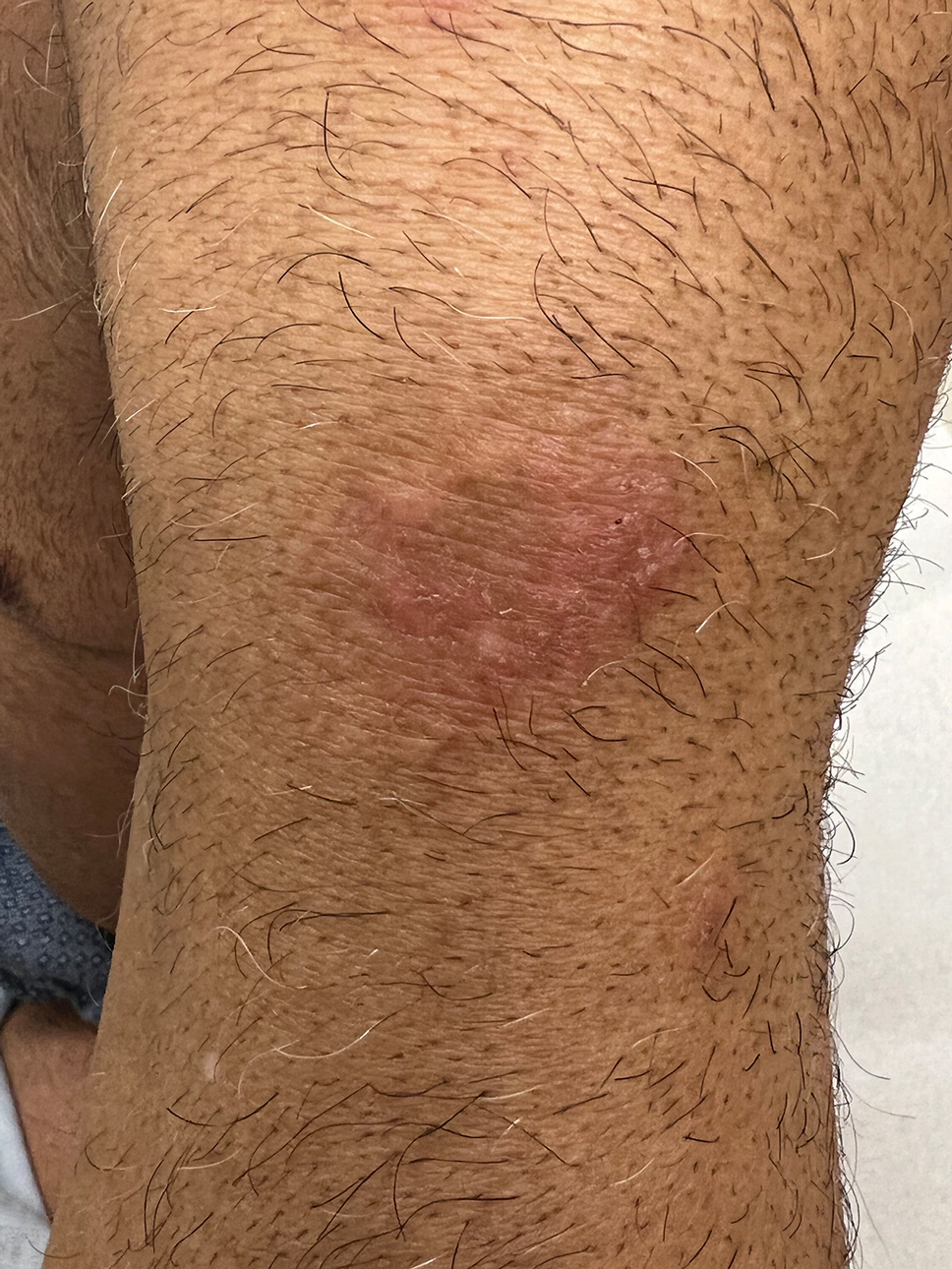
Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.

Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21

Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.

Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
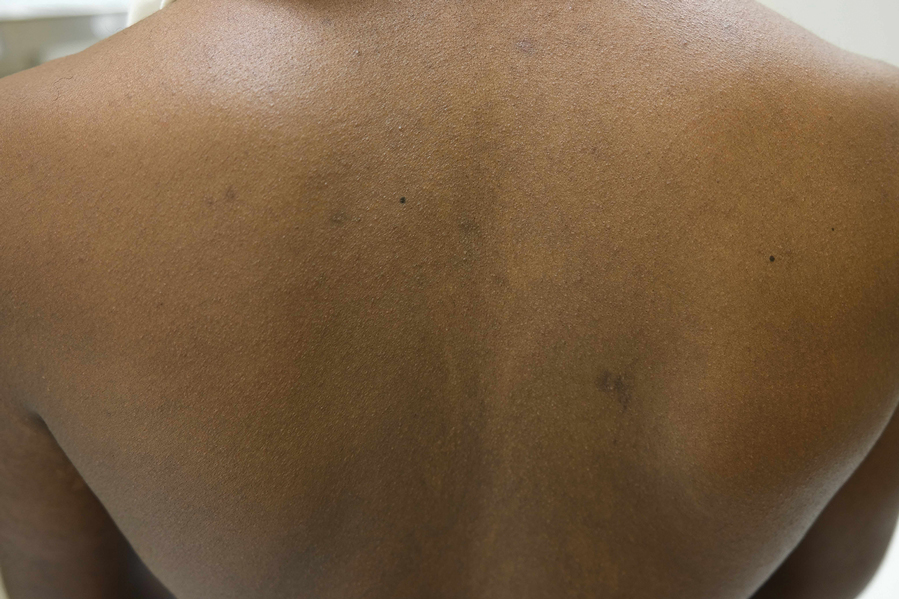
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32

Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.

Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21

Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.

Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
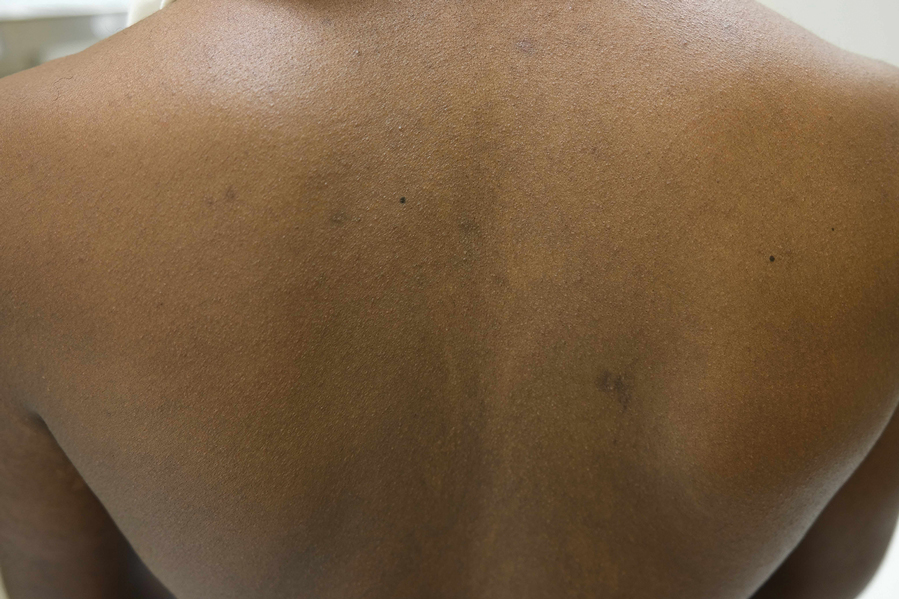
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32

Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
Practice Points
- Social determinants of health play a central role in observed racial and ethnic differences in studies of atopic dermatitis (AD) in patients with skin of color.
- Prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions are among the diverse lesion morphologies seen with AD.
- Key signs of cutaneous inflammation and lesional severity, including erythema, may present differently in darker skin tones and contribute to underestimation of severity.
- Postinflammatory dyspigmentation is common among patients with skin of color, and treatment can substantially improve quality of life.
Recombinant IL-2 shows potential in atopic dermatitis
BERLIN – A novel regulatory T cell–stimulating therapy appears to significantly improve atopic dermatitis in patients with moderate to severe disease and may even benefit quality of life, suggest results from a phase 1b trial.
The research was presented at the annual congress of the European Academy of Dermatology and Venereology.
, after which responders were observed out to 48 weeks. The higher dosage was associated with significant improvements in Eczema Area and Severity Index (EASI) and Body Surface Area (BSA) scores, which were maintained over the course of the study, as well as trends for improved patient-reported outcomes.
“This is the first study to demonstrate the therapeutic potential of rezpegaldesleukin,” said presenter Jonathan Silverberg, MD, PhD, MPH, professor of dermatology and director of clinical research at George Washington University, Washington. He added, “These may be some of the most compelling data to date for the field, proving that, at a high level, if you causally increase regulator T cells, you will take down inflammation and improve a disease state.
“For me, this is proof of concept for so many things, and it gets me very excited.”
Dr. Silverberg noted that with the response maintained out to 48 weeks, despite stopping therapy at week 12, the “hope” with the approach of inducing regulator T cells “is that we could induce tolerance and that we could have some potential for disease modification.”
He continued, “Maybe I daren’t use the word ‘cure,’ but can we at least get to something that is truly remitted, where they can stop the drug and maintain that response?”
Dr. Silverberg said rezpegaldesleukin is now being evaluated in a phase 2b study for moderate to severe atopic dermatitis, and a phase 2b trial for alopecia areata is in development.
Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, who was not involved in the study, told this news organization that “recombinant human interleukin-2 is an original therapy.”
Instead of blocking or inhibiting inflammation, it stimulates the patient’s immune system to “restore a healthy balance.”
He explained that it “stimulates regulatory T cells, which can be seen as the Peace Corps of the immune system, responsible for maintaining the equilibrium and avoiding uncontrolled inflammation.”
At the meeting, Dr. Silverberg told the audience that although they are the “beneficiaries of riches of new advances” in atopic dermatitis, “still, many observational studies have shown that the majority of patients do not achieve adequate control by the end of their induction periods and clinical trials, in the real world,” with currently available treatments.
Moreover, “there are challenges that come up with any of the different therapies,” he said, with adverse effects an important issue. For example, biologic therapies are associated with conjunctivitis, facial erythema, and arthralgia, and there are boxed warnings for Janus kinase inhibitors.
Dr. Silverberg continued, “Even patients with a favorable response can experience a loss of disease control when they come off therapy.” Consequently, “new strategies are certainly welcome that could potentially induce both deep and potentially therapy-free remission.”
To those ends, he explained that regulatory T cells play a central role in immune homeostasis but have not been “therapeutically relevant until very recently,” when it was posited that increasing their function can “induce that homeostasis, to normalize the inflammatory cascades” seen in a range of conditions, including atopic dermatitis.
Rezpegaldesleukin has high selectivity for regulatory T cells, without causing activation of effector T cells, and has been shown to increase cell numbers in a dose-dependent manner that is sustained for up to 30 days.
The current study involved patients aged 18-70 years with moderate to severe atopic dermatitis and a history of inadequate responses or intolerance to topical medications, and an EASI score ≥ 16.
Participants were randomly assigned to receive subcutaneous rezpegaldesleukin 12 mcg/kg or 24 mcg/kg or placebo every 2 weeks for 12 weeks. They then discontinued treatment and were followed up until week 19, when responders, defined as having a reduction in EASI score ≥ 50%, continued follow-up out to week 48.
Seventeen patients were randomized to higher-dose rezpegaldesleukin, whereas 16 received the lower dose and 10 were assigned to placebo. Dr. Silverberg said that the three groups were “fairly well balanced,” with “fairly good representation” across age, race, and ethnicity groups.
The mean baseline EASI score was between 21.9 and 23.7, and the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) suggested that there was an even split between moderate and severe atopic dermatitis, although the higher-dose rezpegaldesleukin group had more patients with moderate disease.
By week 12, rezpegaldesleukin was associated with significantly greater improvements in EASI scores vs. placebo. Patients on the higher dose had a mean 83% improvement over baseline vs. 65% with the lower dose and 47% with placebo (P = .002 for the higher dose vs. placebo).
Crucially, these differences were maintained up to week 48 in patients, particularly in the higher-dose group.
There was also a nonsignificant increase in the proportion of patients who achieved a reduction in EASI scores ≥ 75% over baseline with the active drug: 41% at week 12 with higher-dose rezpegaldesleukin, 25% with the lower dose, and 20% with placebo. Again, the benefit was maintained up to week 48.
The mean improvement in BSA score from baseline with rezpegaldesleukin was significantly greater than that seen with placebo, at 72% with the higher dose, 55% with the lower dose, and 36% with placebo (P = .0158 for the higher dose vs. placebo).
Although improvements in vIGA-AD scores over baseline with rezpegaldesleukin were not substantial at week 12, by week 48 there was a marked difference between higher-dose rezpegaldesleukin and placebo, with 40.0% of patients responding to the drug vs. 0% in the latter group.
A similar pattern was seen for the Itch Numeric Rating Scale, in which 55.6% of patients treated with higher-dose rezpegaldesleukin responding by week 48, compared with 0% of those who received placebo.
Greater improvements in the Dermatology Life Quality Index (DLQI) and Patient Oriented Eczema Measure (POEM) over baseline with higher-dose rezpegaldesleukin vs. plain placebo were also noted, despite a strong response in the latter group.
Dr. Silverberg reported that all treatment-emergent adverse effects in the two rezpegaldesleukin treatment arms were mild to moderate, with no severe or serious events observed.
The most common adverse events were mild to moderate injection-site reactions, seen in 75.0% of the lower-dose rezpegaldesleukin group and 58.8% the of higher-dose group. There were no cases of conjunctivitis.
The study was sponsored by Eli Lilly and Company in collaboration with Nektar Therapeutics.
Dr. Silverberg declares relationships with AbbVie, Alamar, Aldena, Amgen, AOBiome, Arcutis, Arena, Asana, ASLAN, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, CorEvitas, Dermavant, DermTech, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, Target RWE, Union, and UpToDate.
A version of this article appeared on Medscape.com.
BERLIN – A novel regulatory T cell–stimulating therapy appears to significantly improve atopic dermatitis in patients with moderate to severe disease and may even benefit quality of life, suggest results from a phase 1b trial.
The research was presented at the annual congress of the European Academy of Dermatology and Venereology.
, after which responders were observed out to 48 weeks. The higher dosage was associated with significant improvements in Eczema Area and Severity Index (EASI) and Body Surface Area (BSA) scores, which were maintained over the course of the study, as well as trends for improved patient-reported outcomes.
“This is the first study to demonstrate the therapeutic potential of rezpegaldesleukin,” said presenter Jonathan Silverberg, MD, PhD, MPH, professor of dermatology and director of clinical research at George Washington University, Washington. He added, “These may be some of the most compelling data to date for the field, proving that, at a high level, if you causally increase regulator T cells, you will take down inflammation and improve a disease state.
“For me, this is proof of concept for so many things, and it gets me very excited.”
Dr. Silverberg noted that with the response maintained out to 48 weeks, despite stopping therapy at week 12, the “hope” with the approach of inducing regulator T cells “is that we could induce tolerance and that we could have some potential for disease modification.”
He continued, “Maybe I daren’t use the word ‘cure,’ but can we at least get to something that is truly remitted, where they can stop the drug and maintain that response?”
Dr. Silverberg said rezpegaldesleukin is now being evaluated in a phase 2b study for moderate to severe atopic dermatitis, and a phase 2b trial for alopecia areata is in development.
Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, who was not involved in the study, told this news organization that “recombinant human interleukin-2 is an original therapy.”
Instead of blocking or inhibiting inflammation, it stimulates the patient’s immune system to “restore a healthy balance.”
He explained that it “stimulates regulatory T cells, which can be seen as the Peace Corps of the immune system, responsible for maintaining the equilibrium and avoiding uncontrolled inflammation.”
At the meeting, Dr. Silverberg told the audience that although they are the “beneficiaries of riches of new advances” in atopic dermatitis, “still, many observational studies have shown that the majority of patients do not achieve adequate control by the end of their induction periods and clinical trials, in the real world,” with currently available treatments.
Moreover, “there are challenges that come up with any of the different therapies,” he said, with adverse effects an important issue. For example, biologic therapies are associated with conjunctivitis, facial erythema, and arthralgia, and there are boxed warnings for Janus kinase inhibitors.
Dr. Silverberg continued, “Even patients with a favorable response can experience a loss of disease control when they come off therapy.” Consequently, “new strategies are certainly welcome that could potentially induce both deep and potentially therapy-free remission.”
To those ends, he explained that regulatory T cells play a central role in immune homeostasis but have not been “therapeutically relevant until very recently,” when it was posited that increasing their function can “induce that homeostasis, to normalize the inflammatory cascades” seen in a range of conditions, including atopic dermatitis.
Rezpegaldesleukin has high selectivity for regulatory T cells, without causing activation of effector T cells, and has been shown to increase cell numbers in a dose-dependent manner that is sustained for up to 30 days.
The current study involved patients aged 18-70 years with moderate to severe atopic dermatitis and a history of inadequate responses or intolerance to topical medications, and an EASI score ≥ 16.
Participants were randomly assigned to receive subcutaneous rezpegaldesleukin 12 mcg/kg or 24 mcg/kg or placebo every 2 weeks for 12 weeks. They then discontinued treatment and were followed up until week 19, when responders, defined as having a reduction in EASI score ≥ 50%, continued follow-up out to week 48.
Seventeen patients were randomized to higher-dose rezpegaldesleukin, whereas 16 received the lower dose and 10 were assigned to placebo. Dr. Silverberg said that the three groups were “fairly well balanced,” with “fairly good representation” across age, race, and ethnicity groups.
The mean baseline EASI score was between 21.9 and 23.7, and the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) suggested that there was an even split between moderate and severe atopic dermatitis, although the higher-dose rezpegaldesleukin group had more patients with moderate disease.
By week 12, rezpegaldesleukin was associated with significantly greater improvements in EASI scores vs. placebo. Patients on the higher dose had a mean 83% improvement over baseline vs. 65% with the lower dose and 47% with placebo (P = .002 for the higher dose vs. placebo).
Crucially, these differences were maintained up to week 48 in patients, particularly in the higher-dose group.
There was also a nonsignificant increase in the proportion of patients who achieved a reduction in EASI scores ≥ 75% over baseline with the active drug: 41% at week 12 with higher-dose rezpegaldesleukin, 25% with the lower dose, and 20% with placebo. Again, the benefit was maintained up to week 48.
The mean improvement in BSA score from baseline with rezpegaldesleukin was significantly greater than that seen with placebo, at 72% with the higher dose, 55% with the lower dose, and 36% with placebo (P = .0158 for the higher dose vs. placebo).
Although improvements in vIGA-AD scores over baseline with rezpegaldesleukin were not substantial at week 12, by week 48 there was a marked difference between higher-dose rezpegaldesleukin and placebo, with 40.0% of patients responding to the drug vs. 0% in the latter group.
A similar pattern was seen for the Itch Numeric Rating Scale, in which 55.6% of patients treated with higher-dose rezpegaldesleukin responding by week 48, compared with 0% of those who received placebo.
Greater improvements in the Dermatology Life Quality Index (DLQI) and Patient Oriented Eczema Measure (POEM) over baseline with higher-dose rezpegaldesleukin vs. plain placebo were also noted, despite a strong response in the latter group.
Dr. Silverberg reported that all treatment-emergent adverse effects in the two rezpegaldesleukin treatment arms were mild to moderate, with no severe or serious events observed.
The most common adverse events were mild to moderate injection-site reactions, seen in 75.0% of the lower-dose rezpegaldesleukin group and 58.8% the of higher-dose group. There were no cases of conjunctivitis.
The study was sponsored by Eli Lilly and Company in collaboration with Nektar Therapeutics.
Dr. Silverberg declares relationships with AbbVie, Alamar, Aldena, Amgen, AOBiome, Arcutis, Arena, Asana, ASLAN, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, CorEvitas, Dermavant, DermTech, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, Target RWE, Union, and UpToDate.
A version of this article appeared on Medscape.com.
BERLIN – A novel regulatory T cell–stimulating therapy appears to significantly improve atopic dermatitis in patients with moderate to severe disease and may even benefit quality of life, suggest results from a phase 1b trial.
The research was presented at the annual congress of the European Academy of Dermatology and Venereology.
, after which responders were observed out to 48 weeks. The higher dosage was associated with significant improvements in Eczema Area and Severity Index (EASI) and Body Surface Area (BSA) scores, which were maintained over the course of the study, as well as trends for improved patient-reported outcomes.
“This is the first study to demonstrate the therapeutic potential of rezpegaldesleukin,” said presenter Jonathan Silverberg, MD, PhD, MPH, professor of dermatology and director of clinical research at George Washington University, Washington. He added, “These may be some of the most compelling data to date for the field, proving that, at a high level, if you causally increase regulator T cells, you will take down inflammation and improve a disease state.
“For me, this is proof of concept for so many things, and it gets me very excited.”
Dr. Silverberg noted that with the response maintained out to 48 weeks, despite stopping therapy at week 12, the “hope” with the approach of inducing regulator T cells “is that we could induce tolerance and that we could have some potential for disease modification.”
He continued, “Maybe I daren’t use the word ‘cure,’ but can we at least get to something that is truly remitted, where they can stop the drug and maintain that response?”
Dr. Silverberg said rezpegaldesleukin is now being evaluated in a phase 2b study for moderate to severe atopic dermatitis, and a phase 2b trial for alopecia areata is in development.
Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, who was not involved in the study, told this news organization that “recombinant human interleukin-2 is an original therapy.”
Instead of blocking or inhibiting inflammation, it stimulates the patient’s immune system to “restore a healthy balance.”
He explained that it “stimulates regulatory T cells, which can be seen as the Peace Corps of the immune system, responsible for maintaining the equilibrium and avoiding uncontrolled inflammation.”
At the meeting, Dr. Silverberg told the audience that although they are the “beneficiaries of riches of new advances” in atopic dermatitis, “still, many observational studies have shown that the majority of patients do not achieve adequate control by the end of their induction periods and clinical trials, in the real world,” with currently available treatments.
Moreover, “there are challenges that come up with any of the different therapies,” he said, with adverse effects an important issue. For example, biologic therapies are associated with conjunctivitis, facial erythema, and arthralgia, and there are boxed warnings for Janus kinase inhibitors.
Dr. Silverberg continued, “Even patients with a favorable response can experience a loss of disease control when they come off therapy.” Consequently, “new strategies are certainly welcome that could potentially induce both deep and potentially therapy-free remission.”
To those ends, he explained that regulatory T cells play a central role in immune homeostasis but have not been “therapeutically relevant until very recently,” when it was posited that increasing their function can “induce that homeostasis, to normalize the inflammatory cascades” seen in a range of conditions, including atopic dermatitis.
Rezpegaldesleukin has high selectivity for regulatory T cells, without causing activation of effector T cells, and has been shown to increase cell numbers in a dose-dependent manner that is sustained for up to 30 days.
The current study involved patients aged 18-70 years with moderate to severe atopic dermatitis and a history of inadequate responses or intolerance to topical medications, and an EASI score ≥ 16.
Participants were randomly assigned to receive subcutaneous rezpegaldesleukin 12 mcg/kg or 24 mcg/kg or placebo every 2 weeks for 12 weeks. They then discontinued treatment and were followed up until week 19, when responders, defined as having a reduction in EASI score ≥ 50%, continued follow-up out to week 48.
Seventeen patients were randomized to higher-dose rezpegaldesleukin, whereas 16 received the lower dose and 10 were assigned to placebo. Dr. Silverberg said that the three groups were “fairly well balanced,” with “fairly good representation” across age, race, and ethnicity groups.
The mean baseline EASI score was between 21.9 and 23.7, and the Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) suggested that there was an even split between moderate and severe atopic dermatitis, although the higher-dose rezpegaldesleukin group had more patients with moderate disease.
By week 12, rezpegaldesleukin was associated with significantly greater improvements in EASI scores vs. placebo. Patients on the higher dose had a mean 83% improvement over baseline vs. 65% with the lower dose and 47% with placebo (P = .002 for the higher dose vs. placebo).
Crucially, these differences were maintained up to week 48 in patients, particularly in the higher-dose group.
There was also a nonsignificant increase in the proportion of patients who achieved a reduction in EASI scores ≥ 75% over baseline with the active drug: 41% at week 12 with higher-dose rezpegaldesleukin, 25% with the lower dose, and 20% with placebo. Again, the benefit was maintained up to week 48.
The mean improvement in BSA score from baseline with rezpegaldesleukin was significantly greater than that seen with placebo, at 72% with the higher dose, 55% with the lower dose, and 36% with placebo (P = .0158 for the higher dose vs. placebo).
Although improvements in vIGA-AD scores over baseline with rezpegaldesleukin were not substantial at week 12, by week 48 there was a marked difference between higher-dose rezpegaldesleukin and placebo, with 40.0% of patients responding to the drug vs. 0% in the latter group.
A similar pattern was seen for the Itch Numeric Rating Scale, in which 55.6% of patients treated with higher-dose rezpegaldesleukin responding by week 48, compared with 0% of those who received placebo.
Greater improvements in the Dermatology Life Quality Index (DLQI) and Patient Oriented Eczema Measure (POEM) over baseline with higher-dose rezpegaldesleukin vs. plain placebo were also noted, despite a strong response in the latter group.
Dr. Silverberg reported that all treatment-emergent adverse effects in the two rezpegaldesleukin treatment arms were mild to moderate, with no severe or serious events observed.
The most common adverse events were mild to moderate injection-site reactions, seen in 75.0% of the lower-dose rezpegaldesleukin group and 58.8% the of higher-dose group. There were no cases of conjunctivitis.
The study was sponsored by Eli Lilly and Company in collaboration with Nektar Therapeutics.
Dr. Silverberg declares relationships with AbbVie, Alamar, Aldena, Amgen, AOBiome, Arcutis, Arena, Asana, ASLAN, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, CorEvitas, Dermavant, DermTech, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, Target RWE, Union, and UpToDate.
A version of this article appeared on Medscape.com.
AT THE EADV CONGRESS
Teledermatology model takes hold with grants to underserved areas
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
No association between atopic dermatitis and non-alcoholic fatty liver disease
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Dupilumab shows long-term safety and efficacy in severe pediatric atopic dermatitis
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Key clinical point: Long-term dupilumab treatment provides sustained clinical benefits and acceptable safety in children age 6-11 years with uncontrolled severe atopic dermatitis (AD).
Major finding: By week 52, 41% of patients achieved an Investigator’s Global Assessment score of 0 or 1, and 82% of patients achieved ≥75% improvement in the Eczema Area and Severity Index scores compared with the LIBERTY AD PEDS baseline. Treatment-emergent adverse events were mostly of mild or moderate severity.
Study details: This analysis of data from the LIBERTY AD PED-OLE study included 321 children (age 6-11 years) with severe AD who previously participated in LIBERTY AD PEDS and received 300 mg dupilumab every 4 weeks or an up-titrated weight-tiered dose of 200 or 300 mg dupilumab every 2 weeks.
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Seven authors declared being employees of or holding stocks or stock options in Sanofi or Regeneron. The other authors declared ties with various sources, including Sanofi and Regeneron.
Source: Cork MJ et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Sep 26). doi: 10.1007/s13555-023-01016-9
Tralokinumab improves clinical responses at week 16 in moderate-to-severe AD despite IGA 0/1 nonachievement
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0
Key clinical point: Tralokinumab led to clinically meaningful responses in adults with moderate-to-severe atopic dermatitis (AD) who failed to achieve an Investigator’s Global Assessment (IGA) score of 0 or 1 at 16 weeks without rescue medication.
Major finding: At week 16, a significantly greater proportion of patients receiving tralokinumab vs placebo achieved ≥ 50% improvement in the Eczema Area and Severity Index scores (33.0% vs 13.0%; P < .0001) and ≥ 3-point improvement in the itch Numerical Rating Scale scores (22.6% vs 9.4%; P < .0001).
Study details: This post hoc analysis of data from ECZTRA 1 and 2 trials included adults with moderate-to-severe AD who were randomized to receive tralokinumab (n = 966) or placebo (n = 362) and failed to achieve an IGA score of 0 or 1 at week 16 without rescue medication.
Disclosures: ECZTRA 1 and 2 were sponsored by LEO Pharma A/S, Denmark. Several authors declared ties with LEO Pharma, among others. T Mark declared being an employee and stockholder of LEO Pharma A/S.
Source: Simpson EL et al. Tralokinumab provides clinically meaningful responses at week 16 in adults with moderate-to-severe atopic dermatitis who do not achieve IGA 0/1. Am J Clin Dermatol. 2023 (Oct 7). doi: 10.1007/s40257-023-00817-0