User login
Methotrexate reduces epidermal hyperplasia and alters cutaneous IL-31 and IL-31RA expression in AD
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Key clinical point: Methotrexate treatment decreased epidermal hyperplasia and altered the cutaneous expression of pruritus-related inflammatory cytokines and receptors, including interleukin-31 (IL-31) and IL-31 alpha receptor subunits (RA), in adults with moderate-to-severe refractory atopic dermatitis (AD).
Major finding: At 24 weeks of treatment, methotrexate led to a significant increase in IL-31RA expression in the epidermis (P = .016), decrease in IL-31 gene expression in lesional skin (P = .019), and reduction in the mean epidermal thickness (P = .021).
Study details: Findings are from a prospective cohort study including 12 adults with moderate-to-severe refractory AD who orally received 15 mg methotrexate per week and were matched with 10 control individuals without AD.
Disclosures: This study was supported by Fundo de Apoio à Dermatologia de São Paulo, Brazil. The authors declared no conflicts of interest.
Source: Samorano LP et al. Methotrexate for refractory adult atopic dermatitis leads to alterations in cutaneous IL-31 and IL-31RA expression. An Bras Dermatol. 2023 (Sep 18). doi: 10.1016/j.abd.2023.01.002
Tacrolimus tops hydrocortisone in pediatric atopic dermatitis treatment
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Key clinical point: Topical treatment with tacrolimus vs hydrocortisone was more efficacious in reducing inflammatory marker levels and had a better safety profile in children with atopic dermatitis (AD).
Major finding: After 3 weeks, patients receiving tacrolimus vs hydrocortisone showed a significantly greater decrease in the mean serum levels of IL-10 (P = .05), IL-17 (P = .021), and IL-23 (P = .03), lower rates of skin atrophy (P < .05), and higher but manageable incidences of mild-to-moderate transient stinging and erythema (P < .05).
Study details: Findings are from a prospective randomized clinical trial including 200 children with AD (age 2-16 years) who were randomly assigned in a 1:1 ratio to receive either 0.03% topical tacrolimus ointment or 1% hydrocortisone cream twice daily.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Mohamed AA et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol. 2023;14:1202325 (Sep 20). doi: 10.3389/fphar.2023.1202325
Atopic dermatitis affects outcomes in occupational contact dermatitis
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Key clinical point: Atopic dermatitis (AD) negatively affects the prognosis, quality of life (QoL), and work life in young workers with occupational contact dermatitis (OCD).
Major finding: The prevalence of previously diagnosed AD was 41.8%. A higher proportion of workers with vs without AD experienced eczema during the last 3 months of response submission (adjusted odds ratio [aOR] 1.7; P < .001) and reported that OCD had negatively affected their choice of jobs and occupations (aOR 1.4; P < .001). Workers with vs without AD had significantly higher mean scores in the emotions (P < .01) and symptoms (P < .001) subscales of the Skindex-29 assessment of QoL.
Study details: Findings are from a retrospective questionnaire-based study including 2392 workers age < 35 years with OCD who answered a question about being previously diagnosed with AD.
Disclosures: This study was funded by the Danish Working Environment Research Fund. The authors declared no conflicts of interest.
Source: Dietz JB et al. Impact of atopic dermatitis on occupational contact dermatitis among young people: A retrospective cohort study. Contact Dermatitis. 2023 (Sep 26). doi: 10.1111/cod.14426
Xyloglucan-pea protein a possible steroid-sparing alternative for treating pediatric AD
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Key clinical point: Xyloglucan and pea protein (XG-PP)-based topical treatment shows safety and efficacy outcomes comparable with those of hydrocortisone in pediatric patients with atopic dermatitis (AD).
Major finding: At 8 and 15 days of treatment, both XG-PP and hydrocortisone led to significant decreases in the AD Severity Index (ADSI) score (all P = .00001). Both treatment arms showed similar decrease in ADSI scores at 8 (P = .91) and 15 (P = .92) days. No adverse events were reported in the XG-PP treatment arm.
Study details: Findings are from a prospective multicenter study including 42 pediatric patients with mild-to-moderate AD (age 6 months-12 years) who were randomly assigned to receive either topical XG-PP-based cream or hydrocortisone twice daily for 14 consecutive days.
Disclosures: This study was sponsored by Novintethical Pharma SA. The authors declared no conflicts of interest.
Source: Sowlati M et al. Efficacy and tolerability of a novel topical treatment containing pea protein and xyloglucan in the management of atopic dermatitis in children: A prospective, multicenter clinical study. Dermatol Ther (Heidelb). 2023 (Sep 23). doi: 10.1007/s13555-023-01035-6
Increased risk for neuropsychiatric disorders in adults with AD
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk for multiple neuropsychiatric conditions; however, the risk profiles for specific neuropsychiatric conditions differ with AD severity.
Major finding: Adults with AD (of any severity level) vs without AD had a higher risk for anxiety (adjusted hazard ratio [aHR] 1.14, 95% CI 1.13-1.15), depression (aHR 1.14, 95% CI 1.13-1.15), and obsessive-compulsive disorder (aHR 1.48, 95% CI 1.38-1.58); the risk for autism increased in patients with mild (aHR 1.55; 95% CI 1.26-1.89) and moderate (aHR 1.40; 95% CI 1.07-1.83) AD and that for attention-deficit/hyperactivity disorder increased in those with mild AD (aHR 1.27; 95% CI 1.03-1.55].
Study details: This population-based cohort study included 625,083 adults with AD who were matched with 2,678,888 control adults without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared receiving research or fellowship funding or consultation honoraria from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer.
Source: Wan J et al. Neuropsychiatric disorders in adults with atopic dermatitis: A population-based cohort study. J Eur Acad Dermatol Venereol. 2023 (Sep 20). doi: 10.1111/jdv.19518
Methotrexate is a safe and efficacious alternative to ciclosporin in children with severe AD
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Children with atopic dermatitis more prone to allergic contact dermatitis
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
Atopic Dermatitis Triggered by Omalizumab and Treated With Dupilumab
To the Editor:
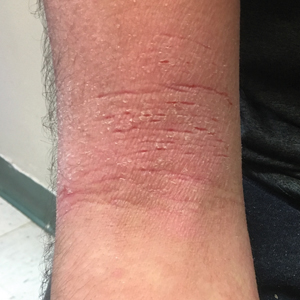
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

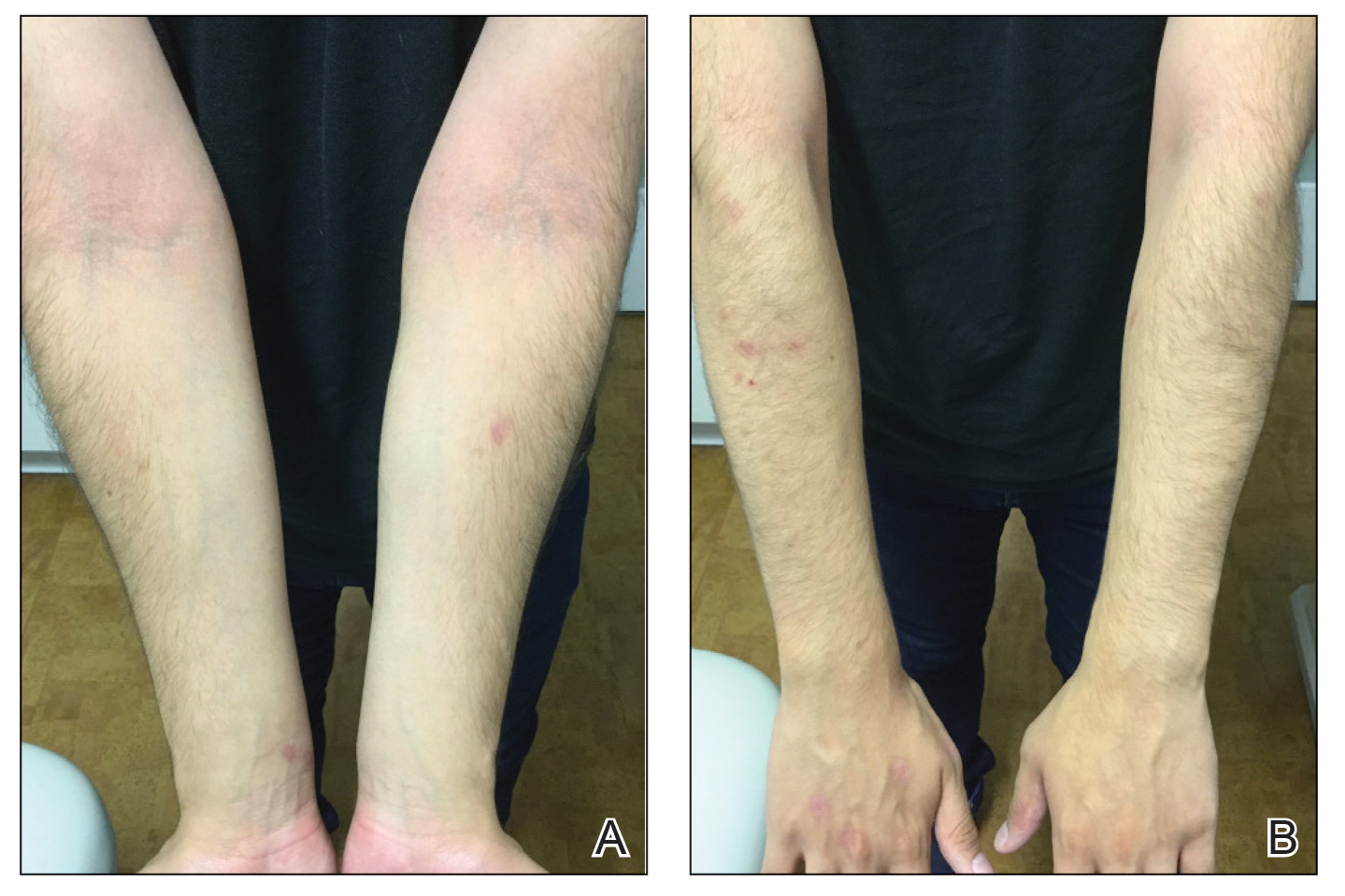
Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
Practice Points
- Monoclonal antibodies are promising therapies for atopic conditions, although its efficacy for atopic dermatitis (AD) is debated and the side-effect profile is not entirely known.
- Omalizumab may cause a paradoxical exacerbation of AD in select patients analogous to tumor necrosis factor α inhibitor–induced psoriasis.
Topical botanical drug coacillium curbs childhood alopecia
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Patch testing finds higher prevalence of ACD among children with AD
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY