User login
Daptomycin beats infective endocarditis caused by several pathogens
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
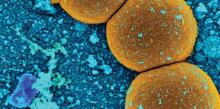
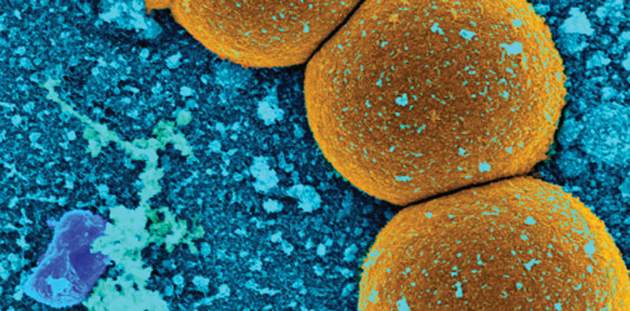
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
Key clinical point: Daptomycin had a high cure rate for infective endocarditis caused by MRSA, MSSA, coagulase-negative staph, and enterococci.
Major finding: The 2-year clinical cure rate was 90% for S. aureus infections.
Data source: Retrospective analysis of EUCORE, which comprised 198 patients.
Disclosures: Dr. Guleri had no financial disclosures.
MRSA prevalence in asymptomatic athletes comparable to dialysis, HIV patients
The prevalence of methicillin-resistant Staphylococcus aureus colonization among asymptomatic athletes is more than three times higher than the rate reported for the community population overall, a systematic review and meta-analysis showed.
Investigators searched PubMed and EMBASE looking for studies on MRSA colonization among the athletic community. They did not include studies involving individuals who previously were infected or had active MRSA infections. The database search yielded 382 studies, and of those, 15 were included in the meta-analysis, reported Dr. Styliani Karanika of Rhode Island Hospital’s infectious diseases division at Brown University, Providence, R.I. (Clin Infect Dis. 2016 April 18. doi: 10.1093/cid/ciw240).
By conducting a statistical analysis among 1,495 screened asymptomatic athletic team members (athletes and staff), Dr. Karanika and colleagues were able to see how the prevalence of MRSA colonization differed among athletes by level of playing experience and sport. The investigators found that the 6% prevalence of MRSA colonization among asymptomatic athletes was comparable to the prevalence among patients on dialysis (6%) and those with HIV (6.9%). Among college athletes, the 13% prevalence of MRSA was almost twice the rate found among patients in intensive care units (7%).
When it came to individual sports, the highest prevalence was found in wrestling (22%), followed by football (8%) and basketball (8%). The risk for subsequent MRSA skin and soft tissue infection among colonized athletes was more than seven times higher than the risk of MRSA skin and soft tissue infection among noncolonized athletes within a 3-month follow-up period upon documented MRSA colonization. Decolonization treatment was effective in reducing the risk of infection in colonized individuals.
“Our findings highlight the importance of controlling the spread of MRSA in the athletic setting, particularly among collegiate athletes,” Dr. Karanika said in an interview.
Dr. Karanika noted that athletes are more susceptible to MRSA because of the frequency of skin abrasions, close contact, shared equipment and training facilities, and poor hygiene practices that can result from the intense demands and time restrictions. Because the prevalence of MRSA colonization is high among this group, coaches, athletes, and athletic trainers should be aware of the early symptoms of a MRSA skin and soft tissue infection, and they should be educated about proper hygiene and prevention and control protocols to halt the spread of MRSA.
Though researchers found decolonization to be effective at reducing the risk of subsequent infection, they believe more research is needed to determine the durability and feasibility of decolonization regimens. Until these protocols are established, they said, strategies including implementing MRSA surveillance in athletes, environmental surveys, and regularly occurring physical examinations of athletes over the course of the season might help break the cycle of MRSA colonization-infection-transmission in athletic settings.
The investigators declared no conflicts of interest.
The prevalence of methicillin-resistant Staphylococcus aureus colonization among asymptomatic athletes is more than three times higher than the rate reported for the community population overall, a systematic review and meta-analysis showed.
Investigators searched PubMed and EMBASE looking for studies on MRSA colonization among the athletic community. They did not include studies involving individuals who previously were infected or had active MRSA infections. The database search yielded 382 studies, and of those, 15 were included in the meta-analysis, reported Dr. Styliani Karanika of Rhode Island Hospital’s infectious diseases division at Brown University, Providence, R.I. (Clin Infect Dis. 2016 April 18. doi: 10.1093/cid/ciw240).
By conducting a statistical analysis among 1,495 screened asymptomatic athletic team members (athletes and staff), Dr. Karanika and colleagues were able to see how the prevalence of MRSA colonization differed among athletes by level of playing experience and sport. The investigators found that the 6% prevalence of MRSA colonization among asymptomatic athletes was comparable to the prevalence among patients on dialysis (6%) and those with HIV (6.9%). Among college athletes, the 13% prevalence of MRSA was almost twice the rate found among patients in intensive care units (7%).
When it came to individual sports, the highest prevalence was found in wrestling (22%), followed by football (8%) and basketball (8%). The risk for subsequent MRSA skin and soft tissue infection among colonized athletes was more than seven times higher than the risk of MRSA skin and soft tissue infection among noncolonized athletes within a 3-month follow-up period upon documented MRSA colonization. Decolonization treatment was effective in reducing the risk of infection in colonized individuals.
“Our findings highlight the importance of controlling the spread of MRSA in the athletic setting, particularly among collegiate athletes,” Dr. Karanika said in an interview.
Dr. Karanika noted that athletes are more susceptible to MRSA because of the frequency of skin abrasions, close contact, shared equipment and training facilities, and poor hygiene practices that can result from the intense demands and time restrictions. Because the prevalence of MRSA colonization is high among this group, coaches, athletes, and athletic trainers should be aware of the early symptoms of a MRSA skin and soft tissue infection, and they should be educated about proper hygiene and prevention and control protocols to halt the spread of MRSA.
Though researchers found decolonization to be effective at reducing the risk of subsequent infection, they believe more research is needed to determine the durability and feasibility of decolonization regimens. Until these protocols are established, they said, strategies including implementing MRSA surveillance in athletes, environmental surveys, and regularly occurring physical examinations of athletes over the course of the season might help break the cycle of MRSA colonization-infection-transmission in athletic settings.
The investigators declared no conflicts of interest.
The prevalence of methicillin-resistant Staphylococcus aureus colonization among asymptomatic athletes is more than three times higher than the rate reported for the community population overall, a systematic review and meta-analysis showed.
Investigators searched PubMed and EMBASE looking for studies on MRSA colonization among the athletic community. They did not include studies involving individuals who previously were infected or had active MRSA infections. The database search yielded 382 studies, and of those, 15 were included in the meta-analysis, reported Dr. Styliani Karanika of Rhode Island Hospital’s infectious diseases division at Brown University, Providence, R.I. (Clin Infect Dis. 2016 April 18. doi: 10.1093/cid/ciw240).
By conducting a statistical analysis among 1,495 screened asymptomatic athletic team members (athletes and staff), Dr. Karanika and colleagues were able to see how the prevalence of MRSA colonization differed among athletes by level of playing experience and sport. The investigators found that the 6% prevalence of MRSA colonization among asymptomatic athletes was comparable to the prevalence among patients on dialysis (6%) and those with HIV (6.9%). Among college athletes, the 13% prevalence of MRSA was almost twice the rate found among patients in intensive care units (7%).
When it came to individual sports, the highest prevalence was found in wrestling (22%), followed by football (8%) and basketball (8%). The risk for subsequent MRSA skin and soft tissue infection among colonized athletes was more than seven times higher than the risk of MRSA skin and soft tissue infection among noncolonized athletes within a 3-month follow-up period upon documented MRSA colonization. Decolonization treatment was effective in reducing the risk of infection in colonized individuals.
“Our findings highlight the importance of controlling the spread of MRSA in the athletic setting, particularly among collegiate athletes,” Dr. Karanika said in an interview.
Dr. Karanika noted that athletes are more susceptible to MRSA because of the frequency of skin abrasions, close contact, shared equipment and training facilities, and poor hygiene practices that can result from the intense demands and time restrictions. Because the prevalence of MRSA colonization is high among this group, coaches, athletes, and athletic trainers should be aware of the early symptoms of a MRSA skin and soft tissue infection, and they should be educated about proper hygiene and prevention and control protocols to halt the spread of MRSA.
Though researchers found decolonization to be effective at reducing the risk of subsequent infection, they believe more research is needed to determine the durability and feasibility of decolonization regimens. Until these protocols are established, they said, strategies including implementing MRSA surveillance in athletes, environmental surveys, and regularly occurring physical examinations of athletes over the course of the season might help break the cycle of MRSA colonization-infection-transmission in athletic settings.
The investigators declared no conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: The prevalence of methicillin-resistant Staphylococcus aureus colonization among asymptomatic athletes is comparable to that among individuals with chronic illnesses.
Major finding: The prevalence of MRSA colonization was 8% among U.S. athletes and 13% among U.S. collegiate athletes. The prevalence of MRSA in the total athletic population was comparable to MRSA in patients with illnesses such as kidney disease and HIV.
Data source: A PubMed and EMBASE search yielded 382 studies and was narrowed to 15 analyses.
Disclosures: The investigators declared no conflicts of interest.
USDA to release more funds for antibiotic resistance research
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
The U.S. Department of Agriculture has made $6 million available through its Agriculture and Food Research Initiative to fund research on antimicrobial resistance.
“The research projects funded through this announcement will help us succeed in our efforts to preserve the effectiveness of antibiotics and protect public health,” said U.S. Agriculture Secretary Tom Vilsack in a statement.
The funding is authorized by the 2014 Farm Bill and administered by the USDA’s National Institute of Food and Agriculture. Secretary Vilsack said it is one of many ways that the USDA supports the Combating Antimicrobial Resistant Bacteria (CARB) National Action Plan and work of the Task Force for Combating Antibiotic Resistance, which the USDA cochairs. The program priority is to promote the development of sustainable and integrated food safety strategies that reduce public health risks along the entire food chain.
According to the USDA announcement, applications for funding must address one or more of the following:
• Develop novel systems approaches to investigate the ecology of microbial resistance microbes and gene reservoirs in the environment in animals, crops, food products, or farm-raised aquaculture products.
• Develop, evaluate, and implement effective and sustainable resources and strategies, to include alternative practices, techniques, technologies, or tools that mitigate emergence, spread, or persistence of antimicrobial-resistant pathogens within the agricultural ecosystem, in animals, crops, and food.
• Identify critical control points for mitigating antimicrobial resistance in the pre- and postharvest food production environment.
• Design innovative training, education, and outreach resources (including Web-based resources) that can be adapted by users across the food chain, including policy makers, producers, processors, retailers, and consumers.
• Design and conduct studies that evaluate the impact and efficacy of proposed research, education, and extension/outreach interventions on antimicrobial resistance across the food chain, from primary producers to primary consumers.
Since 2009, more than $82 million in food safety research and extension grants has been awarded through the Agriculture and Food Research Initiative, including $3.4 million in fiscal year 2015 for antimicrobial resistance. Previously funded projects include a State University of New York project evaluating critical control points in dairy farm operations and a Texas A&M University project to develop science-based decision aids related to antibiotic stewardship.
Applications are due Aug. 3, 2016. See the request for applications for more information.
On Twitter @richpizzi
Mixing, cycling of antibiotics fails to reduce antibiotic resistance
AMSTERDAM – Neither cycling through a regular schedule of antibiotics on a unit-wide basis, nor randomly mixing them on a patient-level basis reduced the prevalence of antibiotic resistance in eight European intensive care units, a randomized study has determined.
Lead investigator Dr. Pleun Joppe van Duijn of University Medical Center Utrecht (the Netherlands), said he and his colleagues did, however, discover a few common sense findings that seemed to positively affect antibiotic resistance, including compliance with hand hygiene, shorter lengths of stay, staff ratio, and unit occupancy rate. He reported the results of his research at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Many ICUs in Europe have one preferred empirical treatment strategy which, Dr. van Duijn said, may create selective pressure for a single resistance type. “An alternative to this is a program of antibiotic rotation,” he noted. “By constantly changing the preferred first-line treatment, selective pressure is constantly changing, which may reduce selection of antibiotic resistance.”
Dr. van Duijn and his colleagues examined this idea in a randomized crossover trial that compared antibiotic cycling and mixing. The protocols employed three antibiotic classes: third- and fourth-generation cephalosporins, piperacillin/tazobactam, and carbapenems. The trial was conducted in eight ICUs in Belgium, Germany, France, Slovenia, and Portugal.
The sites were randomized to two 9-month interventions of cycling or mixing antibiotics, with a 1-month washout period between the two interventions. In cycling protocol, the preferred empiric antibiotic was changed every 6 weeks. In the mixing protocol, every consecutive patient received a different antibiotic. However, treating physicians were allowed to deviate from any protocol for patient safety or to optimize treatment.
The primary endpoint was the monthly prevalence of perineal and/or respiratory carriage of two classes of bacteria:
• Enterobacteriaceae species that were piperacillin/tazobactam–resistant or that showed extended spectrum beta-lactamase production.
• Pseudomonas aeruginosa and Acinetobacter species that were either piperacillin/tazobactam– or carbapenem-resistant.
In all, 8,945 patients were involved, with 4,238 exposed to cycling and 4,707 to mixing. Patients were a mean of 62 years old, with a mean 7-day length of stay. About 4.5% were already colonized with resistant bacteria upon admission. A quarter were on contact isolation; 2% were on both droplet and respiratory isolation.
The overall mortality rate was 11% and did not differ between the cycling and mixing groups (10.9% vs. 11.6%). Antibiotic resistance developed in 22.6% of the cycling group and 21.5% of the mixing group – not a significant difference. Neither protocol significantly reduced over time the amount of antibiotic resistance that was observed in the baseline period.
A multivariate analysis did, however, find a few things associated with resistance prevalence. Women were about 58% less likely to develop a resistant bacterial strain than men. Patients who stayed less than 48 hours had a 38% decreased risk of developing a resistant strain. Good staff compliance with hand hygiene reduced the risk by 12%, and having one-on-one nursing reduced it by 53%.
The study was funded by the European Community’s Seventh Framework Programme. Dr. van Duijn had no financial declarations.
AMSTERDAM – Neither cycling through a regular schedule of antibiotics on a unit-wide basis, nor randomly mixing them on a patient-level basis reduced the prevalence of antibiotic resistance in eight European intensive care units, a randomized study has determined.
Lead investigator Dr. Pleun Joppe van Duijn of University Medical Center Utrecht (the Netherlands), said he and his colleagues did, however, discover a few common sense findings that seemed to positively affect antibiotic resistance, including compliance with hand hygiene, shorter lengths of stay, staff ratio, and unit occupancy rate. He reported the results of his research at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Many ICUs in Europe have one preferred empirical treatment strategy which, Dr. van Duijn said, may create selective pressure for a single resistance type. “An alternative to this is a program of antibiotic rotation,” he noted. “By constantly changing the preferred first-line treatment, selective pressure is constantly changing, which may reduce selection of antibiotic resistance.”
Dr. van Duijn and his colleagues examined this idea in a randomized crossover trial that compared antibiotic cycling and mixing. The protocols employed three antibiotic classes: third- and fourth-generation cephalosporins, piperacillin/tazobactam, and carbapenems. The trial was conducted in eight ICUs in Belgium, Germany, France, Slovenia, and Portugal.
The sites were randomized to two 9-month interventions of cycling or mixing antibiotics, with a 1-month washout period between the two interventions. In cycling protocol, the preferred empiric antibiotic was changed every 6 weeks. In the mixing protocol, every consecutive patient received a different antibiotic. However, treating physicians were allowed to deviate from any protocol for patient safety or to optimize treatment.
The primary endpoint was the monthly prevalence of perineal and/or respiratory carriage of two classes of bacteria:
• Enterobacteriaceae species that were piperacillin/tazobactam–resistant or that showed extended spectrum beta-lactamase production.
• Pseudomonas aeruginosa and Acinetobacter species that were either piperacillin/tazobactam– or carbapenem-resistant.
In all, 8,945 patients were involved, with 4,238 exposed to cycling and 4,707 to mixing. Patients were a mean of 62 years old, with a mean 7-day length of stay. About 4.5% were already colonized with resistant bacteria upon admission. A quarter were on contact isolation; 2% were on both droplet and respiratory isolation.
The overall mortality rate was 11% and did not differ between the cycling and mixing groups (10.9% vs. 11.6%). Antibiotic resistance developed in 22.6% of the cycling group and 21.5% of the mixing group – not a significant difference. Neither protocol significantly reduced over time the amount of antibiotic resistance that was observed in the baseline period.
A multivariate analysis did, however, find a few things associated with resistance prevalence. Women were about 58% less likely to develop a resistant bacterial strain than men. Patients who stayed less than 48 hours had a 38% decreased risk of developing a resistant strain. Good staff compliance with hand hygiene reduced the risk by 12%, and having one-on-one nursing reduced it by 53%.
The study was funded by the European Community’s Seventh Framework Programme. Dr. van Duijn had no financial declarations.
AMSTERDAM – Neither cycling through a regular schedule of antibiotics on a unit-wide basis, nor randomly mixing them on a patient-level basis reduced the prevalence of antibiotic resistance in eight European intensive care units, a randomized study has determined.
Lead investigator Dr. Pleun Joppe van Duijn of University Medical Center Utrecht (the Netherlands), said he and his colleagues did, however, discover a few common sense findings that seemed to positively affect antibiotic resistance, including compliance with hand hygiene, shorter lengths of stay, staff ratio, and unit occupancy rate. He reported the results of his research at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Many ICUs in Europe have one preferred empirical treatment strategy which, Dr. van Duijn said, may create selective pressure for a single resistance type. “An alternative to this is a program of antibiotic rotation,” he noted. “By constantly changing the preferred first-line treatment, selective pressure is constantly changing, which may reduce selection of antibiotic resistance.”
Dr. van Duijn and his colleagues examined this idea in a randomized crossover trial that compared antibiotic cycling and mixing. The protocols employed three antibiotic classes: third- and fourth-generation cephalosporins, piperacillin/tazobactam, and carbapenems. The trial was conducted in eight ICUs in Belgium, Germany, France, Slovenia, and Portugal.
The sites were randomized to two 9-month interventions of cycling or mixing antibiotics, with a 1-month washout period between the two interventions. In cycling protocol, the preferred empiric antibiotic was changed every 6 weeks. In the mixing protocol, every consecutive patient received a different antibiotic. However, treating physicians were allowed to deviate from any protocol for patient safety or to optimize treatment.
The primary endpoint was the monthly prevalence of perineal and/or respiratory carriage of two classes of bacteria:
• Enterobacteriaceae species that were piperacillin/tazobactam–resistant or that showed extended spectrum beta-lactamase production.
• Pseudomonas aeruginosa and Acinetobacter species that were either piperacillin/tazobactam– or carbapenem-resistant.
In all, 8,945 patients were involved, with 4,238 exposed to cycling and 4,707 to mixing. Patients were a mean of 62 years old, with a mean 7-day length of stay. About 4.5% were already colonized with resistant bacteria upon admission. A quarter were on contact isolation; 2% were on both droplet and respiratory isolation.
The overall mortality rate was 11% and did not differ between the cycling and mixing groups (10.9% vs. 11.6%). Antibiotic resistance developed in 22.6% of the cycling group and 21.5% of the mixing group – not a significant difference. Neither protocol significantly reduced over time the amount of antibiotic resistance that was observed in the baseline period.
A multivariate analysis did, however, find a few things associated with resistance prevalence. Women were about 58% less likely to develop a resistant bacterial strain than men. Patients who stayed less than 48 hours had a 38% decreased risk of developing a resistant strain. Good staff compliance with hand hygiene reduced the risk by 12%, and having one-on-one nursing reduced it by 53%.
The study was funded by the European Community’s Seventh Framework Programme. Dr. van Duijn had no financial declarations.
AT ECCMID 2016
Key clinical point: Neither cycling nor mixing antibiotics reduced the prevalence of resistant bacteria in intensive care units.
Major finding: Antibiotic resistance developed in 22.6% of the cycling group and 21.5% of the mixing group – not a significant difference.
Data source: The randomized crossover trial comprised 8,945 patients in eight ICUs.
Disclosures: The study was funded by the European Community’s Seventh Framework Programme. Dr. van Duijn had no financial declarations.
Infections kill many waiting for liver transplant, force others off list
AMSTERDAM – Infection is a major cause of death among patients waiting for a liver transplant, killing more than half of those who contracted one.
Infection also was the biggest reason that patients with end-stage liver disease withdrew from the transplant waiting list, a 9-year-long study has shown. Patients who developed an infection were six times more likely to withdraw than were those who did not, Dr. Loes Alferink wrote in a poster presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“We need to focus on better prophylactic antibiotic strategies to save lives in patients with end-stage liver disease who are on the waiting list,” said Dr. Alferink of Erasmus Medical Center, Rotterdam, the Netherlands.
She and her colleagues examined the effect of infections on 312 patients who were waiting for a transplant at Erasmus Medical Center from the period of 2006-2013. During that time, a total of 317 infections developed in 144 patients. The infections were fatal in 58% of these patients.
These included spontaneous primary cholangitis (75); spontaneous bacterial peritonitis (61); urogenital (38), respiratory (30), and skin (25) infections; as well as primary bacteremia (22). Also, there were 18 cases of gastroenteritis and 12 cases of Candida esophagitis. The remainder were unspecified infections.
The death rate was highest in primary bacteremia, which killed about 40% of those who developed it. The rate was about 25% in respiratory infections, 20% in spontaneous primary bacteremia, 15% in esophagitis, 10% in gastroenteritis and urinary tract infections, and 10% in patients with multiple site infections.
The pathogens were gram negative (70) and gram positive (37) bacteria; Enterococcus faecium (15) and faecalis (3); yeasts (13); viruses (7); and mold (2). The remainder of the infections yielded a negative culture.
In 24 patients, multiple pathogens were identified. These patients had the highest rate of mortality, with almost half of them dying from their infection; one of the two patients with a mold infection also died. The death rate was 20% in patients with yeast infections, 18% in those with E. faecium, 15% in gram-positive infections, and 10% in gram-negative infections.
A multivariate analysis found several factors that increased the risk of dying from an infection. For every 10 years of increasing age, the risk of infection-related mortality doubled (odds ratio, 2); worse MELD (Model for End-Stage Liver Disease) scores increased the risk by 12%.
Patients with hepatic encephalopathy were 76% more likely to die from an infection, and those with refractory ascites faced a 2.5-fold increased risk. Mechanical ventilation was associated with more than a fivefold increased risk (OR, 5.72).
Patients who developed an infection were almost six times more likely to be withdrawn from the transplant waiting list (hazard ratio, 5.87). The regression analysis for withdrawal identified several factors that significantly increased the risk, including age, MELD score, and serum albumin. The biggest risk factor for withdrawal related to infection was refractory ascites, which more than doubled the risk (HR, 2.2).
Dr. Alferink had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Infection is a major cause of death among patients waiting for a liver transplant, killing more than half of those who contracted one.
Infection also was the biggest reason that patients with end-stage liver disease withdrew from the transplant waiting list, a 9-year-long study has shown. Patients who developed an infection were six times more likely to withdraw than were those who did not, Dr. Loes Alferink wrote in a poster presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“We need to focus on better prophylactic antibiotic strategies to save lives in patients with end-stage liver disease who are on the waiting list,” said Dr. Alferink of Erasmus Medical Center, Rotterdam, the Netherlands.
She and her colleagues examined the effect of infections on 312 patients who were waiting for a transplant at Erasmus Medical Center from the period of 2006-2013. During that time, a total of 317 infections developed in 144 patients. The infections were fatal in 58% of these patients.
These included spontaneous primary cholangitis (75); spontaneous bacterial peritonitis (61); urogenital (38), respiratory (30), and skin (25) infections; as well as primary bacteremia (22). Also, there were 18 cases of gastroenteritis and 12 cases of Candida esophagitis. The remainder were unspecified infections.
The death rate was highest in primary bacteremia, which killed about 40% of those who developed it. The rate was about 25% in respiratory infections, 20% in spontaneous primary bacteremia, 15% in esophagitis, 10% in gastroenteritis and urinary tract infections, and 10% in patients with multiple site infections.
The pathogens were gram negative (70) and gram positive (37) bacteria; Enterococcus faecium (15) and faecalis (3); yeasts (13); viruses (7); and mold (2). The remainder of the infections yielded a negative culture.
In 24 patients, multiple pathogens were identified. These patients had the highest rate of mortality, with almost half of them dying from their infection; one of the two patients with a mold infection also died. The death rate was 20% in patients with yeast infections, 18% in those with E. faecium, 15% in gram-positive infections, and 10% in gram-negative infections.
A multivariate analysis found several factors that increased the risk of dying from an infection. For every 10 years of increasing age, the risk of infection-related mortality doubled (odds ratio, 2); worse MELD (Model for End-Stage Liver Disease) scores increased the risk by 12%.
Patients with hepatic encephalopathy were 76% more likely to die from an infection, and those with refractory ascites faced a 2.5-fold increased risk. Mechanical ventilation was associated with more than a fivefold increased risk (OR, 5.72).
Patients who developed an infection were almost six times more likely to be withdrawn from the transplant waiting list (hazard ratio, 5.87). The regression analysis for withdrawal identified several factors that significantly increased the risk, including age, MELD score, and serum albumin. The biggest risk factor for withdrawal related to infection was refractory ascites, which more than doubled the risk (HR, 2.2).
Dr. Alferink had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Infection is a major cause of death among patients waiting for a liver transplant, killing more than half of those who contracted one.
Infection also was the biggest reason that patients with end-stage liver disease withdrew from the transplant waiting list, a 9-year-long study has shown. Patients who developed an infection were six times more likely to withdraw than were those who did not, Dr. Loes Alferink wrote in a poster presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“We need to focus on better prophylactic antibiotic strategies to save lives in patients with end-stage liver disease who are on the waiting list,” said Dr. Alferink of Erasmus Medical Center, Rotterdam, the Netherlands.
She and her colleagues examined the effect of infections on 312 patients who were waiting for a transplant at Erasmus Medical Center from the period of 2006-2013. During that time, a total of 317 infections developed in 144 patients. The infections were fatal in 58% of these patients.
These included spontaneous primary cholangitis (75); spontaneous bacterial peritonitis (61); urogenital (38), respiratory (30), and skin (25) infections; as well as primary bacteremia (22). Also, there were 18 cases of gastroenteritis and 12 cases of Candida esophagitis. The remainder were unspecified infections.
The death rate was highest in primary bacteremia, which killed about 40% of those who developed it. The rate was about 25% in respiratory infections, 20% in spontaneous primary bacteremia, 15% in esophagitis, 10% in gastroenteritis and urinary tract infections, and 10% in patients with multiple site infections.
The pathogens were gram negative (70) and gram positive (37) bacteria; Enterococcus faecium (15) and faecalis (3); yeasts (13); viruses (7); and mold (2). The remainder of the infections yielded a negative culture.
In 24 patients, multiple pathogens were identified. These patients had the highest rate of mortality, with almost half of them dying from their infection; one of the two patients with a mold infection also died. The death rate was 20% in patients with yeast infections, 18% in those with E. faecium, 15% in gram-positive infections, and 10% in gram-negative infections.
A multivariate analysis found several factors that increased the risk of dying from an infection. For every 10 years of increasing age, the risk of infection-related mortality doubled (odds ratio, 2); worse MELD (Model for End-Stage Liver Disease) scores increased the risk by 12%.
Patients with hepatic encephalopathy were 76% more likely to die from an infection, and those with refractory ascites faced a 2.5-fold increased risk. Mechanical ventilation was associated with more than a fivefold increased risk (OR, 5.72).
Patients who developed an infection were almost six times more likely to be withdrawn from the transplant waiting list (hazard ratio, 5.87). The regression analysis for withdrawal identified several factors that significantly increased the risk, including age, MELD score, and serum albumin. The biggest risk factor for withdrawal related to infection was refractory ascites, which more than doubled the risk (HR, 2.2).
Dr. Alferink had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
Key clinical point: Infections are a major cause of transplant wait-list withdrawal and death in patients with end-stage liver disease.
Major finding: Infections increased the risk of withdrawal by sixfold, and killed 58% of those who developed one.
Data source: A retrospective study of 144 patients who developed a total of 317 infections.
Disclosures: Dr. Alferink had no financial disclosures.
The perils of hospital air
Hospital air is a potential route of transmission of beta-lactam–resistant bacteria (BLRB), which are important causative agents of nosocomial infections, according to research published in the American Journal of Infection Control.
Dr. Mahnaz Nikaeen of the department of environmental health engineering at Isfahan (Iran) University of Medical Sciences, and his coauthors collected and tested 64 air samples from four hospital wards to determine the prevalence of airborne BLRB in different teaching hospitals, to evaluate the frequency of five common beta-lactamase–encoding genes in isolated resistant bacteria, and to identify the most predominant BLRB by 16s rRNA gene sequencing. The sampling locations in each hospital included operating rooms, ICUs, surgery wards, and internal medicine wards.
The investigators detected airborne bacteria by using culture plates with and without beta-lactams.
The prevalence of BLRB in the air samples ranged between 3% and 34%, Dr. Nikaeen said. Oxacillin-resistant bacteria had the highest prevalence, followed by ceftazidime- and cefazolin-resistant bacteria. Acinetobacter spp, Acinetobacter baumannii, and Staphylococcus spp were the most predominant BLRB.
Gene sequencing revealed that the frequency of beta-lactamase–encoding genes in isolated BLRB ranged between 0% and 47%, with the highest and lowest detection for OXA-23, commonly found in Acinetobacter spp, and CTX-m-32, a gene prevalent in extended-spectrum beta-lactamase–producing Enterobacteriaceae, respectively. MecA, a genetic element found in methicillin-resistant Staphylococcus spp, had a relatively high frequency in surgery wards and operating rooms, whereas the frequency of blaTEM, another common extended-spectrum beta-lactamase produced by Enterobacteriaceae, was higher in intensive care units and internal medicine wards. OXA-51, a chromosomally located intrinsic gene in A. baumannii, was detected in four wards.
“Isolation of beta-lactam–resistant Staphylococcus spp and A. baumannii as the most predominant BLRB indicated the potential role of airborne bacteria in dissemination of nosocomial infections,” Dr. Nikaeen and his coauthors said. “The results confirm the necessity for application of effective control measures that significantly decrease the exposure of high-risk patients to potentially airborne nosocomial infections.”
The authors reported having no conflicts.
Read the complete study in the American Journal of Infection Control (doi:10.1016/j.ajic.2016.01.041).
On Twitter @richpizzi
Hospital air is a potential route of transmission of beta-lactam–resistant bacteria (BLRB), which are important causative agents of nosocomial infections, according to research published in the American Journal of Infection Control.
Dr. Mahnaz Nikaeen of the department of environmental health engineering at Isfahan (Iran) University of Medical Sciences, and his coauthors collected and tested 64 air samples from four hospital wards to determine the prevalence of airborne BLRB in different teaching hospitals, to evaluate the frequency of five common beta-lactamase–encoding genes in isolated resistant bacteria, and to identify the most predominant BLRB by 16s rRNA gene sequencing. The sampling locations in each hospital included operating rooms, ICUs, surgery wards, and internal medicine wards.
The investigators detected airborne bacteria by using culture plates with and without beta-lactams.
The prevalence of BLRB in the air samples ranged between 3% and 34%, Dr. Nikaeen said. Oxacillin-resistant bacteria had the highest prevalence, followed by ceftazidime- and cefazolin-resistant bacteria. Acinetobacter spp, Acinetobacter baumannii, and Staphylococcus spp were the most predominant BLRB.
Gene sequencing revealed that the frequency of beta-lactamase–encoding genes in isolated BLRB ranged between 0% and 47%, with the highest and lowest detection for OXA-23, commonly found in Acinetobacter spp, and CTX-m-32, a gene prevalent in extended-spectrum beta-lactamase–producing Enterobacteriaceae, respectively. MecA, a genetic element found in methicillin-resistant Staphylococcus spp, had a relatively high frequency in surgery wards and operating rooms, whereas the frequency of blaTEM, another common extended-spectrum beta-lactamase produced by Enterobacteriaceae, was higher in intensive care units and internal medicine wards. OXA-51, a chromosomally located intrinsic gene in A. baumannii, was detected in four wards.
“Isolation of beta-lactam–resistant Staphylococcus spp and A. baumannii as the most predominant BLRB indicated the potential role of airborne bacteria in dissemination of nosocomial infections,” Dr. Nikaeen and his coauthors said. “The results confirm the necessity for application of effective control measures that significantly decrease the exposure of high-risk patients to potentially airborne nosocomial infections.”
The authors reported having no conflicts.
Read the complete study in the American Journal of Infection Control (doi:10.1016/j.ajic.2016.01.041).
On Twitter @richpizzi
Hospital air is a potential route of transmission of beta-lactam–resistant bacteria (BLRB), which are important causative agents of nosocomial infections, according to research published in the American Journal of Infection Control.
Dr. Mahnaz Nikaeen of the department of environmental health engineering at Isfahan (Iran) University of Medical Sciences, and his coauthors collected and tested 64 air samples from four hospital wards to determine the prevalence of airborne BLRB in different teaching hospitals, to evaluate the frequency of five common beta-lactamase–encoding genes in isolated resistant bacteria, and to identify the most predominant BLRB by 16s rRNA gene sequencing. The sampling locations in each hospital included operating rooms, ICUs, surgery wards, and internal medicine wards.
The investigators detected airborne bacteria by using culture plates with and without beta-lactams.
The prevalence of BLRB in the air samples ranged between 3% and 34%, Dr. Nikaeen said. Oxacillin-resistant bacteria had the highest prevalence, followed by ceftazidime- and cefazolin-resistant bacteria. Acinetobacter spp, Acinetobacter baumannii, and Staphylococcus spp were the most predominant BLRB.
Gene sequencing revealed that the frequency of beta-lactamase–encoding genes in isolated BLRB ranged between 0% and 47%, with the highest and lowest detection for OXA-23, commonly found in Acinetobacter spp, and CTX-m-32, a gene prevalent in extended-spectrum beta-lactamase–producing Enterobacteriaceae, respectively. MecA, a genetic element found in methicillin-resistant Staphylococcus spp, had a relatively high frequency in surgery wards and operating rooms, whereas the frequency of blaTEM, another common extended-spectrum beta-lactamase produced by Enterobacteriaceae, was higher in intensive care units and internal medicine wards. OXA-51, a chromosomally located intrinsic gene in A. baumannii, was detected in four wards.
“Isolation of beta-lactam–resistant Staphylococcus spp and A. baumannii as the most predominant BLRB indicated the potential role of airborne bacteria in dissemination of nosocomial infections,” Dr. Nikaeen and his coauthors said. “The results confirm the necessity for application of effective control measures that significantly decrease the exposure of high-risk patients to potentially airborne nosocomial infections.”
The authors reported having no conflicts.
Read the complete study in the American Journal of Infection Control (doi:10.1016/j.ajic.2016.01.041).
On Twitter @richpizzi
FROM AMERICAN JOURNAL OF INFECTION CONTROL
PPIs associated with antibiotic-resistant bacteria carriage
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
Key clinical point: Proton pump inhibitors may increase the risk of carrying antibiotic-resistant bacteria.
Major finding: PPIs conferred a fourfold increase in the risk of being colonized with extended-spectrum beta-lactamase–producing enterobacteriaceae.
Data source: A cross-sectional study involving 570 patients.
Disclosures: Dr. Huizinga had no relevant financial disclosures.
IDSA, SHEA release inpatient antibiotic stewardship guidelines
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
FROM CLINICAL INFECTIOUS DISEASES
Modifying our behavior
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
Fecal transplant cures most with C. difficile, but one dies
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
Key clinical point: Fecal transplants cured most recurrent C. difficile infections, but could be dangerous as well.
Major finding: A duodenal administered fecal transplant cured 97% of patients with recurrent C. difficile, but one patient died after vomiting the fecal material.
Data source: The prospective study comprised 39 patients.
Disclosures: Dr. van Beurden had no financial disclosures.