User login
Pediatric cancers are on the rise
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
REPORTING FROM ASPHO 2018
Key clinical point: Major finding: From 2001 to 2014, there were 196,200 incident cases of pediatric cancer for an overall cancer incidence rate of 173 per 1 million.
Study details: A review of data from the United States Cancer Statistics for children under age 20.
Disclosures: The CDC supported the study. Dr. Siegel and his coauthors are CDC employees. They reported having no conflicts of interest.
Source: Siegel DA et al. ASPHO 2018, Abstract 605.
Regimen can improve DFS in newly diagnosed T-ALL
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
Children with Down syndrome and ALL have good outcomes today
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
REPORTING FROM ASPHO 2018
Key clinical point:
Major finding: The 5-year event-free survival rate for children with Down syndrome was 86% vs. 89% for children without Down syndrome (P = .025).
Study details: Follow-up of 5,311 children with newly diagnosed ALL in the COG AALL0331 trial.
Disclosures: COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
Source: Maloney K et al. ASPHO 2018, Abstract PP 2001.
Cooperation can drive T-ALL, study shows
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Relapse rate drives stem cell transplant failure in pediatric ALL patients
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
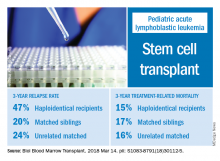
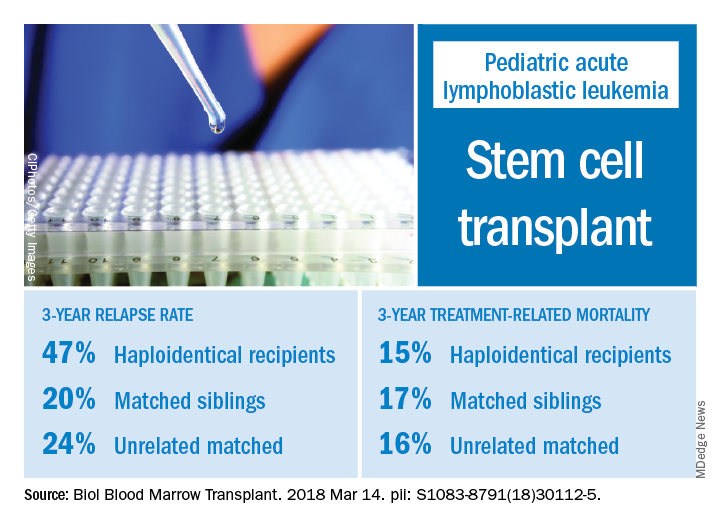
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point: In pediatric acute lymphocytic leukemia (ALL) patients, relapse is the main barrier to successful hematopoietic stem cell transplant (HSCT), especially for those who receive haploidentical donor grafts.
Major finding: The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Study details: A retrospective analysis of 124 transplants performed at a single center during 2008-2016.
Disclosures: Authors had no financial disclosures or conflicts of interest to report.
Source: Yanir AD et al. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2018.03.001.
Drug receives orphan designation for ALL
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Art education benefits blood cancer patients
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
Study reveals gene variants that predispose kids to ALL
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Team uses iPSCs to create ‘universal’ CAR T cells
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
Blinatumomab triggers complete MRD response in ALL
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
FROM BLOOD
Key clinical point:
Major finding: Complete MRD response, seen in 78% of blinatumomab-treated patients, was associated with improved relapse-free and overall survival.
Study details: An open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission, conducted at 46 centers in Europe and Russia.
Disclosures: The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
Source: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.