User login
cfDNA reveals targetable mutations in pediatric neuroblastoma, sarcoma
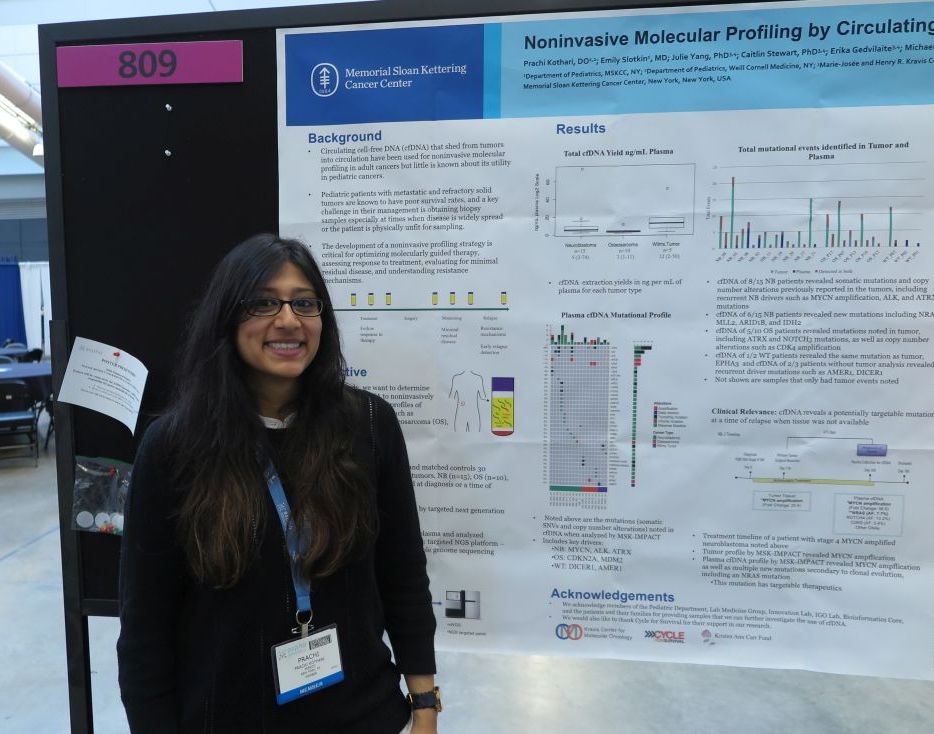
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
REPORTING FROM ASPHO 2018
Key clinical point: Circulating free DNA analysis is a noninvasive method for detecting potential therapeutic targets.
Major finding: cfDNA revealed potentially targetable new mutations in 6 of 15 patients with neuroblastoma.
Study details: Retrospective analysis of tumor and plasma samples in 30 patients with neuroblastoma, osteosarcoma, or Wilms tumor.
Disclosures: The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
Source: Kothari P et al. ASPHO 2018. Abstract #809.
Pediatric cancers are on the rise
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
REPORTING FROM ASPHO 2018
Key clinical point: Major finding: From 2001 to 2014, there were 196,200 incident cases of pediatric cancer for an overall cancer incidence rate of 173 per 1 million.
Study details: A review of data from the United States Cancer Statistics for children under age 20.
Disclosures: The CDC supported the study. Dr. Siegel and his coauthors are CDC employees. They reported having no conflicts of interest.
Source: Siegel DA et al. ASPHO 2018, Abstract 605.
Multiple solid tumors targeted by concept CAR T
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
REPORTING FROM ASPHO 2018
Key clinical point:
Major finding: A single dose of the B7-H3 CAR caused complete regression of osteosarcoma and Ewing sarcoma xenografts and extended survival in mice.
Study details: Preclinical research.
Disclosures: The work is supported by the Sarcoma Alliance for Research through Collaboration, St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
Source: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
Children with Down syndrome and ALL have good outcomes today
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
PITTSBURGH – In the current era, children with Down syndrome who have standard risk B-cell precursor acute lymphoblastic leukemia have event-free and overall survival rates nearly as good as those of other children with standard-risk B–ALL, results of a Children’s Oncology Group study show.
Among 5,311 children enrolled in the COG AALL0331 trial, a study of combination chemotherapy for young patients with newly diagnosed ALL, the 5-year event-free survival (EFS) rate for children with Down syndrome was 86%, compared with 89% for children without Down syndrome (P = .025).
Although the differences in EFS and OS were significant, ”overall in this study, Down syndrome ALL had an excellent outcome that was similar to those patients without Down syndrome,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial confirmed her group’s previous finding that there is a low rate of favorable cytogenetic features in patients with Down syndrome ALL; nonetheless, in the current study, 5-year continuous complete remission rates for standard-risk average, low, and high in patients with Down syndrome were similar to those for patients without Down syndrome, she said.
In the trial, patients were treated with a three-drug induction regimen, and following induction were assigned to standard-risk low, average, or high groups based on leukemia genetics and initial response to therapy.
Of the 5,311 children enrolled, 141 (2.7%) had Down syndrome, and these patients received risk-stratified therapy with additional supportive care guidelines, including leucovorin rescue after intrathecal methotrexate until maintenance. The care team strongly encouraged hospitalizations during high-risk blocks for this subgroup of patients until they experienced neutrophil recovery.
At the end of induction, patients who were judged to be standard-risk average were then randomized in a 2x2 design to either standard or intensified consolidation, and to standard interim maintenance with delayed intensification, or to intensified interim maintenance with delayed intensification.
The intensified interim maintenance with delayed intensification randomization was closed in 2008 because of superior results with escalating intravenous methotrexate during interim maintenance for standard-risk ALL patients treated in the CCG 1991 trial. Subsequently, all patients enrolled in AALL0331 received escalating intravenous methotrexate during interim maintenance.
Also in AALL0331, patients with Down syndrome who had standard-high ALL were given intensified consolidation and a single vs. double intensified interim maintenance with delayed intensification; patients without Down syndrome and standard risk high received the double intensified interim maintenance regimen.
Standard-risk low Down syndrome patients and non–Down syndrome patients participated in a randomization to additional pegaspargase doses during consolidation and interim maintenance.
There were no significant differences between patients with or without Down syndrome in the proportion of either rapid or slow early responses. Significantly fewer patients with Down syndrome had standard-risk low disease, and significantly more had average or high-risk disease.
Patients with Down syndrome initially had 11.5% excess risk for death during induction, but following additional treatment modifications, the excess risk decreased to 1.7%.
Among patients with Down syndrome, one died during intensive consolidation, and two died during delayed intensification. All three deaths were due to infections. No patients with Down syndrome died during maintenance.
Patients with Down syndrome also had a significantly increased risk for infection during induction (P less than .0001).
For patients with standard-risk low disease, 5-year EFS was 100% for those with Down syndrome, compared with 95.35% for patients without. Respective rates for standard risk average and high disease were 88.07% vs. 89.63%. and 82.35% vs. 86.18%.
Down syndrome was not an independent risk factor for survival in multivariate analyses accounting for risk group, Dr. Maloney said.
COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
SOURCE: Maloney K et al. ASPHO 2018, Abstract PP 2001.
REPORTING FROM ASPHO 2018
Key clinical point:
Major finding: The 5-year event-free survival rate for children with Down syndrome was 86% vs. 89% for children without Down syndrome (P = .025).
Study details: Follow-up of 5,311 children with newly diagnosed ALL in the COG AALL0331 trial.
Disclosures: COG AALL0331 was supported by the National Cancer Institute. Dr. Maloney reported having no financial disclosures.
Source: Maloney K et al. ASPHO 2018, Abstract PP 2001.
Hydroxyurea in infancy yields better SCD outcomes
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
REPORTING FROM ASPHO 2018
Key clinical point: Early initiation of hydroxyurea is associated with better SCD outcomes.
Major finding: Patients started on hydroxyurea at a mean of 7.2 months had significantly fewer admissions, pain crises, and transfusions than did patients started after age 1 year.
Study details: Retrospective review of data on 65 children with SCD in a single center.
Disclosures: The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
Source: Schuchard S et al. ASPHO 2018, Poster 342.