User login
Report details financial burden of blood cancers
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).
Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
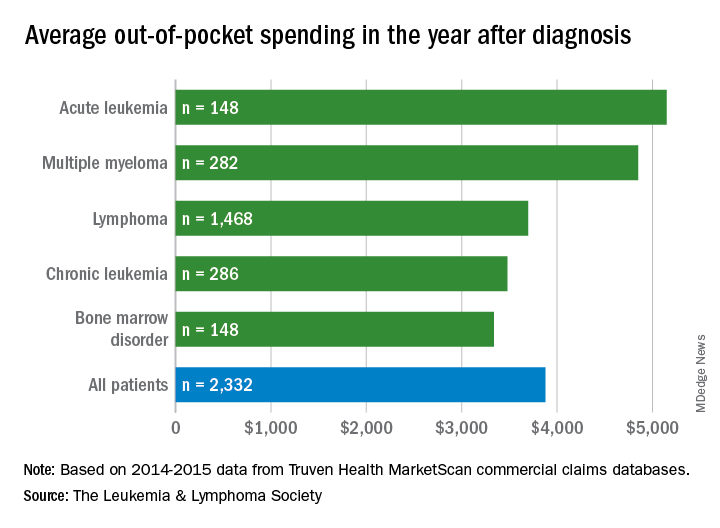
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
Team tracks changes in height, weight in pediatric ALL
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
Cost-effectiveness of CAR T-cell therapy
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
‘Intense’ end-of-life care may be common in HSCT recipients
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Kymriah appears cost effective in analysis
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: The incremental cost-effectiveness ratio for tisagenlecleucel versus clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year gained.
Study details: A cost-effectiveness analysis comparing tisagenlecleucel with clofarabine monotherapy.
Disclosures: The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
Source: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
Researchers consider R/R ALL drugs in the first-line setting
CHICAGO – Novel antibodies are improving outcomes in relapsed and refractory acute lymphoblastic leukemia (ALL), and the hope is that they will also show benefit in the up-front treatment setting and thereby improve overall outcomes, according to Anjali Advani, MD.
“It has been a really exciting time in ALL because several drugs have now been FDA approved: blinatumomab, inotuzumab, and now – for patients who are less than 26 years of age – we actually have CAR [chimeric antigen receptor] T cells that have been approved,” Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic said at the American Society of Hematology Meeting on Hematologic Malignancies.
At the time of relapse, however, the only known cure is allogeneic bone marrow transplant. That may change as more data regarding CAR T cells become available, but the typical goal at this time is to get patients into remission and then to transplant, she said.
Blinatumomab
“Blinatumomab is a very interesting antibody,” Dr. Advani said, explaining that it is a bispecific, T cell–engaging antibody with an anti-CD3 arm that engages the T cell and an anti-CD19 antibody that engages the B lymphoblast.
“Basically this drug then acts as a bridge between the lymphoblast and the T cell to lead to proliferation of the cytotoxic T cell and apoptosis of the lymphoblast,” she said. “It’s interesting because it’s an antibody but it actually works through the immune system through the T cells.”
The largest study to date of blinatumomab in the relapsed/refractory ALL setting showed a 43% complete remission (CR) or CR with partial hematological recovery of peripheral blood counts (CRi) in 189 treated patients with Philadelphia chromosome–negative ALL. It also demonstrated and a 39% rate of salvage status 2 or higher, she said, noting that the response was impressive given that about 30% of participants had a prior transplant (Lancet. 2015 Jan 1;16[1]:57-66).
Of the responders, 40% went on to allogeneic transplant. This was a “fairly impressive” rate given the 30% prior-transplant rate, Dr. Advani said.
“There also was a high minimal residual disease response in those patients achieving CR,” she said, adding that the only significant predictor of response was bone marrow blast count; patients with 50% or more blasts in the bone marrow had a reduced likelihood of responding to blinatumomab.
The agent was approved by the Food and Drug Administration in December 2014 based on these phase 2 findings.
Adverse events mainly included toxicities that are expected in leukemia patients; the most frequent were febrile neutropenia, neutropenia, and anemia. Two patients developed cytokine release syndrome, and about half of the blinatumomab-treated patients experienced neurological events, although the majority of those were grade 1 or 2 and were easily manageable, she noted.
Blinatumomab was further evaluated in the phase 3 TOWER study (NCT02013167), which compared it with standard-of-care chemotherapy regimens. This study showed much higher response rates with blinatumomab than with the chemotherapy regimens (CR with full, partial, or incomplete hematologic recovery, 44% vs. 25%, respectively), Dr. Advani said (N Engl J Med. 2017 Mar 2;376[9]:836-47).
“The main things to remember [are that blinatumomab is] generally very well tolerated and it has been shown to be superior over standard chemotherapy,” she said. “I think it’s a very good drug to use as a bridge to transplant.”
One setting where blinatumomab perhaps should not be used is in patients with central nervous system disease, she noted.
“There is some concern, at least theoretically, that if you have to use concurrent intrathecal chemo along with blinatumomab, there could be some neurotoxicity,” Dr. Advani said, adding that there are no clear data in that setting because patients with CNS disease were not included in the trials.
Patients with high tumor burden may also be poor candidates for blinatumomab because they tend to have lower response rates.
“That doesn’t mean you can’t use it, but you have to kind of think about what the best option would be,” she said.
Additionally, patients treated with CAR T-cell therapy may develop CD19 loss or CD19-negative disease, and blinatumomab should be avoided in these patients.
“The nice thing ... is you don’t have to worry about veno-occlusive disease [VOD] in patients who are proceeding to transplant,” she said, explaining that no increased risk of VOD was seen in these trials.
Inotuzumab
Inotuzumab, which was approved in 2017, differs from blinatumomab in that it is an anti-CD22-calicheamicin conjugate; however, it also showed high response rates in the initial phase 2 trial in relapsed/refractory ALL. The overall response rate was 57%, with 18% achieving a complete response and 63% achieving complete molecular remission.
Of 49 treated patients, 22 patients proceeded to allogeneic transplant, and 5 of those developed VOD.
“Interestingly, four out of five of these patients had received a clofarabine-based preparative regimen, and this likely explains why there was a higher risk of VOD in this study,” she said, noting that the VOD risk has been lower in subsequent studies of inotuzumab.
The international INO-VATE ALL study (NCT01564784) that led to FDA approval was similar in design to the TOWER study in that it compared inotuzumab with standard chemotherapy regimens, and response rates were clearly higher (81% vs. 33%) with inotuzumab (N Engl J Med. 2016 Aug 25;375[8]:740-53).
The VOD risk in the INO-VATE trial was 11%, and it seemed to be higher in those who received dual alkylator–conditioning regimens, which are commonly used in Europe.
Longer-term outcomes after transplant in INO-VATE participants show that median survival has not been reached.
“It’s encouraging that with longer follow-up these patients actually look like they’re doing well,” Dr. Advani said, adding that inotuzumab is a good treatment option for relapsed patients with high disease burden or with CNS disease.
The continuous hookup required for this treatment may be problematic for some younger and older patients, but it is generally not an issue, she noted.
It is important, though, to give as few cycles prior to transplant as possible and to “really think about the preparative regimen to decrease the risk of VOD.”
CAR T-cell therapy
As for CAR T-cell therapy in the relapsed/refractory ALL setting, tisagenlecleucel was approved in 2017 for those up to age 25 years with B-cell precursor ALL that is refractory or in second or later relapse.
Approval was based on a single-arm trial of 63 patients with relapsed or refractory pediatric precursor B-cell ALL, including 35 patients who had prior transplant. The confirmed overall remission rate was 82%, with a 63% CR rate and 19% CRi rate.
“This is a very exciting area,” Dr. Advani said. “There are multiple trials being done in adults with ALL to really look at the older subgroup of patients.”
Overall outcomes
“These treatments we have now really seem to be effective in the relapse setting, but the problem is that once patients relapse and then go to transplant, their overall survival is still poor,” Dr. Advani said. “So the question is how can we improve the up-front treatment of patients so that hopefully they don’t relapse, and hopefully we also can send a smaller number of patients to transplant.”
Two trials seek to address this, she said.
The A041501 study (NCT03150693) is comparing C10403 chemotherapy with C10403 induction followed by two cycles of inotuzumab before continuing with chemotherapy in adults under age 40 years with previously untreated B ALL.
The primary objective is improved 3-year event-free survival, she said, adding that minimal residual disease (MRD) testing will be used and that CD20-positive patients will receive rituximab, as is now standard.
The phase 3 E1910 study (NCT02003222) is evaluating up-front blinatumomab in patients aged 30-70 years with newly diagnosed BCR-ABL–negative B-lineage ALL. This trial was complicated by the recent approval of blinatumomab for MRD-positive disease, which rendered randomization of MRD-positive patients unethical. MRD-negative patients will be randomized, however.
“The hope is that, by incorporating blinatumomab up front, this will again improve outcomes for patients,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – Novel antibodies are improving outcomes in relapsed and refractory acute lymphoblastic leukemia (ALL), and the hope is that they will also show benefit in the up-front treatment setting and thereby improve overall outcomes, according to Anjali Advani, MD.
“It has been a really exciting time in ALL because several drugs have now been FDA approved: blinatumomab, inotuzumab, and now – for patients who are less than 26 years of age – we actually have CAR [chimeric antigen receptor] T cells that have been approved,” Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic said at the American Society of Hematology Meeting on Hematologic Malignancies.
At the time of relapse, however, the only known cure is allogeneic bone marrow transplant. That may change as more data regarding CAR T cells become available, but the typical goal at this time is to get patients into remission and then to transplant, she said.
Blinatumomab
“Blinatumomab is a very interesting antibody,” Dr. Advani said, explaining that it is a bispecific, T cell–engaging antibody with an anti-CD3 arm that engages the T cell and an anti-CD19 antibody that engages the B lymphoblast.
“Basically this drug then acts as a bridge between the lymphoblast and the T cell to lead to proliferation of the cytotoxic T cell and apoptosis of the lymphoblast,” she said. “It’s interesting because it’s an antibody but it actually works through the immune system through the T cells.”
The largest study to date of blinatumomab in the relapsed/refractory ALL setting showed a 43% complete remission (CR) or CR with partial hematological recovery of peripheral blood counts (CRi) in 189 treated patients with Philadelphia chromosome–negative ALL. It also demonstrated and a 39% rate of salvage status 2 or higher, she said, noting that the response was impressive given that about 30% of participants had a prior transplant (Lancet. 2015 Jan 1;16[1]:57-66).
Of the responders, 40% went on to allogeneic transplant. This was a “fairly impressive” rate given the 30% prior-transplant rate, Dr. Advani said.
“There also was a high minimal residual disease response in those patients achieving CR,” she said, adding that the only significant predictor of response was bone marrow blast count; patients with 50% or more blasts in the bone marrow had a reduced likelihood of responding to blinatumomab.
The agent was approved by the Food and Drug Administration in December 2014 based on these phase 2 findings.
Adverse events mainly included toxicities that are expected in leukemia patients; the most frequent were febrile neutropenia, neutropenia, and anemia. Two patients developed cytokine release syndrome, and about half of the blinatumomab-treated patients experienced neurological events, although the majority of those were grade 1 or 2 and were easily manageable, she noted.
Blinatumomab was further evaluated in the phase 3 TOWER study (NCT02013167), which compared it with standard-of-care chemotherapy regimens. This study showed much higher response rates with blinatumomab than with the chemotherapy regimens (CR with full, partial, or incomplete hematologic recovery, 44% vs. 25%, respectively), Dr. Advani said (N Engl J Med. 2017 Mar 2;376[9]:836-47).
“The main things to remember [are that blinatumomab is] generally very well tolerated and it has been shown to be superior over standard chemotherapy,” she said. “I think it’s a very good drug to use as a bridge to transplant.”
One setting where blinatumomab perhaps should not be used is in patients with central nervous system disease, she noted.
“There is some concern, at least theoretically, that if you have to use concurrent intrathecal chemo along with blinatumomab, there could be some neurotoxicity,” Dr. Advani said, adding that there are no clear data in that setting because patients with CNS disease were not included in the trials.
Patients with high tumor burden may also be poor candidates for blinatumomab because they tend to have lower response rates.
“That doesn’t mean you can’t use it, but you have to kind of think about what the best option would be,” she said.
Additionally, patients treated with CAR T-cell therapy may develop CD19 loss or CD19-negative disease, and blinatumomab should be avoided in these patients.
“The nice thing ... is you don’t have to worry about veno-occlusive disease [VOD] in patients who are proceeding to transplant,” she said, explaining that no increased risk of VOD was seen in these trials.
Inotuzumab
Inotuzumab, which was approved in 2017, differs from blinatumomab in that it is an anti-CD22-calicheamicin conjugate; however, it also showed high response rates in the initial phase 2 trial in relapsed/refractory ALL. The overall response rate was 57%, with 18% achieving a complete response and 63% achieving complete molecular remission.
Of 49 treated patients, 22 patients proceeded to allogeneic transplant, and 5 of those developed VOD.
“Interestingly, four out of five of these patients had received a clofarabine-based preparative regimen, and this likely explains why there was a higher risk of VOD in this study,” she said, noting that the VOD risk has been lower in subsequent studies of inotuzumab.
The international INO-VATE ALL study (NCT01564784) that led to FDA approval was similar in design to the TOWER study in that it compared inotuzumab with standard chemotherapy regimens, and response rates were clearly higher (81% vs. 33%) with inotuzumab (N Engl J Med. 2016 Aug 25;375[8]:740-53).
The VOD risk in the INO-VATE trial was 11%, and it seemed to be higher in those who received dual alkylator–conditioning regimens, which are commonly used in Europe.
Longer-term outcomes after transplant in INO-VATE participants show that median survival has not been reached.
“It’s encouraging that with longer follow-up these patients actually look like they’re doing well,” Dr. Advani said, adding that inotuzumab is a good treatment option for relapsed patients with high disease burden or with CNS disease.
The continuous hookup required for this treatment may be problematic for some younger and older patients, but it is generally not an issue, she noted.
It is important, though, to give as few cycles prior to transplant as possible and to “really think about the preparative regimen to decrease the risk of VOD.”
CAR T-cell therapy
As for CAR T-cell therapy in the relapsed/refractory ALL setting, tisagenlecleucel was approved in 2017 for those up to age 25 years with B-cell precursor ALL that is refractory or in second or later relapse.
Approval was based on a single-arm trial of 63 patients with relapsed or refractory pediatric precursor B-cell ALL, including 35 patients who had prior transplant. The confirmed overall remission rate was 82%, with a 63% CR rate and 19% CRi rate.
“This is a very exciting area,” Dr. Advani said. “There are multiple trials being done in adults with ALL to really look at the older subgroup of patients.”
Overall outcomes
“These treatments we have now really seem to be effective in the relapse setting, but the problem is that once patients relapse and then go to transplant, their overall survival is still poor,” Dr. Advani said. “So the question is how can we improve the up-front treatment of patients so that hopefully they don’t relapse, and hopefully we also can send a smaller number of patients to transplant.”
Two trials seek to address this, she said.
The A041501 study (NCT03150693) is comparing C10403 chemotherapy with C10403 induction followed by two cycles of inotuzumab before continuing with chemotherapy in adults under age 40 years with previously untreated B ALL.
The primary objective is improved 3-year event-free survival, she said, adding that minimal residual disease (MRD) testing will be used and that CD20-positive patients will receive rituximab, as is now standard.
The phase 3 E1910 study (NCT02003222) is evaluating up-front blinatumomab in patients aged 30-70 years with newly diagnosed BCR-ABL–negative B-lineage ALL. This trial was complicated by the recent approval of blinatumomab for MRD-positive disease, which rendered randomization of MRD-positive patients unethical. MRD-negative patients will be randomized, however.
“The hope is that, by incorporating blinatumomab up front, this will again improve outcomes for patients,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – Novel antibodies are improving outcomes in relapsed and refractory acute lymphoblastic leukemia (ALL), and the hope is that they will also show benefit in the up-front treatment setting and thereby improve overall outcomes, according to Anjali Advani, MD.
“It has been a really exciting time in ALL because several drugs have now been FDA approved: blinatumomab, inotuzumab, and now – for patients who are less than 26 years of age – we actually have CAR [chimeric antigen receptor] T cells that have been approved,” Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic said at the American Society of Hematology Meeting on Hematologic Malignancies.
At the time of relapse, however, the only known cure is allogeneic bone marrow transplant. That may change as more data regarding CAR T cells become available, but the typical goal at this time is to get patients into remission and then to transplant, she said.
Blinatumomab
“Blinatumomab is a very interesting antibody,” Dr. Advani said, explaining that it is a bispecific, T cell–engaging antibody with an anti-CD3 arm that engages the T cell and an anti-CD19 antibody that engages the B lymphoblast.
“Basically this drug then acts as a bridge between the lymphoblast and the T cell to lead to proliferation of the cytotoxic T cell and apoptosis of the lymphoblast,” she said. “It’s interesting because it’s an antibody but it actually works through the immune system through the T cells.”
The largest study to date of blinatumomab in the relapsed/refractory ALL setting showed a 43% complete remission (CR) or CR with partial hematological recovery of peripheral blood counts (CRi) in 189 treated patients with Philadelphia chromosome–negative ALL. It also demonstrated and a 39% rate of salvage status 2 or higher, she said, noting that the response was impressive given that about 30% of participants had a prior transplant (Lancet. 2015 Jan 1;16[1]:57-66).
Of the responders, 40% went on to allogeneic transplant. This was a “fairly impressive” rate given the 30% prior-transplant rate, Dr. Advani said.
“There also was a high minimal residual disease response in those patients achieving CR,” she said, adding that the only significant predictor of response was bone marrow blast count; patients with 50% or more blasts in the bone marrow had a reduced likelihood of responding to blinatumomab.
The agent was approved by the Food and Drug Administration in December 2014 based on these phase 2 findings.
Adverse events mainly included toxicities that are expected in leukemia patients; the most frequent were febrile neutropenia, neutropenia, and anemia. Two patients developed cytokine release syndrome, and about half of the blinatumomab-treated patients experienced neurological events, although the majority of those were grade 1 or 2 and were easily manageable, she noted.
Blinatumomab was further evaluated in the phase 3 TOWER study (NCT02013167), which compared it with standard-of-care chemotherapy regimens. This study showed much higher response rates with blinatumomab than with the chemotherapy regimens (CR with full, partial, or incomplete hematologic recovery, 44% vs. 25%, respectively), Dr. Advani said (N Engl J Med. 2017 Mar 2;376[9]:836-47).
“The main things to remember [are that blinatumomab is] generally very well tolerated and it has been shown to be superior over standard chemotherapy,” she said. “I think it’s a very good drug to use as a bridge to transplant.”
One setting where blinatumomab perhaps should not be used is in patients with central nervous system disease, she noted.
“There is some concern, at least theoretically, that if you have to use concurrent intrathecal chemo along with blinatumomab, there could be some neurotoxicity,” Dr. Advani said, adding that there are no clear data in that setting because patients with CNS disease were not included in the trials.
Patients with high tumor burden may also be poor candidates for blinatumomab because they tend to have lower response rates.
“That doesn’t mean you can’t use it, but you have to kind of think about what the best option would be,” she said.
Additionally, patients treated with CAR T-cell therapy may develop CD19 loss or CD19-negative disease, and blinatumomab should be avoided in these patients.
“The nice thing ... is you don’t have to worry about veno-occlusive disease [VOD] in patients who are proceeding to transplant,” she said, explaining that no increased risk of VOD was seen in these trials.
Inotuzumab
Inotuzumab, which was approved in 2017, differs from blinatumomab in that it is an anti-CD22-calicheamicin conjugate; however, it also showed high response rates in the initial phase 2 trial in relapsed/refractory ALL. The overall response rate was 57%, with 18% achieving a complete response and 63% achieving complete molecular remission.
Of 49 treated patients, 22 patients proceeded to allogeneic transplant, and 5 of those developed VOD.
“Interestingly, four out of five of these patients had received a clofarabine-based preparative regimen, and this likely explains why there was a higher risk of VOD in this study,” she said, noting that the VOD risk has been lower in subsequent studies of inotuzumab.
The international INO-VATE ALL study (NCT01564784) that led to FDA approval was similar in design to the TOWER study in that it compared inotuzumab with standard chemotherapy regimens, and response rates were clearly higher (81% vs. 33%) with inotuzumab (N Engl J Med. 2016 Aug 25;375[8]:740-53).
The VOD risk in the INO-VATE trial was 11%, and it seemed to be higher in those who received dual alkylator–conditioning regimens, which are commonly used in Europe.
Longer-term outcomes after transplant in INO-VATE participants show that median survival has not been reached.
“It’s encouraging that with longer follow-up these patients actually look like they’re doing well,” Dr. Advani said, adding that inotuzumab is a good treatment option for relapsed patients with high disease burden or with CNS disease.
The continuous hookup required for this treatment may be problematic for some younger and older patients, but it is generally not an issue, she noted.
It is important, though, to give as few cycles prior to transplant as possible and to “really think about the preparative regimen to decrease the risk of VOD.”
CAR T-cell therapy
As for CAR T-cell therapy in the relapsed/refractory ALL setting, tisagenlecleucel was approved in 2017 for those up to age 25 years with B-cell precursor ALL that is refractory or in second or later relapse.
Approval was based on a single-arm trial of 63 patients with relapsed or refractory pediatric precursor B-cell ALL, including 35 patients who had prior transplant. The confirmed overall remission rate was 82%, with a 63% CR rate and 19% CRi rate.
“This is a very exciting area,” Dr. Advani said. “There are multiple trials being done in adults with ALL to really look at the older subgroup of patients.”
Overall outcomes
“These treatments we have now really seem to be effective in the relapse setting, but the problem is that once patients relapse and then go to transplant, their overall survival is still poor,” Dr. Advani said. “So the question is how can we improve the up-front treatment of patients so that hopefully they don’t relapse, and hopefully we also can send a smaller number of patients to transplant.”
Two trials seek to address this, she said.
The A041501 study (NCT03150693) is comparing C10403 chemotherapy with C10403 induction followed by two cycles of inotuzumab before continuing with chemotherapy in adults under age 40 years with previously untreated B ALL.
The primary objective is improved 3-year event-free survival, she said, adding that minimal residual disease (MRD) testing will be used and that CD20-positive patients will receive rituximab, as is now standard.
The phase 3 E1910 study (NCT02003222) is evaluating up-front blinatumomab in patients aged 30-70 years with newly diagnosed BCR-ABL–negative B-lineage ALL. This trial was complicated by the recent approval of blinatumomab for MRD-positive disease, which rendered randomization of MRD-positive patients unethical. MRD-negative patients will be randomized, however.
“The hope is that, by incorporating blinatumomab up front, this will again improve outcomes for patients,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
EXPERT ANALYSIS FROM MHM 2018
FDA issues draft guidance on MRD
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
Bacteremic sepsis in ALL tied to neurocognitive dysfunction
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all patients participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other patients, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% CI, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear.
“In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” the investigators said in their report.
Further study is needed to look at potential brain injury mechanisms and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all patients participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other patients, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% CI, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear.
“In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” the investigators said in their report.
Further study is needed to look at potential brain injury mechanisms and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all patients participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other patients, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% CI, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear.
“In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” the investigators said in their report.
Further study is needed to look at potential brain injury mechanisms and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
Phase 1 NHL, ALL trials placed on clinical hold
Update: On October 12, 2018, Affimed N.V. received a notification from the U.S. Food and Drug Administration (FDA) saying the agency concurred with Affimed’s decision and formally placed the investigational new drug application for AFM11 on full clinical hold. Affimed said it will comply with the FDA and other global health authorities’ requests for information to resolve the clinical hold.
Affimed N.V. has placed trials of AFM11 on clinical hold and notified the global health authorities of its decision.
AFM11 is a CD19/CD3-targeting T-cell engager being evaluated in two phase 1 trials—one in patients with relapsed or refractory, CD19-positive B-cell non-Hodgkin lymphoma (NHL) and one in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia (ALL).
Affimed initiated the clinical hold on these trials after serious adverse events occurred in three patients treated with AFM11.
This included a death in the ALL study and two life-threatening events in the NHL study.
The serious adverse events occurred in patients enrolled in the highest dose cohorts of each study.
A total of 33 patients have been treated in the two studies (NCT02848911 and NCT02106091), and preliminary signs of clinical activity have been observed in several patients.
Affimed said it will be working closely with the global health authorities, safety monitoring committees, and the studies’ clinical investigators to review the adverse events, assess all the data, and determine next steps for the AFM11 program.
Affimed intends to provide an update on AFM11 upon completing the evaluation.
Update: On October 12, 2018, Affimed N.V. received a notification from the U.S. Food and Drug Administration (FDA) saying the agency concurred with Affimed’s decision and formally placed the investigational new drug application for AFM11 on full clinical hold. Affimed said it will comply with the FDA and other global health authorities’ requests for information to resolve the clinical hold.
Affimed N.V. has placed trials of AFM11 on clinical hold and notified the global health authorities of its decision.
AFM11 is a CD19/CD3-targeting T-cell engager being evaluated in two phase 1 trials—one in patients with relapsed or refractory, CD19-positive B-cell non-Hodgkin lymphoma (NHL) and one in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia (ALL).
Affimed initiated the clinical hold on these trials after serious adverse events occurred in three patients treated with AFM11.
This included a death in the ALL study and two life-threatening events in the NHL study.
The serious adverse events occurred in patients enrolled in the highest dose cohorts of each study.
A total of 33 patients have been treated in the two studies (NCT02848911 and NCT02106091), and preliminary signs of clinical activity have been observed in several patients.
Affimed said it will be working closely with the global health authorities, safety monitoring committees, and the studies’ clinical investigators to review the adverse events, assess all the data, and determine next steps for the AFM11 program.
Affimed intends to provide an update on AFM11 upon completing the evaluation.
Update: On October 12, 2018, Affimed N.V. received a notification from the U.S. Food and Drug Administration (FDA) saying the agency concurred with Affimed’s decision and formally placed the investigational new drug application for AFM11 on full clinical hold. Affimed said it will comply with the FDA and other global health authorities’ requests for information to resolve the clinical hold.
Affimed N.V. has placed trials of AFM11 on clinical hold and notified the global health authorities of its decision.
AFM11 is a CD19/CD3-targeting T-cell engager being evaluated in two phase 1 trials—one in patients with relapsed or refractory, CD19-positive B-cell non-Hodgkin lymphoma (NHL) and one in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia (ALL).
Affimed initiated the clinical hold on these trials after serious adverse events occurred in three patients treated with AFM11.
This included a death in the ALL study and two life-threatening events in the NHL study.
The serious adverse events occurred in patients enrolled in the highest dose cohorts of each study.
A total of 33 patients have been treated in the two studies (NCT02848911 and NCT02106091), and preliminary signs of clinical activity have been observed in several patients.
Affimed said it will be working closely with the global health authorities, safety monitoring committees, and the studies’ clinical investigators to review the adverse events, assess all the data, and determine next steps for the AFM11 program.
Affimed intends to provide an update on AFM11 upon completing the evaluation.
Weighing the costs of CAR T-cell therapy
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of Stanford University, and his colleagues.
Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the U.S. Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL).
In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects.
However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology.
“Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant.
Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award.
One of the study coauthors reported consulting and research funding from Novartis.
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of Stanford University, and his colleagues.
Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the U.S. Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL).
In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects.
However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology.
“Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant.
Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award.
One of the study coauthors reported consulting and research funding from Novartis.
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of Stanford University, and his colleagues.
Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the U.S. Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL).
In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects.
However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology.
“Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant.
Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award.
One of the study coauthors reported consulting and research funding from Novartis.