User login
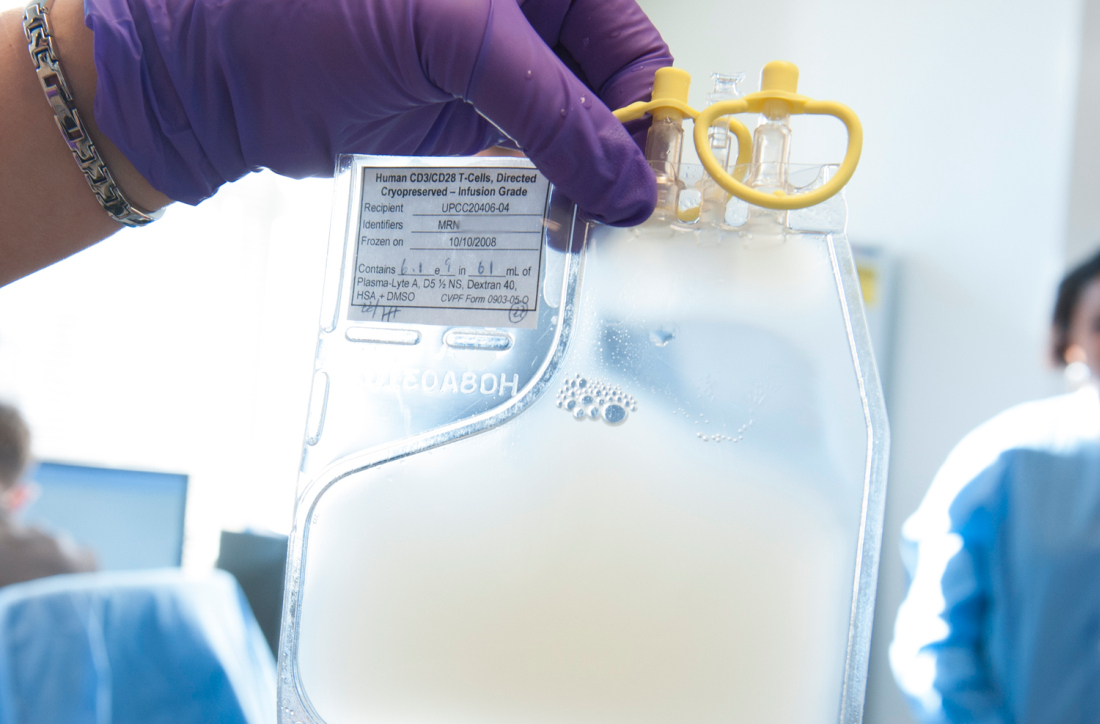
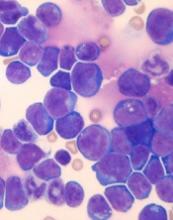
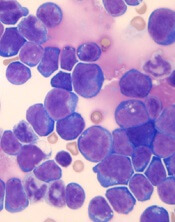
Single leukemic cell can contaminate CAR T-cell product
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
First reported case of induced resistance to tisagenlecleucel
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
FROM NATURE MEDICINE
Key clinical point: Unintentional transduction of a single leukemic B cell induced resistance to CTL019 (tisagenlecleucel) therapy.
Major finding: A patient with B-cell acute lymphoblastic leukemia (B-ALL) had frank relapse 9 months after a CTL019 infusion, with more than 90% bone marrow infiltration of chimeric antigen receptor–transduced B-cell leukemia cells.
Study details: A case study of a 20-year-old male with B-ALL undergoing CTL019 therapy.
Disclosures: Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
Source: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
FDA authorizes ClonoSEQ to detect MRD in ALL, myeloma
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
First NGS assay approved for MRD detection in ALL or MM
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
Factors that may drive relapse in AYAs with ALL
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
Blinatumomab approved to treat ALL in Japan
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
FDA grants OBI-3424 orphan designation for ALL
The Food and Drug Administration has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL. In addition, OBI-3424 significantly reduced leukemia bone marrow infiltration in four of six evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
The Food and Drug Administration has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL. In addition, OBI-3424 significantly reduced leukemia bone marrow infiltration in four of six evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
The Food and Drug Administration has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL. In addition, OBI-3424 significantly reduced leukemia bone marrow infiltration in four of six evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Bacteremic sepsis in ALL linked to later cognitive issues
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: ALL survivors with a sepsis history performed worse than did those with no sepsis history on evaluations of spatial planning (difference, 0.78), verbal fluency (0.38), and attention (0.63).
Study details: Prospective cohort study of 212 ALL survivors who underwent neurocognitive testing at a median of nearly 8 years after diagnosis.
Disclosures: The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
Source: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
CHMP reconsiders new indication for blinatumomab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
OBI-3424 receives orphan designation for ALL
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.