User login
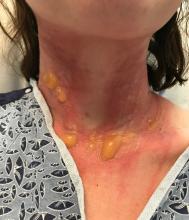
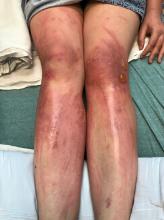
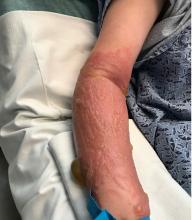
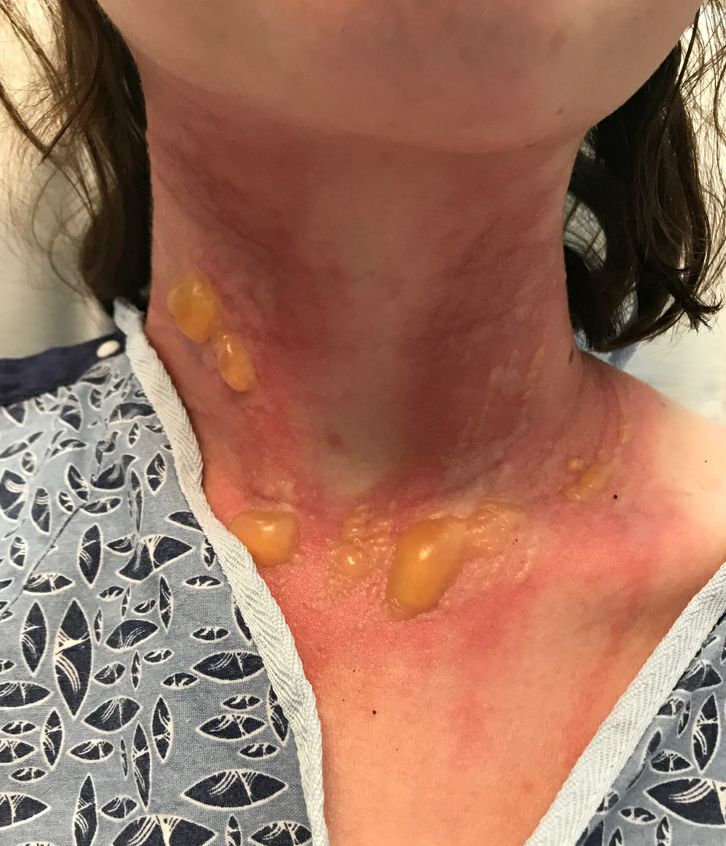
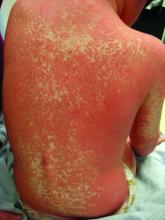
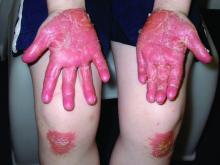
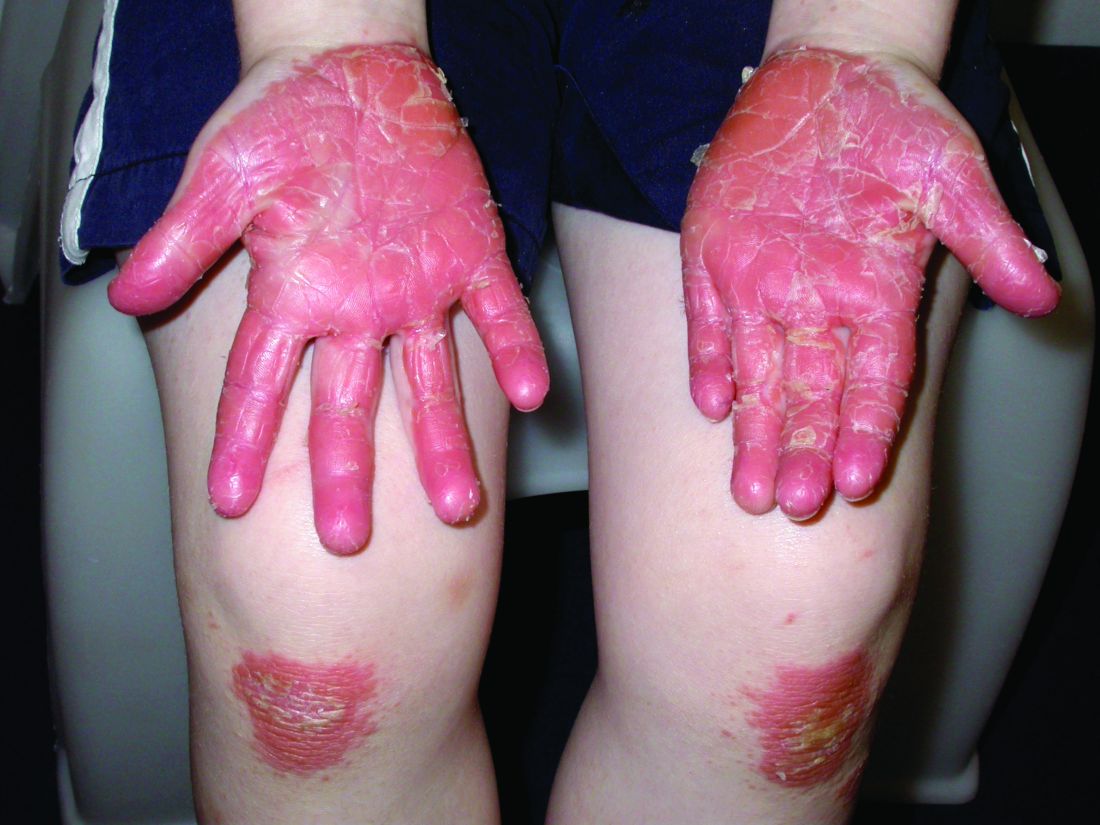
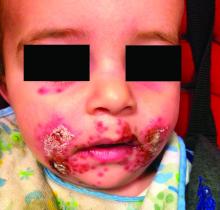
A teen presents with a severe, tender rash on the extremities
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
She started taking lithium for depression and anxiety 3 weeks prior to her developing the rash. She denies taking any other medications, supplements, or recreational drugs.
She denied any prior history of photosensitivity, no history of mouth ulcers, joint pain, muscle weakness, hair loss, or any other symptoms.
Besides her brother, there are no other affected family members, and no history of immune bullous disorders or other skin conditions.
On physical exam, the girl appears in a lot of pain and is uncomfortable. The skin is red and hot, and there are tense bullae on the neck, arms, and legs. There are no ocular or mucosal lesions.
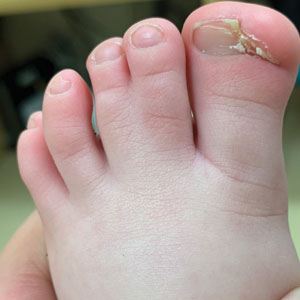
Congenital Defect of the Toenail
The Diagnosis: Onychodystrophy Secondary to Polydactyly
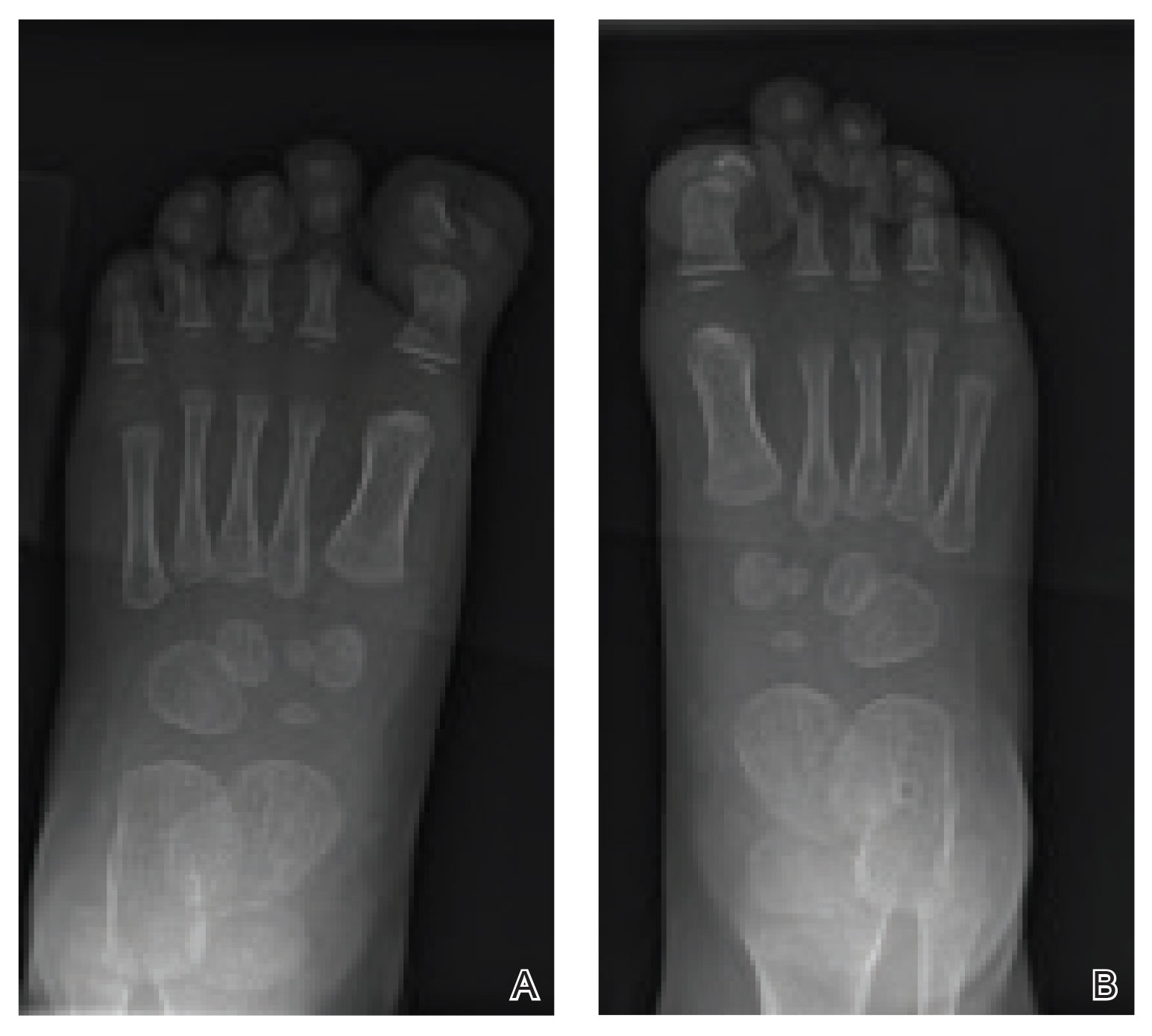
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.
Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
The Diagnosis: Onychodystrophy Secondary to Polydactyly
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.

Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
The Diagnosis: Onychodystrophy Secondary to Polydactyly
Radiographs of the feet demonstrated an accessory distal phalanx of the left great toe with a similar smaller accessory distal phalanx on the right great toe (Figure). The patient was referred to orthopedic surgery, and surgical intervention was recommended for only the left great toe given recurrent skin inflammation and nail complications. An excision of the left great toe polydactyly was performed. The patient healed well without complications.

Many clinically heterogeneous phenotypes exist for polydactyly and syndactyly, which both are common entities with incidences of 1 in 700 to 1000 births and 1 in 2000 to 3000 births, respectively.1 Both polydactyly and syndactyly can be an isolated variant in newborns or present with multiple concurrent malformations as part of a genetic syndrome, with more than 300 syndromic anomalies described. The genetic basis of these conditions is equally diverse, with homeobox genes, hedgehog pathways, fibroblast growth factors, and boneand cartilage-derived morphogenetic proteins implicated in their development.1
The differential diagnosis for our patient included congenital malalignment of the great toenails, nail-patella syndrome, onychodystrophy secondary to polydactyly, and congenital hypertrophy of the lateral nail fold. Given the strong family history, polydactyly was suspected.
Congenital malalignment of the great toenails results in lateral deviation of the nail plates.2 It is an underdiagnosed condition with different etiologies hypothesized, such as genetic factors with possible autosomal-dominant transmission and extrinsic factors.3 One proposed mechanism of pathogenesis is desynchronization during growth of the nail and distal phalanx of the hallux, leading to larger nail plates that grow laterally.4 Typical features associated with this disease are nail discoloration, nail plate thickening, and transversal grooves or ridges, none of which were seen in our patient.2
Children with nail-patella syndrome have dysplastic nails and associated bony abnormalities, such as absent patellae.5 This syndrome results from an autosomaldominant mutation in the LIM homeobox transcription factor 1-beta gene, LMX1B, which is responsible for dorsal-ventral patterning of the limb, as well as patterning of the nails, patellae and long bones, and even the kidney tubule.6 As such, patients with nail-patella syndrome have associated renal abnormalities. The findings in our patient were limited to the feet, making an underlying syndrome unlikely to be the cause.
First described in 1968 by Meadow,7 fetal hydantoin syndrome is a well-documented sequela in women taking phenytoin throughout pregnancy. Multiple malformations are possible, including cardiac defects, cleft lip/palate, digit and nail hypoplasia, abnormal facial features, mental disability, and growth abnormalities.8 The teratogenicity behind phenytoin results from reactive oxygen species that alter embryonic DNA, proteins, and lipids.9 The mother of this child was not on any seizure prophylaxis, eliminating it from the differential.
Congenital hypertrophy of the lateral nail fold is a defect of the soft tissue of the hallux leading to hypertrophy of the nail fold, commonly presenting with inflammation and pain10 possibly due to dyssynchronous growth between the soft tissue and nail plate.11 With this defect, a lip covering the nail plate is common, which was not seen in our patient.
As demonstrated in our patient, family history can help guide the diagnosis. Seven of 9 nonsyndromic forms of syndactyly are inherited in an autosomal-dominant fashion and range from mild presentations, as in our patient, to more severe deformations with underlying bone fusion and functional impairment.12 Polydactyly also often is expressed in an autosomal-dominant pattern, with up to 30% of patients having a positive family history. Polydactyly traditionally is classified by the location of the supernumerary digit as preaxial (radial), central, or postaxial (ulnar), and many further morphologic variations exist within these groups. Overall, preaxial polydactyly is relatively rare and represents 15% of polydactylies, with central and postaxial comprising the other 6% and 79%, respectively.13 Delineation of the underlying anatomy may reveal ray duplications (digit and corresponding metacarpal or metatarsal bone), metatarsal variants, and duplicated phalanges that may be hypoplastic or deformed. Patients may report difficulty finding comfortable footwear, cosmetic concerns, and nail-related complications. Although not always required, surgical intervention may provide definitive treatment but can leave residual deformities in the surrounding altered anatomy; thus, orthopedic or plastic surgery consultations are critical in appropriately counseling patients.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
- Ahmed H, Akbari H, Emanmi A, et al. Genetic overview of syndactyly and polydactyly. Plast Reconstr Surg Glob Open. 2017;5:e1549.
- Catalfo P, Musumeci ML, Lacarrubba F, et al. Congenital malalignment of the great toenails: a review. Skin Appendage Disord. 2018;4:230-235.
- Kus S, Tahmaz E, Gurunluoglu R, et al. Congenital malalignment of the great toenails in dizygotic twins. Pediatr Dermatol. 2005;22:434-435.
- Chaniotakis I, Bonitsis N, Stergiopoulou C, et al. Dizygotic twins with congenital malalignment of the great toenails: reappraisal of the pathogenesis. J Am Acad Dermatol. 2007;57:711-715.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Dreyer SD, Zhou G, Baldini A, et al, Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47-50.
- Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet. 1968;2:1296.
- Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6.
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995;48:112-120.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Martinet C, Pascal M, Civatte J, et al. Lateral nail-pad of the big toe in infants. apropos of 2 cases. Ann Dermatol Venereol. 1984;111:731-732.
- Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824.
- Belthur MV, Linton JL, Barnes DA. The spectrum of preaxial polydactyly of the foot. J Pediatr Orthop. 2011;31:435-447.
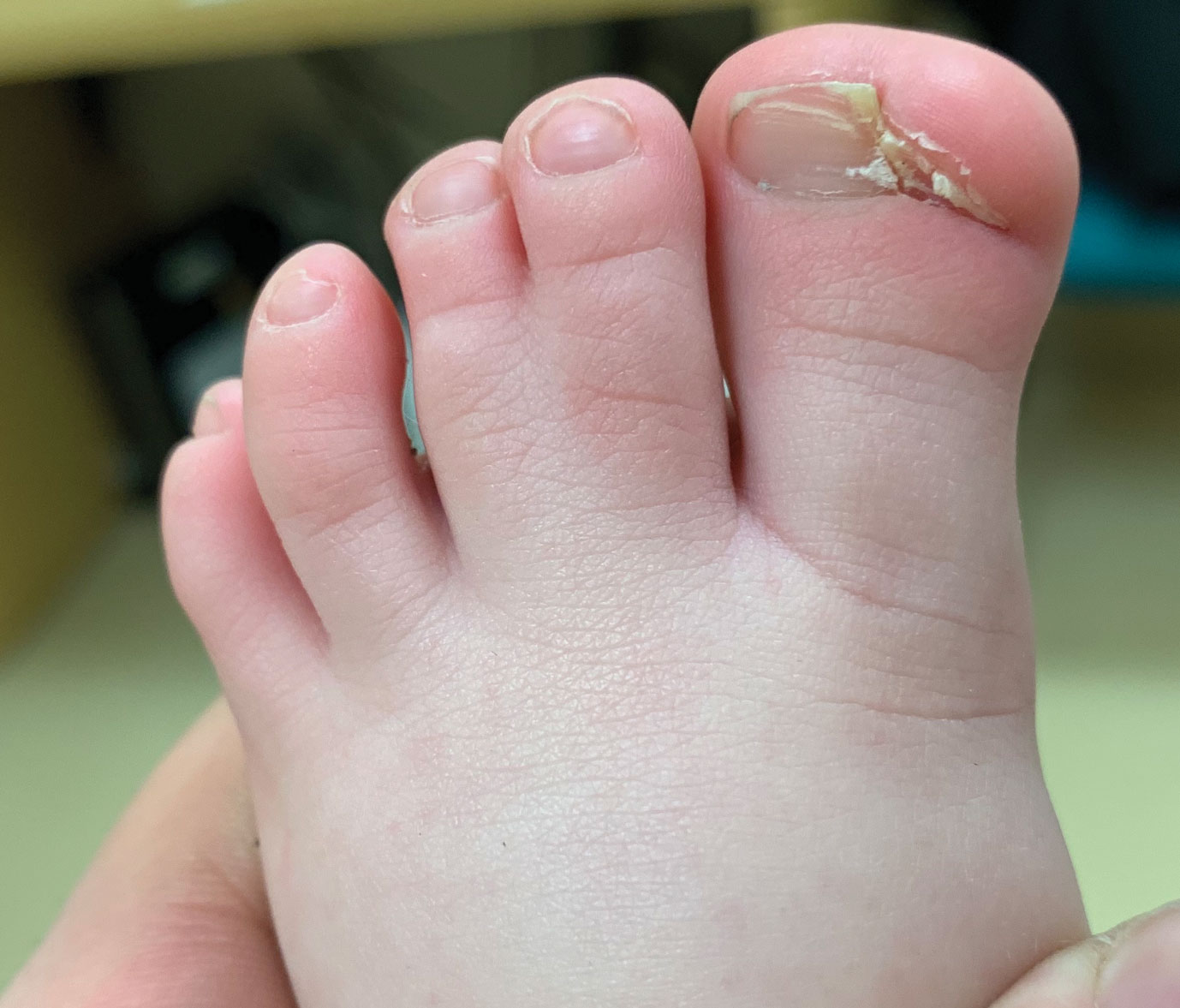
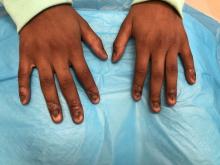
An 18-month-old girl presented for evaluation of nail dystrophy. The patient’s parents stated that the left great toenail had been dystrophic since birth, leading to skin irritation and “snagging” of the toenail on socks and footwear. Additional history revealed that the patient also had webbed toes, and there was a paternal family history of polydactyly and syndactyly. Physical examination revealed webbing of the second and third toes to the distal interphalangeal joints on both feet, marked nail plate dystrophy on the left big toe, and an irregularly shaped nail plate on the right big toe. The patient had no similar findings on the hands.
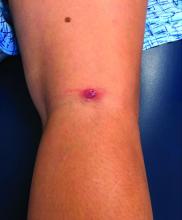
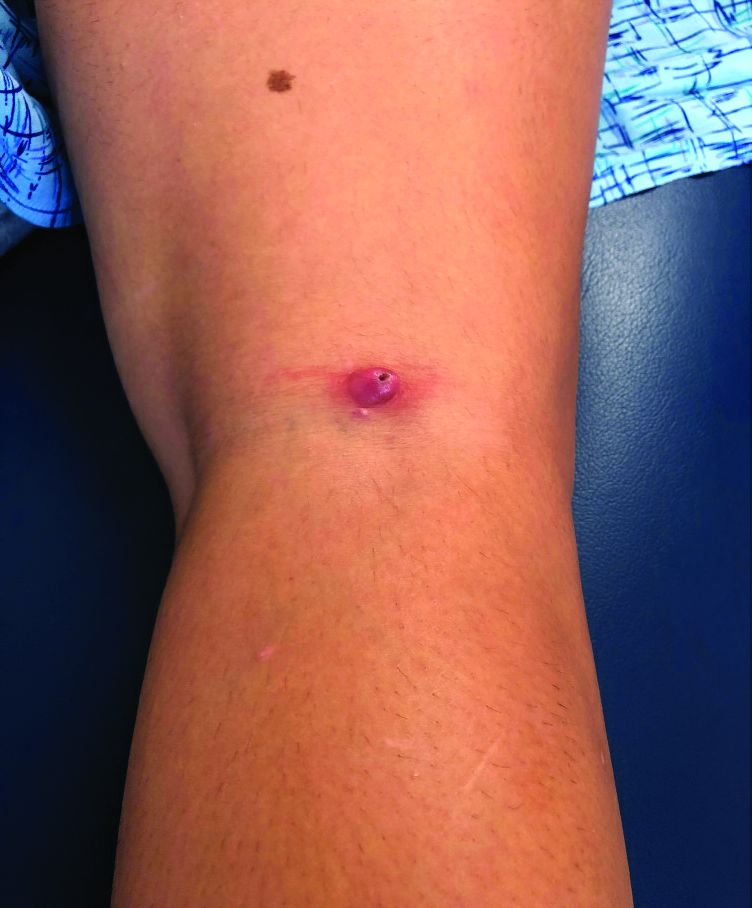
A teen girl presents with a pinkish-red bump on her right leg
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
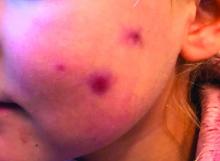
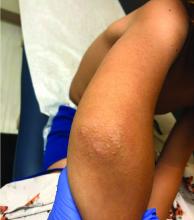
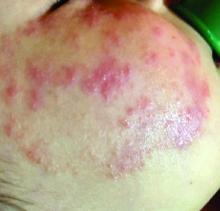
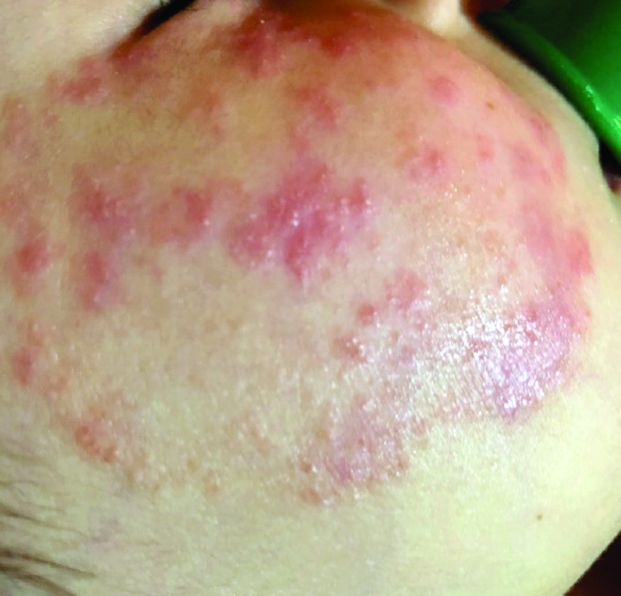
A 4-year-old with a lesion on her cheek, which grew and became firmer over two months
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
A 4-year-old female is brought to our pediatric dermatology clinic for evaluation of a persistent lesion on the cheek.
The mother of the child reports that the lesion started as a small "bug bite" and then started growing and getting firmer for the past 2 months. The girl has developed other smaller red, pimple-like lesions on the cheeks and one of them is starting to increase in size.
She denies any tenderness on the area or any purulent discharge. She has had no fevers, chills, weight loss, nose bleeds, fatigue, or any other symptoms. The mother has not noted any changes on the child's body odor, any rapid growth, or hair on her axillary or pubic area. She was treated with three different courses of oral antibiotics including cephalexin, trimethoprim/sulfamethoxazole, and clindamycin, as well as topical mupirocin, with no improvement.
Her past medical history is significant for several episodes of eyelid cysts that were treated with warm compresses and topical erythromycin ointment. The family history is significant for the father having severe acne as a teenager. She has two cats, she has not traveled, and she has an older sister who has no lesions.
On physical examination she is a lovely 4-year-old female in no acute distress. Her height is on the 70th percentile and weight on the 40th percentile for her age. Her blood pressure is 95/84 with a heart rate of 96. On skin examination she has several pink macules and papules on her bilateral cheeks. On the left cheek there are two pink nodules: One is 1 cm, and the other is 7 mm. The nodules are not tender. There is no warmth, fluctuance, or discharge from the lesions.
She has no cervical lymphadenopathy. She has no axillary or pubic hair. She is Tanner stage I.
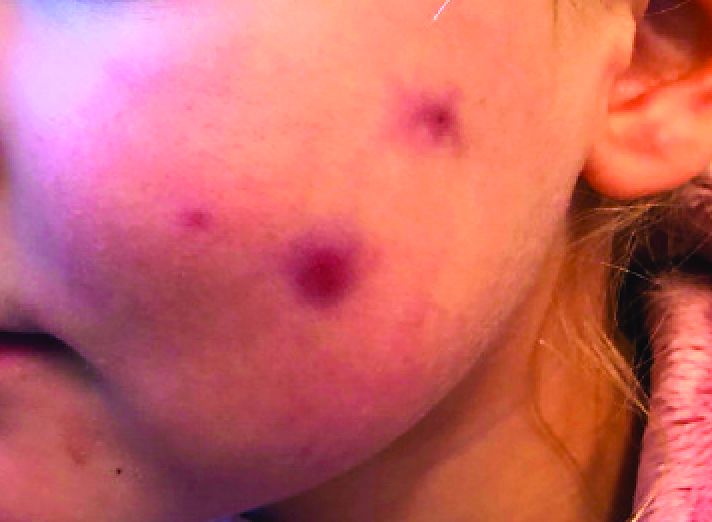
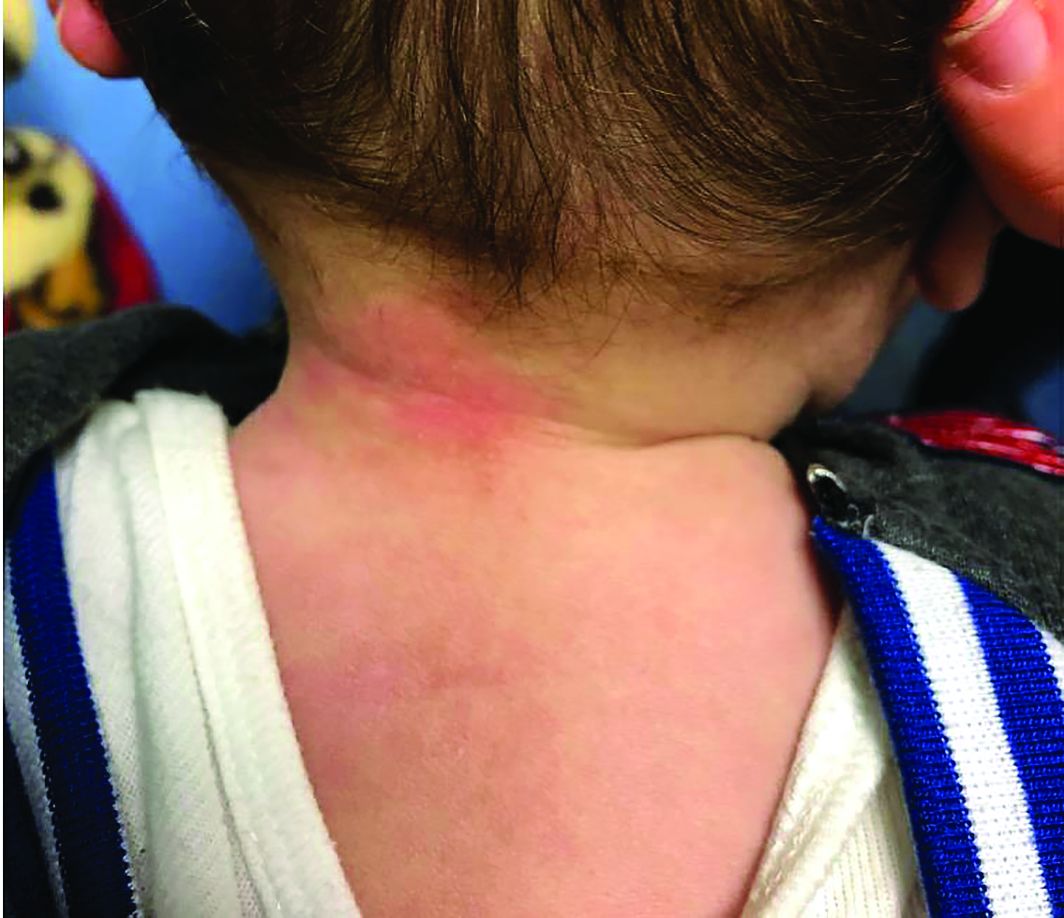
Four-year-old boy presents with itchy rash on face, extremities
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
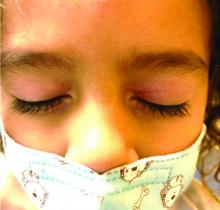
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
A 4-year-old healthy male with no significant prior medical history presents for evaluation of "itchy bumps" on the face and extremities of 2 weeks' duration.
The child was well until around 2 and a half weeks ago when he presented for evaluation of two lesions on the lower eyelids, diagnosed as hordeolum (a stye). He was prescribed ofloxacin ophthalmic solution.
One week later he developed bilateral itchy red eyes with red, thickened areas on the upper lids, followed several days later by pruritic papules on the ears, wrists, elbows, knees, and ankles. His mother used Vaseline for the eyelids for 1 week with no improvement. Physical exam at the dermatologist's office showed mild erythema, induration, and lichenification of the upper eyelids, and bilateral periocular eczematous patches with overlying scale. Subtle papules were evident on the elbows and feet.
What presents as deep erythematous papules, pustules, and may form an annular or circular plaque?
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
A 3-year-old girl with a known history of eczema presented to our dermatology clinic for evaluation of a persistent rash for about a year on the right cheek.
The mother reported she was treating the lesions with hydrocortisone cream 2.5% as instructed previously for her eczema. Initially the rash got partially better but then started getting worse again. The area was itchy.
The child was later seen in the emergency department, where she was recommended to treat the area with a combination cream of terbinafine 1% and betamethasone dipropionate 0.05%. The mother applied this cream as instructed for 3 weeks with some improvement of the lesions on the cheek.
A few weeks later, pimples started coming back. The mother tried the medication again but this time it was not helpful and the rash continued to expand.
The mother reported having a rash on her hand months back, which she successfully treated with the combination cream provided at the emergency department. They have no pets at home.
The child has a little sister who also has mild eczema.
She goes to day care and dances ballet.
What's your diagnosis?
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
A 10-year-old, otherwise healthy female with no prior significant medical history is brought into clinic for evaluation of orange-red scaly papules and plaques that first started on the face, neck, and fingers and began spreading to the trunk, arms, and knees. The mother of the patient also had noticed thickening of the skin on her palms and soles. The rash has been present for 2 months. Patient does not appear to be itchy, and otherwise is in normal state without pain, fever, drainage from sites, or known exposures. She was initially treated with topical triamcinolone with minimal improvement.
On physical exam, she is noted to have reddish-orange hyperkeratotic scaling papules coalescing into large plaques with follicular prominence diffusely on the face, neck, trunk, and upper extremities with smaller islands of skin that are normal-appearing. There is diffuse fine scale throughout the scalp and thickening of the skin on the palms and soles with a yellowish waxy appearance.
What's your diagnosis?
A punch biopsy of one of the lesions showed a superficial and deep mixed inflammatory cell infiltrate, including neutrophils and eosinophils. There was also vasculitis, karyorrhexis and extravasated red blood cells. The findings are those of leukocytoclastic vasculitis, suggestive of acute hemorrhagic edema of infancy. Direct immunofluorescence was positive for IgM, C3, and fibrinogen, but negative for IgA.
Acute hemorrhagic edema of infancy (AHEI), also known as Finkelstein disease, is form of leukocytoclastic vasculitis that occurs in infants and toddlers aged between4 months and 3 years.
The lesions start as petechiae or edematous, erythematous to violaceous nodules that later coalesce and form “cockade”-like plaques with a central clearing on the face and extremities. Gastrointestinal, renal, and joint involvement are rare.1 AHEI follows a benign course with resolution of the lesions and symptoms within days to weeks. The etiology of this condition is not known but infection triggers have been reported including coronavirus infections, coxsackie virus infections, Escherichia coli urinary tract infections, herpes simplex virus stomatitis, and pneumococcal bacteremia.2,3 Our patient had a prior history of pneumococcal pneumonia and metapneumovirus infection. MMR vaccine also has been reported as a possible trigger, as well as some medications.
Laboratory results are usually normal, but some patients may have elevated inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), as noted in our patient, and leukocytosis, thrombocytosis, and eosinophilia. Microscopic analysis demonstrates leukocytoclastic vasculitis of small vessels with associated karyorrhexis and extravasated red blood cells.
The differential diagnosis includes other vasculitic conditions, primarily Henoch-Schönlein purpura (HSP). Patients with HSP tend to be older in age and the lesions described as palpable purpura commonly affect the lower extremities and buttocks. These patients can present with abdominal pain and arthritis; renal compromise also can occur. Direct immunofluorescence can commonly be positive for IgA, which was negative in our patient.
AHEI and HSP are considered different entities, but both present with leukocytoclastic vasculitis.1 Another condition to consider in patients with fever, rash, and edema is Kawasaki disease, also a form of vasculitis, that affects small- and medium-size muscular vessels with predilection for the coronary arteries. Patients with Kawasaki disease present with fever (usually longer than 5 days), facial and extremity edema (similar to AHEI), skin lesions (which may have multiple presentations, the most common being macular, papular and erythematous, and urticarial eruptions), but also lymphadenopathy and conjunctivitis. These patients appear sicker than children with AHEI. Their laboratory results show leukocytosis, thrombocytosis or thrombocytopenia, elevated inflammatory markers, and sterile pyuria.4
Patients with erythema nodosum present with tender erythematous nodules, which can look like early AHEI lesions. The most common location is the lower extremities, but in children erythema nodosum can occur on the face, trunk, and arms. The lesions can occur secondary to infections such as streptococcus, mycoplasma, tuberculosis, coccidioidomycosis, and sarcoidosis, as well as to malignancy or medications. These patients do not appear sick, are not febrile, and are rarely seen under 2 years of age.5
Acute febrile neutrophilic dermatosis – Sweets’ syndrome – also should be considered in a patient with tender nodules, fever, and leukocytosis. The skin lesions in Sweets’ syndrome, compared with those in AHEI, are painful and can present as papules, nodules, and bullae on the face and extremities. A prior history of an upper respiratory infection is commonly described in children with Sweets’ syndrome. These patients present with fever, which may start days to weeks prior to the lesions starting. Children with Sweets’ syndrome also can have conjunctivitis, myalgias, polyarthritis, and in severe cases septic shock and multiorgan dysfunction. Sweets’ syndrome can be seen in patients with inflammatory bowel disease, systemic lupus erythematosus, chronic multifocal osteomyelitis, and malignancy; it also may be induced by certain medications.6
As mentioned above, the course of AHEI is benign, and the condition resolves within days to weeks. Treatment is supportive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. F1000Res. 2019;8:1771.
2. Pediatr Dermatol. 2006 Jul-Aug;23(4):361-4.
3. Pediatr Dermatol. 2015 Nov-Dec;32(6):e309-11.
4. Clin Dermatol. 2017 Nov-Dec;35(6):530-40.
5. Yonsei Med J. 2019 Mar;60(3):312-4.
6. Pediatr Dermatol. 2015 Jul-Aug;32(4):437-46.
A punch biopsy of one of the lesions showed a superficial and deep mixed inflammatory cell infiltrate, including neutrophils and eosinophils. There was also vasculitis, karyorrhexis and extravasated red blood cells. The findings are those of leukocytoclastic vasculitis, suggestive of acute hemorrhagic edema of infancy. Direct immunofluorescence was positive for IgM, C3, and fibrinogen, but negative for IgA.
Acute hemorrhagic edema of infancy (AHEI), also known as Finkelstein disease, is form of leukocytoclastic vasculitis that occurs in infants and toddlers aged between4 months and 3 years.
The lesions start as petechiae or edematous, erythematous to violaceous nodules that later coalesce and form “cockade”-like plaques with a central clearing on the face and extremities. Gastrointestinal, renal, and joint involvement are rare.1 AHEI follows a benign course with resolution of the lesions and symptoms within days to weeks. The etiology of this condition is not known but infection triggers have been reported including coronavirus infections, coxsackie virus infections, Escherichia coli urinary tract infections, herpes simplex virus stomatitis, and pneumococcal bacteremia.2,3 Our patient had a prior history of pneumococcal pneumonia and metapneumovirus infection. MMR vaccine also has been reported as a possible trigger, as well as some medications.
Laboratory results are usually normal, but some patients may have elevated inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), as noted in our patient, and leukocytosis, thrombocytosis, and eosinophilia. Microscopic analysis demonstrates leukocytoclastic vasculitis of small vessels with associated karyorrhexis and extravasated red blood cells.
The differential diagnosis includes other vasculitic conditions, primarily Henoch-Schönlein purpura (HSP). Patients with HSP tend to be older in age and the lesions described as palpable purpura commonly affect the lower extremities and buttocks. These patients can present with abdominal pain and arthritis; renal compromise also can occur. Direct immunofluorescence can commonly be positive for IgA, which was negative in our patient.
AHEI and HSP are considered different entities, but both present with leukocytoclastic vasculitis.1 Another condition to consider in patients with fever, rash, and edema is Kawasaki disease, also a form of vasculitis, that affects small- and medium-size muscular vessels with predilection for the coronary arteries. Patients with Kawasaki disease present with fever (usually longer than 5 days), facial and extremity edema (similar to AHEI), skin lesions (which may have multiple presentations, the most common being macular, papular and erythematous, and urticarial eruptions), but also lymphadenopathy and conjunctivitis. These patients appear sicker than children with AHEI. Their laboratory results show leukocytosis, thrombocytosis or thrombocytopenia, elevated inflammatory markers, and sterile pyuria.4
Patients with erythema nodosum present with tender erythematous nodules, which can look like early AHEI lesions. The most common location is the lower extremities, but in children erythema nodosum can occur on the face, trunk, and arms. The lesions can occur secondary to infections such as streptococcus, mycoplasma, tuberculosis, coccidioidomycosis, and sarcoidosis, as well as to malignancy or medications. These patients do not appear sick, are not febrile, and are rarely seen under 2 years of age.5
Acute febrile neutrophilic dermatosis – Sweets’ syndrome – also should be considered in a patient with tender nodules, fever, and leukocytosis. The skin lesions in Sweets’ syndrome, compared with those in AHEI, are painful and can present as papules, nodules, and bullae on the face and extremities. A prior history of an upper respiratory infection is commonly described in children with Sweets’ syndrome. These patients present with fever, which may start days to weeks prior to the lesions starting. Children with Sweets’ syndrome also can have conjunctivitis, myalgias, polyarthritis, and in severe cases septic shock and multiorgan dysfunction. Sweets’ syndrome can be seen in patients with inflammatory bowel disease, systemic lupus erythematosus, chronic multifocal osteomyelitis, and malignancy; it also may be induced by certain medications.6
As mentioned above, the course of AHEI is benign, and the condition resolves within days to weeks. Treatment is supportive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. F1000Res. 2019;8:1771.
2. Pediatr Dermatol. 2006 Jul-Aug;23(4):361-4.
3. Pediatr Dermatol. 2015 Nov-Dec;32(6):e309-11.
4. Clin Dermatol. 2017 Nov-Dec;35(6):530-40.
5. Yonsei Med J. 2019 Mar;60(3):312-4.
6. Pediatr Dermatol. 2015 Jul-Aug;32(4):437-46.
A punch biopsy of one of the lesions showed a superficial and deep mixed inflammatory cell infiltrate, including neutrophils and eosinophils. There was also vasculitis, karyorrhexis and extravasated red blood cells. The findings are those of leukocytoclastic vasculitis, suggestive of acute hemorrhagic edema of infancy. Direct immunofluorescence was positive for IgM, C3, and fibrinogen, but negative for IgA.
Acute hemorrhagic edema of infancy (AHEI), also known as Finkelstein disease, is form of leukocytoclastic vasculitis that occurs in infants and toddlers aged between4 months and 3 years.
The lesions start as petechiae or edematous, erythematous to violaceous nodules that later coalesce and form “cockade”-like plaques with a central clearing on the face and extremities. Gastrointestinal, renal, and joint involvement are rare.1 AHEI follows a benign course with resolution of the lesions and symptoms within days to weeks. The etiology of this condition is not known but infection triggers have been reported including coronavirus infections, coxsackie virus infections, Escherichia coli urinary tract infections, herpes simplex virus stomatitis, and pneumococcal bacteremia.2,3 Our patient had a prior history of pneumococcal pneumonia and metapneumovirus infection. MMR vaccine also has been reported as a possible trigger, as well as some medications.
Laboratory results are usually normal, but some patients may have elevated inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), as noted in our patient, and leukocytosis, thrombocytosis, and eosinophilia. Microscopic analysis demonstrates leukocytoclastic vasculitis of small vessels with associated karyorrhexis and extravasated red blood cells.
The differential diagnosis includes other vasculitic conditions, primarily Henoch-Schönlein purpura (HSP). Patients with HSP tend to be older in age and the lesions described as palpable purpura commonly affect the lower extremities and buttocks. These patients can present with abdominal pain and arthritis; renal compromise also can occur. Direct immunofluorescence can commonly be positive for IgA, which was negative in our patient.
AHEI and HSP are considered different entities, but both present with leukocytoclastic vasculitis.1 Another condition to consider in patients with fever, rash, and edema is Kawasaki disease, also a form of vasculitis, that affects small- and medium-size muscular vessels with predilection for the coronary arteries. Patients with Kawasaki disease present with fever (usually longer than 5 days), facial and extremity edema (similar to AHEI), skin lesions (which may have multiple presentations, the most common being macular, papular and erythematous, and urticarial eruptions), but also lymphadenopathy and conjunctivitis. These patients appear sicker than children with AHEI. Their laboratory results show leukocytosis, thrombocytosis or thrombocytopenia, elevated inflammatory markers, and sterile pyuria.4
Patients with erythema nodosum present with tender erythematous nodules, which can look like early AHEI lesions. The most common location is the lower extremities, but in children erythema nodosum can occur on the face, trunk, and arms. The lesions can occur secondary to infections such as streptococcus, mycoplasma, tuberculosis, coccidioidomycosis, and sarcoidosis, as well as to malignancy or medications. These patients do not appear sick, are not febrile, and are rarely seen under 2 years of age.5
Acute febrile neutrophilic dermatosis – Sweets’ syndrome – also should be considered in a patient with tender nodules, fever, and leukocytosis. The skin lesions in Sweets’ syndrome, compared with those in AHEI, are painful and can present as papules, nodules, and bullae on the face and extremities. A prior history of an upper respiratory infection is commonly described in children with Sweets’ syndrome. These patients present with fever, which may start days to weeks prior to the lesions starting. Children with Sweets’ syndrome also can have conjunctivitis, myalgias, polyarthritis, and in severe cases septic shock and multiorgan dysfunction. Sweets’ syndrome can be seen in patients with inflammatory bowel disease, systemic lupus erythematosus, chronic multifocal osteomyelitis, and malignancy; it also may be induced by certain medications.6
As mentioned above, the course of AHEI is benign, and the condition resolves within days to weeks. Treatment is supportive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. F1000Res. 2019;8:1771.
2. Pediatr Dermatol. 2006 Jul-Aug;23(4):361-4.
3. Pediatr Dermatol. 2015 Nov-Dec;32(6):e309-11.
4. Clin Dermatol. 2017 Nov-Dec;35(6):530-40.
5. Yonsei Med J. 2019 Mar;60(3):312-4.
6. Pediatr Dermatol. 2015 Jul-Aug;32(4):437-46.
At 3 a.m., you receive a call from the ED for a baby with a new rash on the arms, legs, and face. Some of the lesions appear to be tender. He has a mild fever of 38.4° C (101.1° F) and is not in acute distress. He is drinking, but not eating much.
The parents also have noted some swelling on the hands and the feet. He has no upper respiratory or gastrointestinal symptoms. He is not walking yet.
He was admitted to the hospital 3 weeks prior for streptococcal pneumonia and metapneumovirus infection. He was treated with ceftriaxone, supportive respiratory care, and an albuterol inhaler. Influenza and respiratory syncytial virus tests were negative.
On physical exam, the child is tired and sleeping in his mom's arms. He has red and some purpuric papules on the face. On the arms and legs, he has purpuric papules and nodules. There is some edema on the face, hands, and feet. His conjunctiva is normal, and he has no oral lesions. He has no lymphadenopathy or hepatosplenomegaly.
Blood work shows normal complete blood count, coagulation tests, comprehensive metabolic panel, and urinalysis, but he has an elevated C-reactive protein of 114 mg/L and an elevated erythrocyte sedimentation rate of 71 mm/hour.
A 7-month-old male presents with perioral rash and fever
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Nail dystrophy and nail plate thinning
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
An 8-year-old female child comes to our pediatric dermatology clinic for evaluation of onychomycosis on her fingernails. The mother stated the child started developing funny-looking nails 1 year prior to the visit. It started with only two fingernails affected and now has spread to all her fingernails. Her toenails are not involved.
She denied any pain or itching. She initially was treated with topical antifungal medications as well as tea tree oil, apple cider vinegar, and a 6-week course of oral griseofulvin without any improvement. Her nails progressively have gotten much worse. She has no history of atopic dermatitis or any other skin conditions. She denied any joint pain, sun sensitivity, hair loss, or any other symptoms. The mother denied any family history of nail fungus, ringworm, psoriasis, or eczema.
She likes to play basketball and enjoys arts and crafts. She has a cat and a dog; neither of them have any skin problems.
On physical examination, there is nail dystrophy with nail plate thinning and longitudinal fissuring of all fingernails but not of the toenails. She also has hyperpigmented violaceous plaques on the surrounding periungual skin. There are no other skin lesions, and there are no oral or genital lesions. There is no scalp involvement or hair loss. At follow-up several months later, she had complete destruction of the nail plate with scar formation.
A fungal culture was performed, as well as microscopic analysis of the nail with periodic acid fast and giemsa stains, which showed no fungal organisms.
She initially was treated with topical betamethasone twice a day for 6 weeks and then 2 weeks on and 2 weeks off without much change.