User login
December 2018
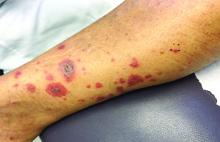
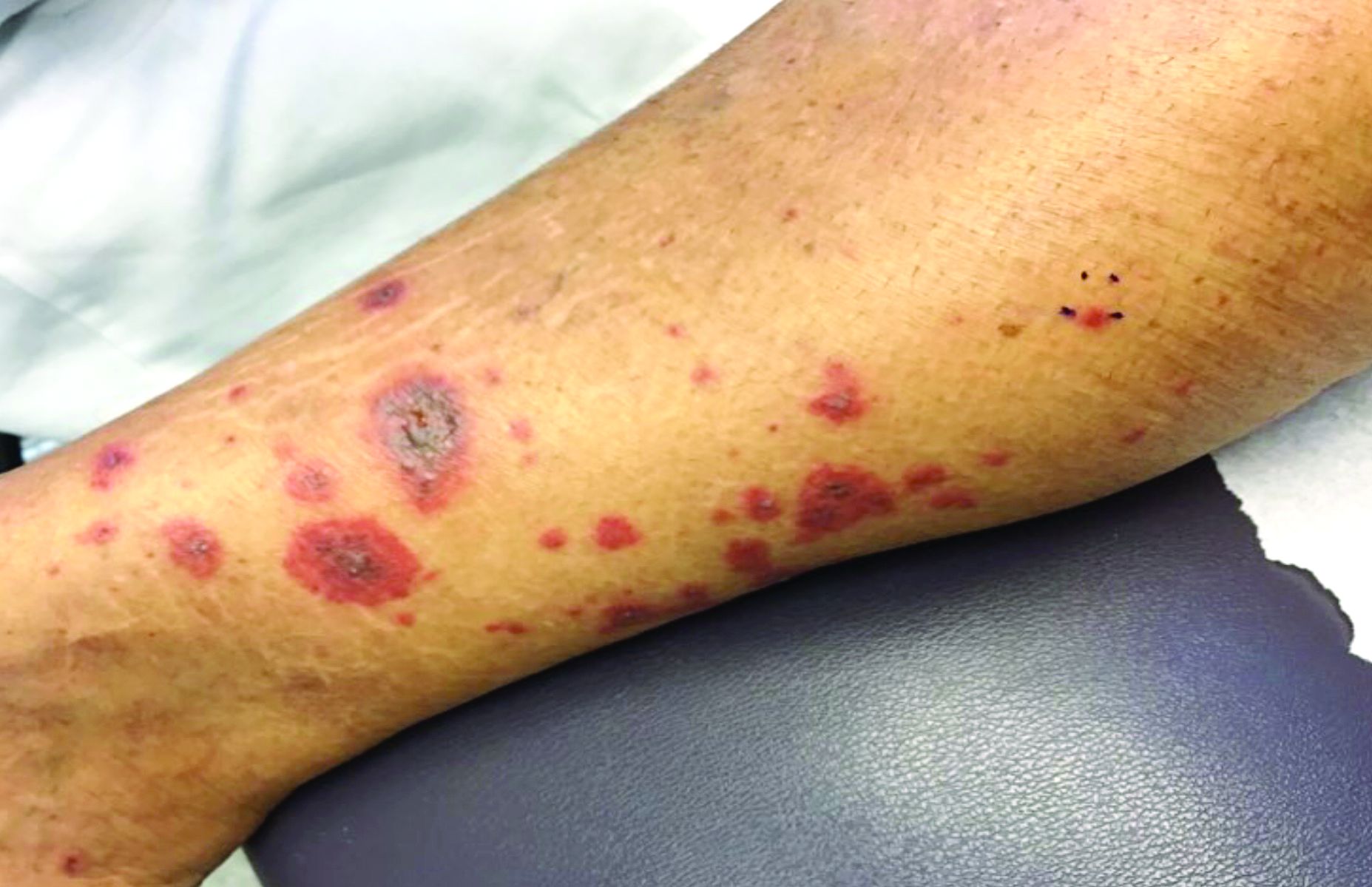
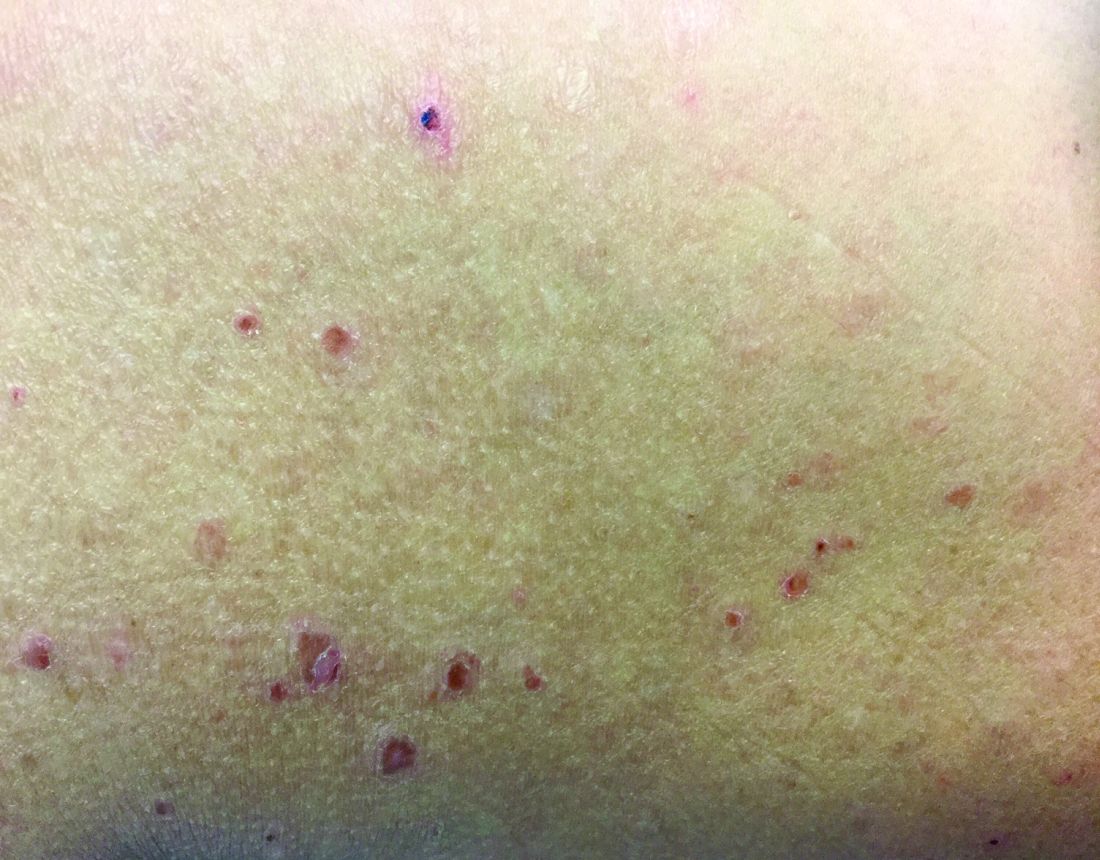
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
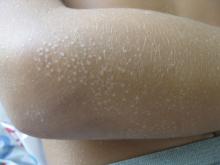
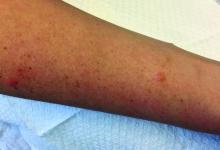
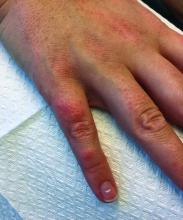
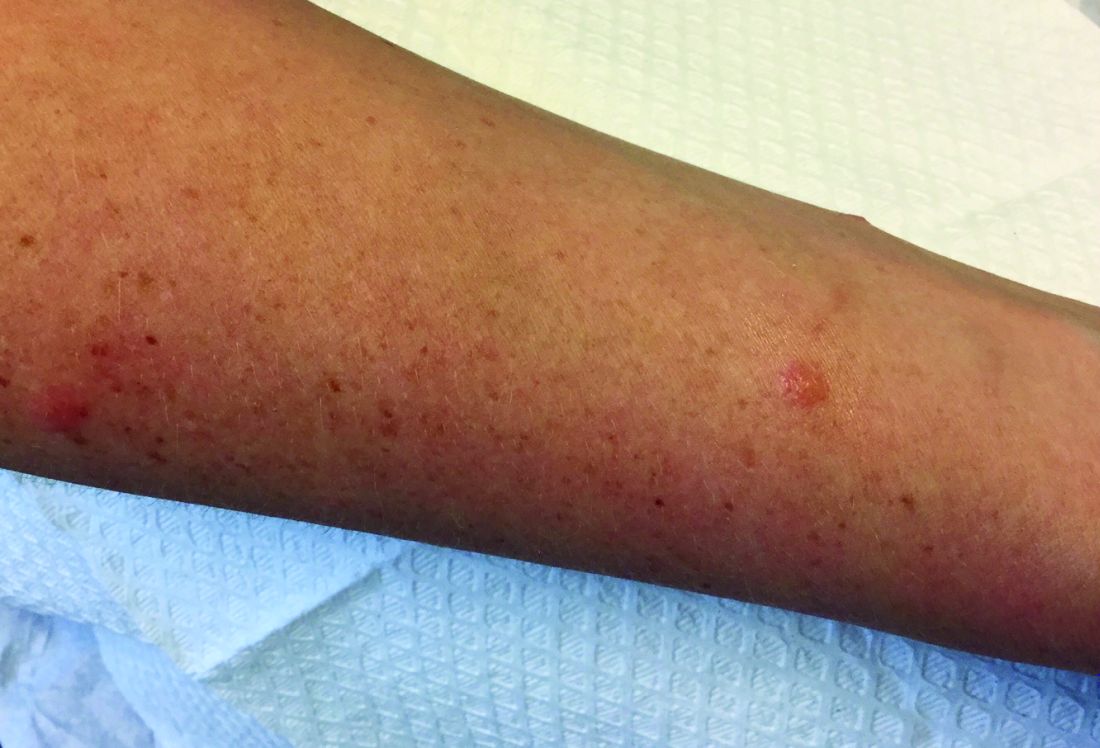
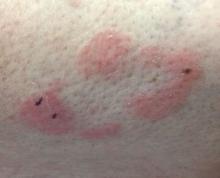
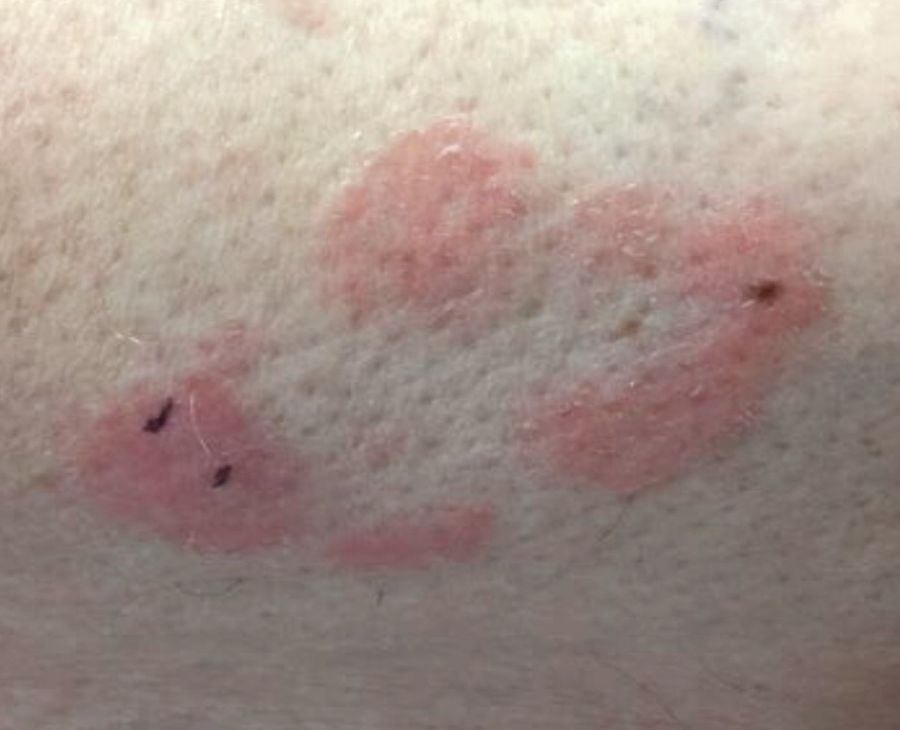
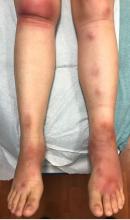
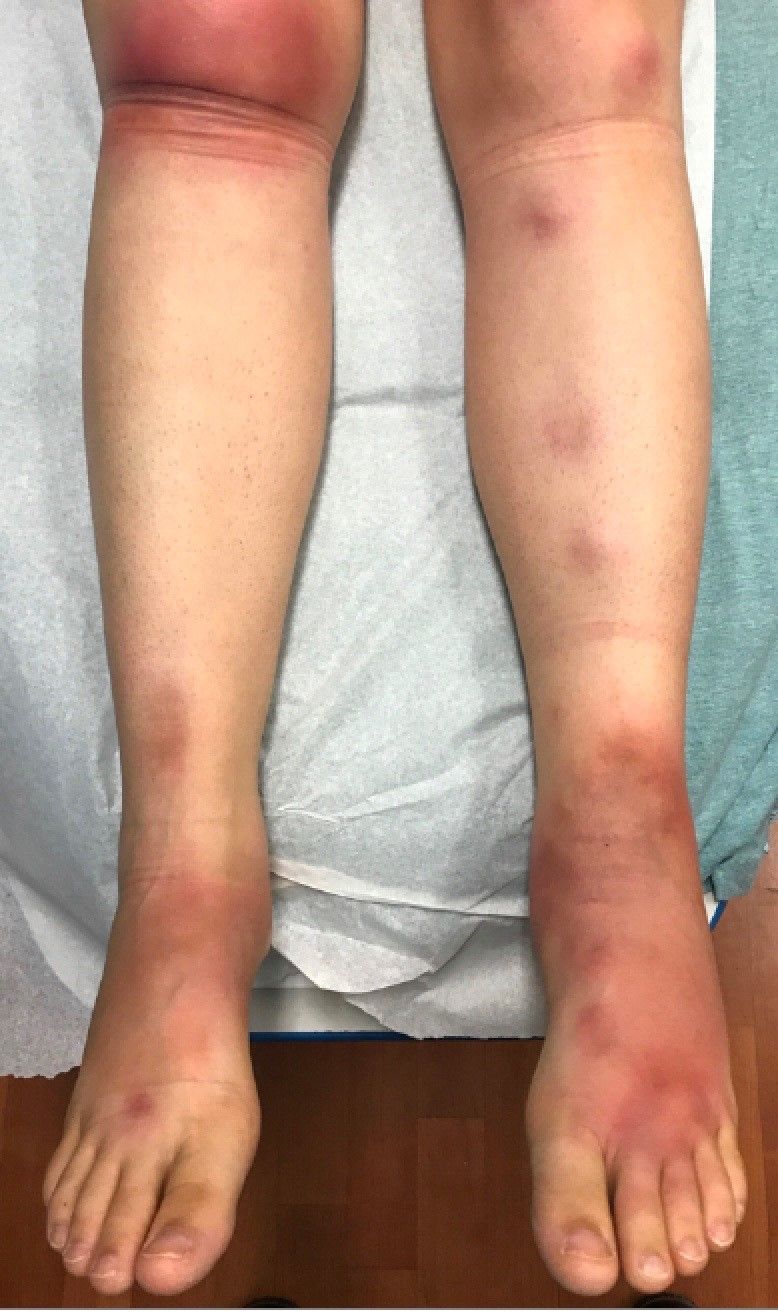
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
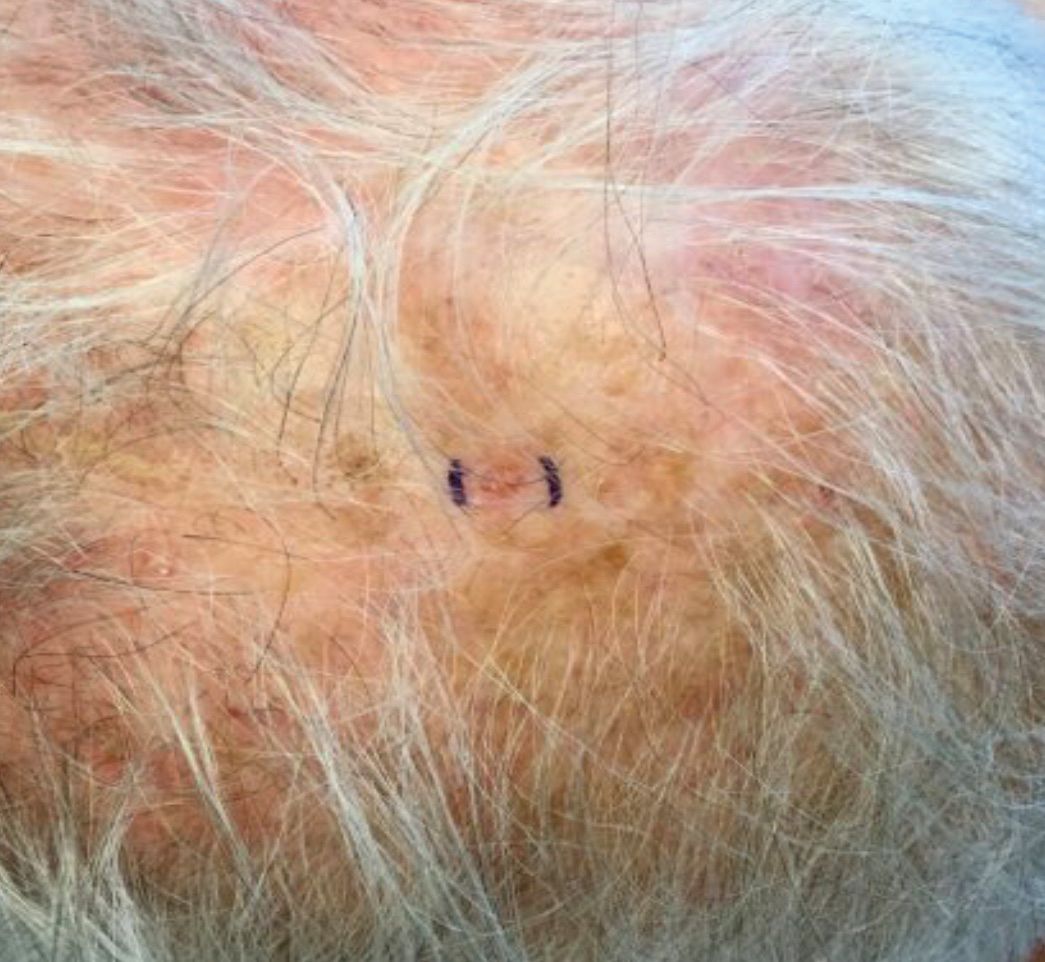
A 63-year-old white female presented with a 2-week history of hemorrhagic purpuric lesions and necrotic vesicles on the bilateral lower extremities.
Nearly 1 year prior to presentation, the patient underwent surgical resection for lung cancer. The patient also complained of joint swelling and pain in her ankles. She denied abdominal pain. She denied recent illness, including sore throat and upper respiratory infection. Skin biopsies were performed, including for direct immunofluorescence.
What is your diagnosis?
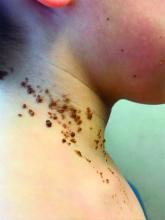
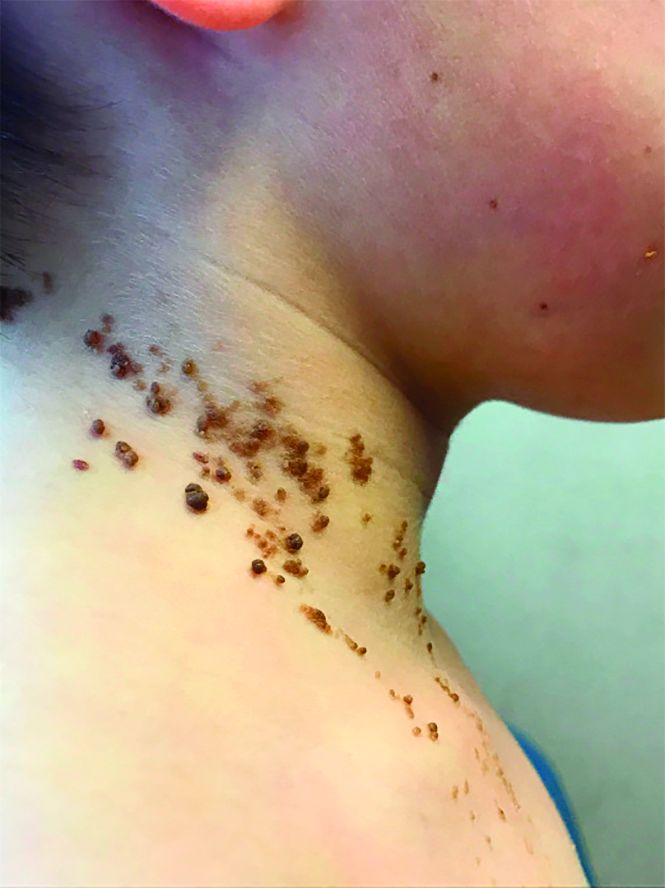
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
A 6-year-old, otherwise-healthy male is brought into clinic for evaluation of papules on his neck. The rash has been present since 1 year of age and has been growing in size proportionately. He claims there is occasional itching but no pain or redness. He does not seem to be disturbed by his rash. He has two siblings, aged 2 and 4 years, without lesions.
On physical exam, he is noted to have a linear plaque of hyperpigmented verrucous papules on his neck.
What is your diagnosis? - December 2018
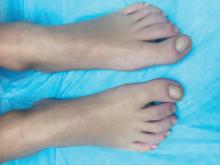
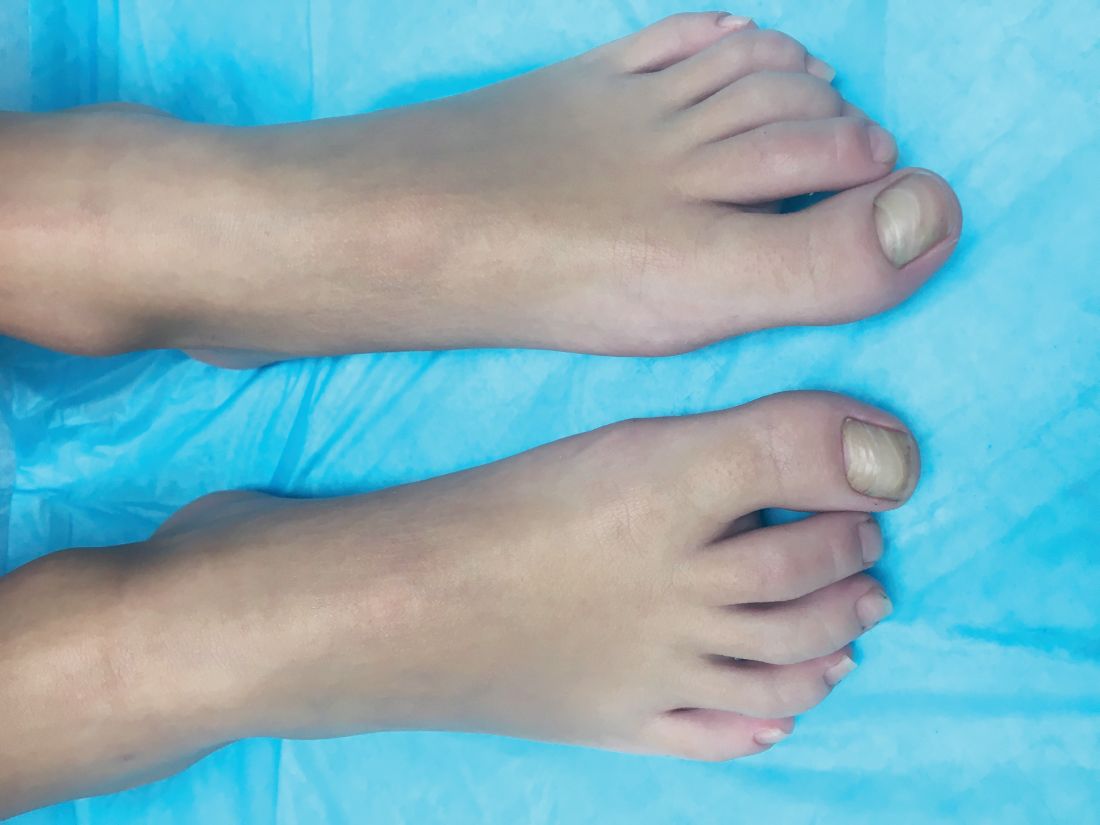
A KOH (potassium hydroxide) test done at the visit was negative as well as a fungal culture of each toenail.
The patient was diagnosed with congenital malalignment of the great toenails (CMGTN) based on history and morphologic appearance.
Congenital malalignment of the great toenails is an underrecognized and underreported nail disorder characterized by lateral deviation of the nail plate, which is not parallel to the longitudinal axis of the distal phalanx.1 The cause is unknown. Some reports suggest a genetic cause being transmitted in an autosomal dominant fashion with variable expression.2 There have been reports of CMGTN in monozygotic and dizygotic twins making this theory likely.3 Other authors consider an external cause such as amniotic bands, neonatal asphyxia, vascular malformations, and uterine pressure. This condition also has been reported in patients with Rubinstein-Taybi syndrome.4
The nail changes can occur at birth but in some cases, such as our patient, the nails become dystrophic months to years after birth. Characteristic nail changes include shorter, discolored, hyperkeratotic nails with transverse groove or ridges. In some cases, the dystrophic nails may cause inflammation and tenderness and is the most common cause of ingrown toenails in children.
The differential diagnosis includes onychomycosis, traumatic nails, nail psoriasis, pachyonychia congenital (PC), and onychomadesis. Onychomycosis can present with white or yellow discoloration of the nail that in some cases can be associated with nail breakage, hyperkeratosis, onycholysis, and subungual debris. Either fungal culture or periodic acid shift stain of nail clippings can help confirm or exclude this diagnosis. Psoriatic nails present with nail pits, oils spots, and onycholysis. Traumatic nail changes may occur from using small shoes and trauma from running or playing soccer, and presents with subungual hemorrhage and nail dystrophy of the first or second toenail. PC is a genetic disorder caused by a mutation in certain keratin proteins of the skin (k6a, k6b, K16 and K17). These patients usually have other skin findings including palmoplantar keratoderma, white plaques on the mouth, and skin cysts (steatocystoma multiplex and vellus hair cysts). Nail changes characteristic of PC includes subungual hyperkeratosis that causes a wedge shape thickening of the nail bed (pincer nails).5 Onychomadesis can be seen after viral infections such as hand-foot-mouth disease or in patients taking chemotherapy drugs that affect nail growth.
CMGTN usually resolves with time, but some patients with severe deviation and paronychia may need surgical correction.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2014 Jan 15;20(1):21251.
2. J Dtsch Dermatol Ges. 2012 May;10(5):326-30.
3. J Am Acad Dermatol. 2007 Oct;57(4):711-5.
4. Pediatr Dermatol. 2004 Jan-Feb;21(1):44-7.
5. Curr Opin Pediatr. 2014 Aug;26(4):440-5.
6. Skin Appendage Disord. 2018 Oct;4(4):230-5.
A KOH (potassium hydroxide) test done at the visit was negative as well as a fungal culture of each toenail.
The patient was diagnosed with congenital malalignment of the great toenails (CMGTN) based on history and morphologic appearance.
Congenital malalignment of the great toenails is an underrecognized and underreported nail disorder characterized by lateral deviation of the nail plate, which is not parallel to the longitudinal axis of the distal phalanx.1 The cause is unknown. Some reports suggest a genetic cause being transmitted in an autosomal dominant fashion with variable expression.2 There have been reports of CMGTN in monozygotic and dizygotic twins making this theory likely.3 Other authors consider an external cause such as amniotic bands, neonatal asphyxia, vascular malformations, and uterine pressure. This condition also has been reported in patients with Rubinstein-Taybi syndrome.4
The nail changes can occur at birth but in some cases, such as our patient, the nails become dystrophic months to years after birth. Characteristic nail changes include shorter, discolored, hyperkeratotic nails with transverse groove or ridges. In some cases, the dystrophic nails may cause inflammation and tenderness and is the most common cause of ingrown toenails in children.
The differential diagnosis includes onychomycosis, traumatic nails, nail psoriasis, pachyonychia congenital (PC), and onychomadesis. Onychomycosis can present with white or yellow discoloration of the nail that in some cases can be associated with nail breakage, hyperkeratosis, onycholysis, and subungual debris. Either fungal culture or periodic acid shift stain of nail clippings can help confirm or exclude this diagnosis. Psoriatic nails present with nail pits, oils spots, and onycholysis. Traumatic nail changes may occur from using small shoes and trauma from running or playing soccer, and presents with subungual hemorrhage and nail dystrophy of the first or second toenail. PC is a genetic disorder caused by a mutation in certain keratin proteins of the skin (k6a, k6b, K16 and K17). These patients usually have other skin findings including palmoplantar keratoderma, white plaques on the mouth, and skin cysts (steatocystoma multiplex and vellus hair cysts). Nail changes characteristic of PC includes subungual hyperkeratosis that causes a wedge shape thickening of the nail bed (pincer nails).5 Onychomadesis can be seen after viral infections such as hand-foot-mouth disease or in patients taking chemotherapy drugs that affect nail growth.
CMGTN usually resolves with time, but some patients with severe deviation and paronychia may need surgical correction.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2014 Jan 15;20(1):21251.
2. J Dtsch Dermatol Ges. 2012 May;10(5):326-30.
3. J Am Acad Dermatol. 2007 Oct;57(4):711-5.
4. Pediatr Dermatol. 2004 Jan-Feb;21(1):44-7.
5. Curr Opin Pediatr. 2014 Aug;26(4):440-5.
6. Skin Appendage Disord. 2018 Oct;4(4):230-5.
A KOH (potassium hydroxide) test done at the visit was negative as well as a fungal culture of each toenail.
The patient was diagnosed with congenital malalignment of the great toenails (CMGTN) based on history and morphologic appearance.
Congenital malalignment of the great toenails is an underrecognized and underreported nail disorder characterized by lateral deviation of the nail plate, which is not parallel to the longitudinal axis of the distal phalanx.1 The cause is unknown. Some reports suggest a genetic cause being transmitted in an autosomal dominant fashion with variable expression.2 There have been reports of CMGTN in monozygotic and dizygotic twins making this theory likely.3 Other authors consider an external cause such as amniotic bands, neonatal asphyxia, vascular malformations, and uterine pressure. This condition also has been reported in patients with Rubinstein-Taybi syndrome.4
The nail changes can occur at birth but in some cases, such as our patient, the nails become dystrophic months to years after birth. Characteristic nail changes include shorter, discolored, hyperkeratotic nails with transverse groove or ridges. In some cases, the dystrophic nails may cause inflammation and tenderness and is the most common cause of ingrown toenails in children.
The differential diagnosis includes onychomycosis, traumatic nails, nail psoriasis, pachyonychia congenital (PC), and onychomadesis. Onychomycosis can present with white or yellow discoloration of the nail that in some cases can be associated with nail breakage, hyperkeratosis, onycholysis, and subungual debris. Either fungal culture or periodic acid shift stain of nail clippings can help confirm or exclude this diagnosis. Psoriatic nails present with nail pits, oils spots, and onycholysis. Traumatic nail changes may occur from using small shoes and trauma from running or playing soccer, and presents with subungual hemorrhage and nail dystrophy of the first or second toenail. PC is a genetic disorder caused by a mutation in certain keratin proteins of the skin (k6a, k6b, K16 and K17). These patients usually have other skin findings including palmoplantar keratoderma, white plaques on the mouth, and skin cysts (steatocystoma multiplex and vellus hair cysts). Nail changes characteristic of PC includes subungual hyperkeratosis that causes a wedge shape thickening of the nail bed (pincer nails).5 Onychomadesis can be seen after viral infections such as hand-foot-mouth disease or in patients taking chemotherapy drugs that affect nail growth.
CMGTN usually resolves with time, but some patients with severe deviation and paronychia may need surgical correction.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. Dermatol Online J. 2014 Jan 15;20(1):21251.
2. J Dtsch Dermatol Ges. 2012 May;10(5):326-30.
3. J Am Acad Dermatol. 2007 Oct;57(4):711-5.
4. Pediatr Dermatol. 2004 Jan-Feb;21(1):44-7.
5. Curr Opin Pediatr. 2014 Aug;26(4):440-5.
6. Skin Appendage Disord. 2018 Oct;4(4):230-5.
A 4-year-old boy is brought to our pediatric dermatology clinic by his mother with the concern of difficult to treat toenail fungus.
The mother reported that she started noticing the toenail changes at around 8 months of age, and it has been progressively getting worse.
He has been treated with several courses of topical antifungals and 3 months of oral terbinafine without success.
A fungal culture done 1 year prior showed slight growth of Cladosporium Sp., but the nails failed to improve after systemic therapy. He denied any associated pain or inflammation. He likes playing softball and plays soccer sometimes. The mother is very worried because the father also has a history of onychomycosis that he has not been able to clear for years.
On physical exam, he is a very pleasant young boy. His cutaneous exam is normal including hair and teeth except for thickening of the bilateral first toenails associated with transverse ridging and yellow discoloration.
December 2018
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
November 2018
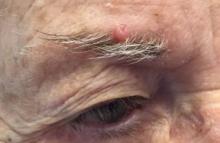
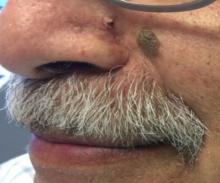
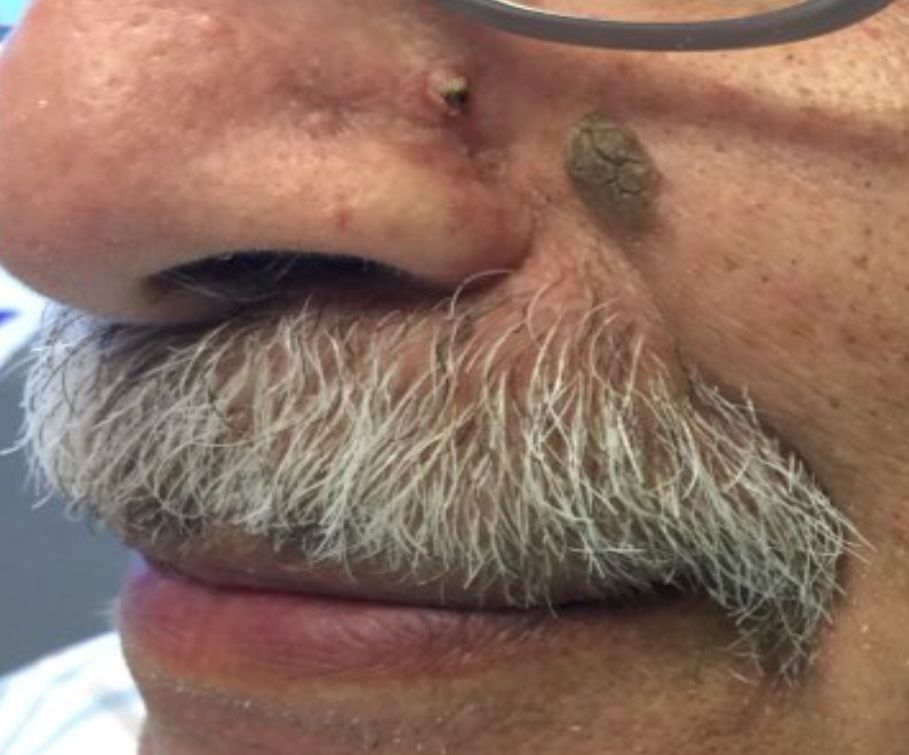
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
What is your diagnosis?
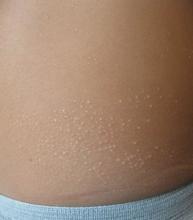
Lichen nitidus, which literally means “shiny moss,” is a relatively rare, chronic skin eruption that is characterized clinically by asymptomatic, flat-topped, sharply-demarcated, skin-colored papules, which are sometimes described as being “pinpoint.”
Lichen nitidus mainly affects children and young adults. The most common sites of involvement are the trunk, flexor aspects of upper extremities, dorsal aspects of hands, and genitalia, but lesions can occur anywhere on the skin. The lesions also can develop in sites of trauma (Koebner phenomenon), and this can be a significant clinical clue to aid in the diagnosis of lichen nitidus in favor of other conditions which may present as many small papules.1 Nail changes can occur but are rare, presenting as dystrophy, pitting, riding, or loss of nail(s).2
Lichen nitidus can be a challenging diagnosis to make, especially if a practitioner is not used to seeing it. Many dermatologic conditions present with many fine papules, including the other answer choices in the given quiz question (molluscum contagiosum, keratosis pilaris, verruca vulgaris, papular eczema). What allows for a clearer diagnosis of lichen nitidus is the history provided by the patient as well as the exam. Lichen nitidus lesions typically arise without a known trigger and often persist for months while remaining asymptomatic.
Molluscum contagiosum tends to include papules that are larger and more substantial than lichen nitidus papules and may be accompanied by background hyperpigmentation or erythema, known as the “beginning of the end” sign. Keratosis pilaris is commonly thought of as a skin type more so than a skin condition, and is more commonly seen in fair-skinned individuals along the lateral arms and cheeks. It is commonly paired with a background of erythema and skin than tends to be more xerotic. Verruca are typically larger lesions, sometimes with a rough surface, and are not typically shiny. Verruca are more likely to present as a single lesion or a few lesions at a given location, as opposed to lichen nitidus which has many individual papules at a single location. Papular eczema typically is intensely pruritic and is associated with xerosis and atopy.
The cause of lichen nitidus is unknown, and there are no reported genetic factors that contribute to its presentation.3 It is thought to be a subtype of lichen planus, although this is still debated. There is more work that needs to be done to find answers to these questions and to assess what triggers these fine papules to present and in whom.
The dermatoscopic features of lichen nitidus were reported in a series of eight cases and include absent dermatoglyphics, radial ridges, ill-defined hypopigmentation, diffuse erythema, linear vessels within the lesion, and peripheral scaling.4 These features are distinctive in combination and can in some cases be used to clinically diagnose lichen nitidus without the need for a skin biopsy, which is an invasive procedure that should be avoided when possible, especially given the benign nature of lichen nitidus.
If a biopsy is performed, the histologic features that commonly are seen include well-circumscribed granuloma-like lymphohistiocytic infiltrates in the papillary dermis adjacent to ridges, mimicking a “ball-and-claw” formation.1,5
Lichen nitidus generally is self-limiting, with minimal cosmetic disruption; therefore, treatment usually is not necessary. The lesions typically resolve within 1 year of presentation – and often sooner. Topical steroids can be used for symptomatic relief of pruritus, but generally do not hasten resolution of the papules themselves. Additionally, there have been reports in the literature of the successful resolution of lesions using topical calcineurin inhibitors and UVB therapy.
In a case report of an 8-year-old child with histologically confirmed lichen nitidus that had been present for 2 years, pimecrolimus 1% cream was used twice daily for 2 months with improvement and flattening of the papules.6 This report is compelling because the lesions persisted for twice the expected time of resolution without improvement and then showed relatively quick response to pimecrolimus. In a case of a 32-year-old male with lichen nitidus on his penis, tacrolimus 0.1% was used for 4 weeks with resolution of the papules.7 Although given that lichen nitidus can self-resolve in this same time period, it is unclear in this case whether the tacrolimus was the independent cause of the resolution.
With regard to UVB therapy, there have been reports of lichen nitidus resolution after 17-30 irradiation sessions in patients with lesions present for 3-6 months, although again, it is possible that the resolution observed was simply the natural course of the lichen nitidus for these patients rather than a therapeutic benefit of UVB therapy.8,9
Given that lichen nitidus is benign and typically asymptomatic or with mild pruritus, it is reasonable to monitor the lesions without treating them. If the lesions persist beyond 1 year, it also is reasonable to trial therapies, such as topical calcineurin inhibitors and UVB therapy, although using these treatments earlier in the disease course has only limited supporting data and any improvement seen within 1 year of onset may be attributed to the natural disease course as opposed to an effect of the intervention. Considerations of the cost of therapy, as well as the degree to which the patient is bothered by the lesions and how long the lesions have persisted, should be undertaken when considering whether an intervention should be made.
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Natsis or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Cutis. 1999 Aug 1;64(2):135-6.
2. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
3. Papulosquamous diseases, in “Pediatric Dermatology,” 4th ed. (St Louis: Mosby; 2011, Vol. 2.
4. Pediatr Dermatol. 2018. doi: 10.1111/pde.13576.
5. Cutis. 2013 Dec;92(6):288, 297-8.
6. Dermatol Online J. 2011 Jul 15;17(7):11.
7. J Drugs Dermatol. 2004 Nov-Dec;3(6):683-4.
8. Int J Dermatol. 2004 Dec 23;45:615-7.
9. Photodermatol Photoimmunol Photomed. 2013 Aug;29(4):215-7.
Lichen nitidus, which literally means “shiny moss,” is a relatively rare, chronic skin eruption that is characterized clinically by asymptomatic, flat-topped, sharply-demarcated, skin-colored papules, which are sometimes described as being “pinpoint.”
Lichen nitidus mainly affects children and young adults. The most common sites of involvement are the trunk, flexor aspects of upper extremities, dorsal aspects of hands, and genitalia, but lesions can occur anywhere on the skin. The lesions also can develop in sites of trauma (Koebner phenomenon), and this can be a significant clinical clue to aid in the diagnosis of lichen nitidus in favor of other conditions which may present as many small papules.1 Nail changes can occur but are rare, presenting as dystrophy, pitting, riding, or loss of nail(s).2
Lichen nitidus can be a challenging diagnosis to make, especially if a practitioner is not used to seeing it. Many dermatologic conditions present with many fine papules, including the other answer choices in the given quiz question (molluscum contagiosum, keratosis pilaris, verruca vulgaris, papular eczema). What allows for a clearer diagnosis of lichen nitidus is the history provided by the patient as well as the exam. Lichen nitidus lesions typically arise without a known trigger and often persist for months while remaining asymptomatic.
Molluscum contagiosum tends to include papules that are larger and more substantial than lichen nitidus papules and may be accompanied by background hyperpigmentation or erythema, known as the “beginning of the end” sign. Keratosis pilaris is commonly thought of as a skin type more so than a skin condition, and is more commonly seen in fair-skinned individuals along the lateral arms and cheeks. It is commonly paired with a background of erythema and skin than tends to be more xerotic. Verruca are typically larger lesions, sometimes with a rough surface, and are not typically shiny. Verruca are more likely to present as a single lesion or a few lesions at a given location, as opposed to lichen nitidus which has many individual papules at a single location. Papular eczema typically is intensely pruritic and is associated with xerosis and atopy.
The cause of lichen nitidus is unknown, and there are no reported genetic factors that contribute to its presentation.3 It is thought to be a subtype of lichen planus, although this is still debated. There is more work that needs to be done to find answers to these questions and to assess what triggers these fine papules to present and in whom.
The dermatoscopic features of lichen nitidus were reported in a series of eight cases and include absent dermatoglyphics, radial ridges, ill-defined hypopigmentation, diffuse erythema, linear vessels within the lesion, and peripheral scaling.4 These features are distinctive in combination and can in some cases be used to clinically diagnose lichen nitidus without the need for a skin biopsy, which is an invasive procedure that should be avoided when possible, especially given the benign nature of lichen nitidus.
If a biopsy is performed, the histologic features that commonly are seen include well-circumscribed granuloma-like lymphohistiocytic infiltrates in the papillary dermis adjacent to ridges, mimicking a “ball-and-claw” formation.1,5
Lichen nitidus generally is self-limiting, with minimal cosmetic disruption; therefore, treatment usually is not necessary. The lesions typically resolve within 1 year of presentation – and often sooner. Topical steroids can be used for symptomatic relief of pruritus, but generally do not hasten resolution of the papules themselves. Additionally, there have been reports in the literature of the successful resolution of lesions using topical calcineurin inhibitors and UVB therapy.
In a case report of an 8-year-old child with histologically confirmed lichen nitidus that had been present for 2 years, pimecrolimus 1% cream was used twice daily for 2 months with improvement and flattening of the papules.6 This report is compelling because the lesions persisted for twice the expected time of resolution without improvement and then showed relatively quick response to pimecrolimus. In a case of a 32-year-old male with lichen nitidus on his penis, tacrolimus 0.1% was used for 4 weeks with resolution of the papules.7 Although given that lichen nitidus can self-resolve in this same time period, it is unclear in this case whether the tacrolimus was the independent cause of the resolution.
With regard to UVB therapy, there have been reports of lichen nitidus resolution after 17-30 irradiation sessions in patients with lesions present for 3-6 months, although again, it is possible that the resolution observed was simply the natural course of the lichen nitidus for these patients rather than a therapeutic benefit of UVB therapy.8,9
Given that lichen nitidus is benign and typically asymptomatic or with mild pruritus, it is reasonable to monitor the lesions without treating them. If the lesions persist beyond 1 year, it also is reasonable to trial therapies, such as topical calcineurin inhibitors and UVB therapy, although using these treatments earlier in the disease course has only limited supporting data and any improvement seen within 1 year of onset may be attributed to the natural disease course as opposed to an effect of the intervention. Considerations of the cost of therapy, as well as the degree to which the patient is bothered by the lesions and how long the lesions have persisted, should be undertaken when considering whether an intervention should be made.
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Natsis or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Cutis. 1999 Aug 1;64(2):135-6.
2. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
3. Papulosquamous diseases, in “Pediatric Dermatology,” 4th ed. (St Louis: Mosby; 2011, Vol. 2.
4. Pediatr Dermatol. 2018. doi: 10.1111/pde.13576.
5. Cutis. 2013 Dec;92(6):288, 297-8.
6. Dermatol Online J. 2011 Jul 15;17(7):11.
7. J Drugs Dermatol. 2004 Nov-Dec;3(6):683-4.
8. Int J Dermatol. 2004 Dec 23;45:615-7.
9. Photodermatol Photoimmunol Photomed. 2013 Aug;29(4):215-7.
Lichen nitidus, which literally means “shiny moss,” is a relatively rare, chronic skin eruption that is characterized clinically by asymptomatic, flat-topped, sharply-demarcated, skin-colored papules, which are sometimes described as being “pinpoint.”
Lichen nitidus mainly affects children and young adults. The most common sites of involvement are the trunk, flexor aspects of upper extremities, dorsal aspects of hands, and genitalia, but lesions can occur anywhere on the skin. The lesions also can develop in sites of trauma (Koebner phenomenon), and this can be a significant clinical clue to aid in the diagnosis of lichen nitidus in favor of other conditions which may present as many small papules.1 Nail changes can occur but are rare, presenting as dystrophy, pitting, riding, or loss of nail(s).2
Lichen nitidus can be a challenging diagnosis to make, especially if a practitioner is not used to seeing it. Many dermatologic conditions present with many fine papules, including the other answer choices in the given quiz question (molluscum contagiosum, keratosis pilaris, verruca vulgaris, papular eczema). What allows for a clearer diagnosis of lichen nitidus is the history provided by the patient as well as the exam. Lichen nitidus lesions typically arise without a known trigger and often persist for months while remaining asymptomatic.
Molluscum contagiosum tends to include papules that are larger and more substantial than lichen nitidus papules and may be accompanied by background hyperpigmentation or erythema, known as the “beginning of the end” sign. Keratosis pilaris is commonly thought of as a skin type more so than a skin condition, and is more commonly seen in fair-skinned individuals along the lateral arms and cheeks. It is commonly paired with a background of erythema and skin than tends to be more xerotic. Verruca are typically larger lesions, sometimes with a rough surface, and are not typically shiny. Verruca are more likely to present as a single lesion or a few lesions at a given location, as opposed to lichen nitidus which has many individual papules at a single location. Papular eczema typically is intensely pruritic and is associated with xerosis and atopy.
The cause of lichen nitidus is unknown, and there are no reported genetic factors that contribute to its presentation.3 It is thought to be a subtype of lichen planus, although this is still debated. There is more work that needs to be done to find answers to these questions and to assess what triggers these fine papules to present and in whom.
The dermatoscopic features of lichen nitidus were reported in a series of eight cases and include absent dermatoglyphics, radial ridges, ill-defined hypopigmentation, diffuse erythema, linear vessels within the lesion, and peripheral scaling.4 These features are distinctive in combination and can in some cases be used to clinically diagnose lichen nitidus without the need for a skin biopsy, which is an invasive procedure that should be avoided when possible, especially given the benign nature of lichen nitidus.
If a biopsy is performed, the histologic features that commonly are seen include well-circumscribed granuloma-like lymphohistiocytic infiltrates in the papillary dermis adjacent to ridges, mimicking a “ball-and-claw” formation.1,5
Lichen nitidus generally is self-limiting, with minimal cosmetic disruption; therefore, treatment usually is not necessary. The lesions typically resolve within 1 year of presentation – and often sooner. Topical steroids can be used for symptomatic relief of pruritus, but generally do not hasten resolution of the papules themselves. Additionally, there have been reports in the literature of the successful resolution of lesions using topical calcineurin inhibitors and UVB therapy.
In a case report of an 8-year-old child with histologically confirmed lichen nitidus that had been present for 2 years, pimecrolimus 1% cream was used twice daily for 2 months with improvement and flattening of the papules.6 This report is compelling because the lesions persisted for twice the expected time of resolution without improvement and then showed relatively quick response to pimecrolimus. In a case of a 32-year-old male with lichen nitidus on his penis, tacrolimus 0.1% was used for 4 weeks with resolution of the papules.7 Although given that lichen nitidus can self-resolve in this same time period, it is unclear in this case whether the tacrolimus was the independent cause of the resolution.
With regard to UVB therapy, there have been reports of lichen nitidus resolution after 17-30 irradiation sessions in patients with lesions present for 3-6 months, although again, it is possible that the resolution observed was simply the natural course of the lichen nitidus for these patients rather than a therapeutic benefit of UVB therapy.8,9
Given that lichen nitidus is benign and typically asymptomatic or with mild pruritus, it is reasonable to monitor the lesions without treating them. If the lesions persist beyond 1 year, it also is reasonable to trial therapies, such as topical calcineurin inhibitors and UVB therapy, although using these treatments earlier in the disease course has only limited supporting data and any improvement seen within 1 year of onset may be attributed to the natural disease course as opposed to an effect of the intervention. Considerations of the cost of therapy, as well as the degree to which the patient is bothered by the lesions and how long the lesions have persisted, should be undertaken when considering whether an intervention should be made.
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Natsis or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Cutis. 1999 Aug 1;64(2):135-6.
2. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
3. Papulosquamous diseases, in “Pediatric Dermatology,” 4th ed. (St Louis: Mosby; 2011, Vol. 2.
4. Pediatr Dermatol. 2018. doi: 10.1111/pde.13576.
5. Cutis. 2013 Dec;92(6):288, 297-8.
6. Dermatol Online J. 2011 Jul 15;17(7):11.
7. J Drugs Dermatol. 2004 Nov-Dec;3(6):683-4.
8. Int J Dermatol. 2004 Dec 23;45:615-7.
9. Photodermatol Photoimmunol Photomed. 2013 Aug;29(4):215-7.
October 2018
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
A 30-year-old female presented with 2 days of intensely pruritic erythematous papules and vesicles on her bilateral arms and hands. The lesions began appearing 1 day after a camping trip. Her neck, chest, and upper back were clear.
Make The Diagnosis - September 2018
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
What is your diagnosis?
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
A 16-year-old female came to the dermatology clinic for acne follow-up. She reported some improvement on her acne since she started taking OCs. She also had been using benzoyl peroxide and tretinoin on her face. In addition to the acne, she also wanted us to check some tender bumps she had been getting on her shins after she came back from a camping trip. Initially she thought they were bug bites, but the lesions were getting larger, more tender, and not improving with diphenhydramine.
The physical exam did not reveal acute distress. She was afebrile. On skin examination, she had comedones, papules and scars on her face, chest, and back. On her shins there were several erythematous tender nodules and plaques. There was no edema on her legs and pulses were present.
Make The Diagnosis - August 2018
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
A 28-year-old white female with no significant past medical history presents with a 10-year history of asymptomatic erythematous papules and scaly patches that come and go. She has used topical steroids in the past.