User login
How long is it safe to delay gynecologic cancer surgery?
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
How should we monitor for ovarian cancer recurrence?
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Disparity in endometrial cancer outcomes: What can we do?
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
Ovarian tumor markers: What to draw and when
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.
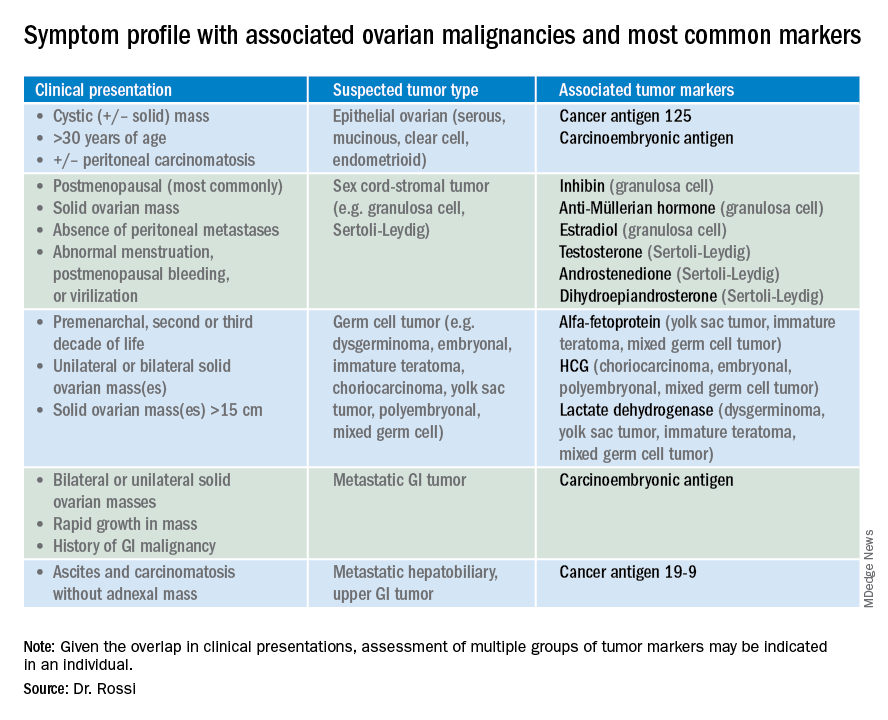
So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Ovarian cancer and perineal talc exposure: An epidemiologic dilemma
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
What is the future of para-aortic lymphadenectomy for endometrial cancer?
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
Preventing delayed genitourinary tract injury during benign hysterectomy
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
Discuss compounded bioidentical hormones and cancer risk
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
What is the ‘microbiome’ and how may it influence gynecologic cancers?
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.