User login
Surgery for early-stage cervical cancer: Are we still too radical?
It has been more than 120 years since Ernst Wertheim, a Viennese surgeon, performed and described what is considered to have been the first radical total hysterectomy with lymphadenectomy for early-stage cervical cancer, yet this morbid procedure remains the standard of care for most early-stage cervical cancers. The rationale for this procedure, which included removal of the parametrial tissue, uterosacral and cardinal ligaments, and upper vagina en bloc with the cervix and uterus, was to obtain margins around a cancer that has a dominant radial growth pattern. The morbidity associated with this procedure is substantial. The parametrium houses important vascular, neural, and urologic structures. Unlike extrafascial hysterectomy, often referred to as “simple” hysterectomy, in which surgeons follow a fascial plane, and therefore a relatively avascular dissection, surgeons performing radical hysterectomy must venture outside of these embryologic fusion planes into less well–defined anatomy. Therefore, surgical complications are relatively common including hemorrhage, ureteral and bladder injury, as well as late-onset devastating complications such as fistula, urinary retention, or incontinence, and sexual dysfunction.1 More recently, variations of the Wertheim-Meigs radical hysterectomy have been described, and objective classifications created, which include modified radical procedures (removing less parametria) and nerve-sparing procedures to facilitate standardized nomenclature for tailoring the most appropriate procedure for any given tumor.2
The trend, and a positive one at that, over the course of the past century, has been a move away from routine radical surgical procedures for most clinical stage 1 cancers. No better example exists than breast cancer, in which the Halsted radical mastectomy has been largely replaced by less morbid breast-conserving or nonradical procedures with adjunct medical and radiation therapies offered to achieve high rates of cure with far more acceptable patient-centered outcomes.3 And so why is it that radical hysterectomy is still considered the standard of care for all but the smallest of microscopic cervical cancers?
The risk of lymph node metastases or recurrence is exceptionally low for women with microscopic (stage IA1) cervical cancers that are less than 3 mm in depth. Therefore, the National Comprehensive Cancer Network guidelines recommend nonradical surgical remedies (such as extrafascial hysterectomy, or cone biopsy or trachelectomy if fertility preservation is desired) for this earlier stage of disease.4 If there is lymphovascular space invasion (an indicator of poor prognosis and potential lymphatic involvement), a lymphadenectomy or sentinel lymph node biopsy is also recommended. For women with stage IA2 or IB lesions, radical excisions (either trachelectomy or hysterectomy) are considered the standard of care. However, this “gold standard” was achieved largely through legacy, and not a result of randomized trials comparing its outcomes with nonradical procedures.
Initial strides away from radical cervical cancer surgery focused on the goal of fertility preservation via radical trachelectomy which allowed women to preserve an intact uterine fundus. This was initially met with skepticism and concern that surgeons could be sacrificing oncologic outcomes in order to preserve a woman’s fertility. Thanks to pioneering work, including prospective research studies by surgeon innovators it has been shown that, in appropriately selected candidates with tumors less than 2 cm, it is an accepted standard of care.4 Radical vaginal or abdominal trachelectomy is associated with cancer recurrence rates of less than 5% and successful pregnancy in approximately three-quarters of patients in whom this is desired.5,6 However, full-term pregnancy is achieved in 50%-75% of cases, reflecting increased obstetric risk, and radical trachelectomy still subjects patients to the morbidity of a radical parametrial resection, despite the fact that many of them will have no residual carcinoma in their final pathological specimens.
Therefore, can we be even more conservative in our surgery for these patients? Are simple hysterectomy or conization potentially adequate treatments for small (<2 cm) stage IA2 and IB1 lesions that have favorable histology (<10 mm stromal invasion, low-risk histology, no lymphovascular space involvement, negative margins on conization and no lymph node metastases)? In patients whose tumor exhibits these histologic features, the likelihood of parametrial involvement is approximately 1%, calling into question the virtue of parametrial resection.7 Observational studies have identified mixed results on the safety of conservative surgical techniques in early-stage cervical cancer. In a study of the National Cancer Database, the outcomes of 2,543 radical hysterectomies and 1,388 extrafascial hysterectomies for women with stage IB1 disease were evaluated and observed a difference in 5-year survival (92.4% vs. 95.3%) favoring the radical procedure.8 Unfortunately, database analyses such as these are limited by potential confounders and discordance between the groups such as rates of lymphadenectomy, known involvement of oncologic surgeon specialists, and margin status. An alternative evaluation of the Surveillance, Epidemiology, and End Results database including 2,571 patients with stage IB1 disease, all of whom had lymphadenectomy performed, showed no difference in 10-year disease-specific survival between the two surgical approaches.9
Ultimately, whether conservative procedures (such as conization or extrafascial hysterectomy) can be offered to women with small, low-risk IB1 or IA2 cervical cancers will be best determined by prospective single-arm or randomized trials. Fortunately, these are underway. Preliminary results from the ConCerv trial in which 100 women with early-stage, low-risk stage IA2 and IB1 cervical cancer were treated with either repeat conization or extrafascial hysterectomy with sentinel lymph node biopsy showed acceptably low rates of recurrence (3%) with this approach.10 If the mature data supports this finding, it seems that, for appropriately selected and well-counseled patients, conservative surgery may become more broadly accepted as a reasonable option for treatment that spares women not only loss of fertility, but also the early and late surgical morbidity from radical procedures.
In the meantime, until more is known about the oncologic safety of nonradical procedures for stage IA2 and IB1 cervical cancer, this option should not be considered standard of care, and only offered to patients with favorable tumor factors who are well counseled regarding the uncertainty of this approach. It is critical that patients with early-stage cervical cancer be evaluated by a gynecologic cancer specialist prior to definitive surgical treatment as they are best equipped to evaluate risk profiles and counsel about her options for surgery, its known and unknown consequences, and the appropriateness of fertility preservation or radicality of surgery. We eagerly await the results of trials evaluating the safety of conservative cervical cancer surgery, which promise to advance us from 19th-century practices, preserving not only fertility, but also quality of life.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures and can be contacted at obnews@mdedge.com.
References
1. Trimbos JB et al. Eur J Cancer. 2004;40(3):375-8.
2. Querleu D and Morrow CP. Lancet Oncol. 2008;9:297-303.
3. Sakorafas GH and Safioleas M. Eur J Cancer Care. 2010 Mar;19(2):145-66.
4. National Comprehensive Cancer Network. Cervical Cancer (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed 2021 Apr 21.
5. Plante M et al. Gynecol Oncol. 2011;121:290-7.
6. Wethington SL et al. Int J Gynecol Cancer. 2012;22:1251-7.
7. Domgue J and Schmeler K. Best Pract Res Clin Obstet Gynaecol. 2019 Feb;55:79-92.
8. Sia TY et al. Obstet Gyenecol. 2019;134(6):1132.
9. Tseng J et al. Gynecol Oncol. 2018;150(1):44.
10. Schmeler K et al. Int J Gynecol Cancer. 2019;29:A14-5.
It has been more than 120 years since Ernst Wertheim, a Viennese surgeon, performed and described what is considered to have been the first radical total hysterectomy with lymphadenectomy for early-stage cervical cancer, yet this morbid procedure remains the standard of care for most early-stage cervical cancers. The rationale for this procedure, which included removal of the parametrial tissue, uterosacral and cardinal ligaments, and upper vagina en bloc with the cervix and uterus, was to obtain margins around a cancer that has a dominant radial growth pattern. The morbidity associated with this procedure is substantial. The parametrium houses important vascular, neural, and urologic structures. Unlike extrafascial hysterectomy, often referred to as “simple” hysterectomy, in which surgeons follow a fascial plane, and therefore a relatively avascular dissection, surgeons performing radical hysterectomy must venture outside of these embryologic fusion planes into less well–defined anatomy. Therefore, surgical complications are relatively common including hemorrhage, ureteral and bladder injury, as well as late-onset devastating complications such as fistula, urinary retention, or incontinence, and sexual dysfunction.1 More recently, variations of the Wertheim-Meigs radical hysterectomy have been described, and objective classifications created, which include modified radical procedures (removing less parametria) and nerve-sparing procedures to facilitate standardized nomenclature for tailoring the most appropriate procedure for any given tumor.2
The trend, and a positive one at that, over the course of the past century, has been a move away from routine radical surgical procedures for most clinical stage 1 cancers. No better example exists than breast cancer, in which the Halsted radical mastectomy has been largely replaced by less morbid breast-conserving or nonradical procedures with adjunct medical and radiation therapies offered to achieve high rates of cure with far more acceptable patient-centered outcomes.3 And so why is it that radical hysterectomy is still considered the standard of care for all but the smallest of microscopic cervical cancers?
The risk of lymph node metastases or recurrence is exceptionally low for women with microscopic (stage IA1) cervical cancers that are less than 3 mm in depth. Therefore, the National Comprehensive Cancer Network guidelines recommend nonradical surgical remedies (such as extrafascial hysterectomy, or cone biopsy or trachelectomy if fertility preservation is desired) for this earlier stage of disease.4 If there is lymphovascular space invasion (an indicator of poor prognosis and potential lymphatic involvement), a lymphadenectomy or sentinel lymph node biopsy is also recommended. For women with stage IA2 or IB lesions, radical excisions (either trachelectomy or hysterectomy) are considered the standard of care. However, this “gold standard” was achieved largely through legacy, and not a result of randomized trials comparing its outcomes with nonradical procedures.
Initial strides away from radical cervical cancer surgery focused on the goal of fertility preservation via radical trachelectomy which allowed women to preserve an intact uterine fundus. This was initially met with skepticism and concern that surgeons could be sacrificing oncologic outcomes in order to preserve a woman’s fertility. Thanks to pioneering work, including prospective research studies by surgeon innovators it has been shown that, in appropriately selected candidates with tumors less than 2 cm, it is an accepted standard of care.4 Radical vaginal or abdominal trachelectomy is associated with cancer recurrence rates of less than 5% and successful pregnancy in approximately three-quarters of patients in whom this is desired.5,6 However, full-term pregnancy is achieved in 50%-75% of cases, reflecting increased obstetric risk, and radical trachelectomy still subjects patients to the morbidity of a radical parametrial resection, despite the fact that many of them will have no residual carcinoma in their final pathological specimens.
Therefore, can we be even more conservative in our surgery for these patients? Are simple hysterectomy or conization potentially adequate treatments for small (<2 cm) stage IA2 and IB1 lesions that have favorable histology (<10 mm stromal invasion, low-risk histology, no lymphovascular space involvement, negative margins on conization and no lymph node metastases)? In patients whose tumor exhibits these histologic features, the likelihood of parametrial involvement is approximately 1%, calling into question the virtue of parametrial resection.7 Observational studies have identified mixed results on the safety of conservative surgical techniques in early-stage cervical cancer. In a study of the National Cancer Database, the outcomes of 2,543 radical hysterectomies and 1,388 extrafascial hysterectomies for women with stage IB1 disease were evaluated and observed a difference in 5-year survival (92.4% vs. 95.3%) favoring the radical procedure.8 Unfortunately, database analyses such as these are limited by potential confounders and discordance between the groups such as rates of lymphadenectomy, known involvement of oncologic surgeon specialists, and margin status. An alternative evaluation of the Surveillance, Epidemiology, and End Results database including 2,571 patients with stage IB1 disease, all of whom had lymphadenectomy performed, showed no difference in 10-year disease-specific survival between the two surgical approaches.9
Ultimately, whether conservative procedures (such as conization or extrafascial hysterectomy) can be offered to women with small, low-risk IB1 or IA2 cervical cancers will be best determined by prospective single-arm or randomized trials. Fortunately, these are underway. Preliminary results from the ConCerv trial in which 100 women with early-stage, low-risk stage IA2 and IB1 cervical cancer were treated with either repeat conization or extrafascial hysterectomy with sentinel lymph node biopsy showed acceptably low rates of recurrence (3%) with this approach.10 If the mature data supports this finding, it seems that, for appropriately selected and well-counseled patients, conservative surgery may become more broadly accepted as a reasonable option for treatment that spares women not only loss of fertility, but also the early and late surgical morbidity from radical procedures.
In the meantime, until more is known about the oncologic safety of nonradical procedures for stage IA2 and IB1 cervical cancer, this option should not be considered standard of care, and only offered to patients with favorable tumor factors who are well counseled regarding the uncertainty of this approach. It is critical that patients with early-stage cervical cancer be evaluated by a gynecologic cancer specialist prior to definitive surgical treatment as they are best equipped to evaluate risk profiles and counsel about her options for surgery, its known and unknown consequences, and the appropriateness of fertility preservation or radicality of surgery. We eagerly await the results of trials evaluating the safety of conservative cervical cancer surgery, which promise to advance us from 19th-century practices, preserving not only fertility, but also quality of life.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures and can be contacted at obnews@mdedge.com.
References
1. Trimbos JB et al. Eur J Cancer. 2004;40(3):375-8.
2. Querleu D and Morrow CP. Lancet Oncol. 2008;9:297-303.
3. Sakorafas GH and Safioleas M. Eur J Cancer Care. 2010 Mar;19(2):145-66.
4. National Comprehensive Cancer Network. Cervical Cancer (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed 2021 Apr 21.
5. Plante M et al. Gynecol Oncol. 2011;121:290-7.
6. Wethington SL et al. Int J Gynecol Cancer. 2012;22:1251-7.
7. Domgue J and Schmeler K. Best Pract Res Clin Obstet Gynaecol. 2019 Feb;55:79-92.
8. Sia TY et al. Obstet Gyenecol. 2019;134(6):1132.
9. Tseng J et al. Gynecol Oncol. 2018;150(1):44.
10. Schmeler K et al. Int J Gynecol Cancer. 2019;29:A14-5.
It has been more than 120 years since Ernst Wertheim, a Viennese surgeon, performed and described what is considered to have been the first radical total hysterectomy with lymphadenectomy for early-stage cervical cancer, yet this morbid procedure remains the standard of care for most early-stage cervical cancers. The rationale for this procedure, which included removal of the parametrial tissue, uterosacral and cardinal ligaments, and upper vagina en bloc with the cervix and uterus, was to obtain margins around a cancer that has a dominant radial growth pattern. The morbidity associated with this procedure is substantial. The parametrium houses important vascular, neural, and urologic structures. Unlike extrafascial hysterectomy, often referred to as “simple” hysterectomy, in which surgeons follow a fascial plane, and therefore a relatively avascular dissection, surgeons performing radical hysterectomy must venture outside of these embryologic fusion planes into less well–defined anatomy. Therefore, surgical complications are relatively common including hemorrhage, ureteral and bladder injury, as well as late-onset devastating complications such as fistula, urinary retention, or incontinence, and sexual dysfunction.1 More recently, variations of the Wertheim-Meigs radical hysterectomy have been described, and objective classifications created, which include modified radical procedures (removing less parametria) and nerve-sparing procedures to facilitate standardized nomenclature for tailoring the most appropriate procedure for any given tumor.2
The trend, and a positive one at that, over the course of the past century, has been a move away from routine radical surgical procedures for most clinical stage 1 cancers. No better example exists than breast cancer, in which the Halsted radical mastectomy has been largely replaced by less morbid breast-conserving or nonradical procedures with adjunct medical and radiation therapies offered to achieve high rates of cure with far more acceptable patient-centered outcomes.3 And so why is it that radical hysterectomy is still considered the standard of care for all but the smallest of microscopic cervical cancers?
The risk of lymph node metastases or recurrence is exceptionally low for women with microscopic (stage IA1) cervical cancers that are less than 3 mm in depth. Therefore, the National Comprehensive Cancer Network guidelines recommend nonradical surgical remedies (such as extrafascial hysterectomy, or cone biopsy or trachelectomy if fertility preservation is desired) for this earlier stage of disease.4 If there is lymphovascular space invasion (an indicator of poor prognosis and potential lymphatic involvement), a lymphadenectomy or sentinel lymph node biopsy is also recommended. For women with stage IA2 or IB lesions, radical excisions (either trachelectomy or hysterectomy) are considered the standard of care. However, this “gold standard” was achieved largely through legacy, and not a result of randomized trials comparing its outcomes with nonradical procedures.
Initial strides away from radical cervical cancer surgery focused on the goal of fertility preservation via radical trachelectomy which allowed women to preserve an intact uterine fundus. This was initially met with skepticism and concern that surgeons could be sacrificing oncologic outcomes in order to preserve a woman’s fertility. Thanks to pioneering work, including prospective research studies by surgeon innovators it has been shown that, in appropriately selected candidates with tumors less than 2 cm, it is an accepted standard of care.4 Radical vaginal or abdominal trachelectomy is associated with cancer recurrence rates of less than 5% and successful pregnancy in approximately three-quarters of patients in whom this is desired.5,6 However, full-term pregnancy is achieved in 50%-75% of cases, reflecting increased obstetric risk, and radical trachelectomy still subjects patients to the morbidity of a radical parametrial resection, despite the fact that many of them will have no residual carcinoma in their final pathological specimens.
Therefore, can we be even more conservative in our surgery for these patients? Are simple hysterectomy or conization potentially adequate treatments for small (<2 cm) stage IA2 and IB1 lesions that have favorable histology (<10 mm stromal invasion, low-risk histology, no lymphovascular space involvement, negative margins on conization and no lymph node metastases)? In patients whose tumor exhibits these histologic features, the likelihood of parametrial involvement is approximately 1%, calling into question the virtue of parametrial resection.7 Observational studies have identified mixed results on the safety of conservative surgical techniques in early-stage cervical cancer. In a study of the National Cancer Database, the outcomes of 2,543 radical hysterectomies and 1,388 extrafascial hysterectomies for women with stage IB1 disease were evaluated and observed a difference in 5-year survival (92.4% vs. 95.3%) favoring the radical procedure.8 Unfortunately, database analyses such as these are limited by potential confounders and discordance between the groups such as rates of lymphadenectomy, known involvement of oncologic surgeon specialists, and margin status. An alternative evaluation of the Surveillance, Epidemiology, and End Results database including 2,571 patients with stage IB1 disease, all of whom had lymphadenectomy performed, showed no difference in 10-year disease-specific survival between the two surgical approaches.9
Ultimately, whether conservative procedures (such as conization or extrafascial hysterectomy) can be offered to women with small, low-risk IB1 or IA2 cervical cancers will be best determined by prospective single-arm or randomized trials. Fortunately, these are underway. Preliminary results from the ConCerv trial in which 100 women with early-stage, low-risk stage IA2 and IB1 cervical cancer were treated with either repeat conization or extrafascial hysterectomy with sentinel lymph node biopsy showed acceptably low rates of recurrence (3%) with this approach.10 If the mature data supports this finding, it seems that, for appropriately selected and well-counseled patients, conservative surgery may become more broadly accepted as a reasonable option for treatment that spares women not only loss of fertility, but also the early and late surgical morbidity from radical procedures.
In the meantime, until more is known about the oncologic safety of nonradical procedures for stage IA2 and IB1 cervical cancer, this option should not be considered standard of care, and only offered to patients with favorable tumor factors who are well counseled regarding the uncertainty of this approach. It is critical that patients with early-stage cervical cancer be evaluated by a gynecologic cancer specialist prior to definitive surgical treatment as they are best equipped to evaluate risk profiles and counsel about her options for surgery, its known and unknown consequences, and the appropriateness of fertility preservation or radicality of surgery. We eagerly await the results of trials evaluating the safety of conservative cervical cancer surgery, which promise to advance us from 19th-century practices, preserving not only fertility, but also quality of life.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures and can be contacted at obnews@mdedge.com.
References
1. Trimbos JB et al. Eur J Cancer. 2004;40(3):375-8.
2. Querleu D and Morrow CP. Lancet Oncol. 2008;9:297-303.
3. Sakorafas GH and Safioleas M. Eur J Cancer Care. 2010 Mar;19(2):145-66.
4. National Comprehensive Cancer Network. Cervical Cancer (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed 2021 Apr 21.
5. Plante M et al. Gynecol Oncol. 2011;121:290-7.
6. Wethington SL et al. Int J Gynecol Cancer. 2012;22:1251-7.
7. Domgue J and Schmeler K. Best Pract Res Clin Obstet Gynaecol. 2019 Feb;55:79-92.
8. Sia TY et al. Obstet Gyenecol. 2019;134(6):1132.
9. Tseng J et al. Gynecol Oncol. 2018;150(1):44.
10. Schmeler K et al. Int J Gynecol Cancer. 2019;29:A14-5.
The significance of mismatch repair deficiency in endometrial cancer
Women with Lynch syndrome are known to carry an approximately 60% lifetime risk of endometrial cancer. These cancers result from inherited deleterious mutations in genes that code for mismatch repair proteins. However, mismatch repair deficiency (MMR-d) is not exclusively found in the tumors of patients with Lynch syndrome, and much is being learned about this group of endometrial cancers, their behavior, and their vulnerability to targeted therapies.
During the processes of DNA replication, recombination, or chemical and physical damage, mismatches in base pairs frequently occurs. Mismatch repair proteins function to identify and repair such errors, and the loss of their function causes the accumulation of the insertions or deletions of short, repetitive sequences of DNA. This phenomenon can be measured using polymerase chain reaction (PCR) screening of known microsatellites to look for the accumulation of errors, a phenotype which is called microsatellite instability (MSI). The accumulation of errors in DNA sequences is thought to lead to mutations in cancer-related genes.
The four predominant mismatch repair genes include MLH1, MSH2, MSH 6, and PMS2. These genes may possess loss of function through a germline/inherited mechanism, such as Lynch syndrome, or can be sporadically acquired. Approximately 20%-30% of endometrial cancers exhibit MMR-d with acquired, sporadic losses in function being the majority of cases and only approximately 10% a result of Lynch syndrome. Mutations in PMS2 are the dominant genotype of Lynch syndrome, whereas loss of function in MLH1 is most frequent aberration in sporadic cases of MMR-d endometrial cancer.1
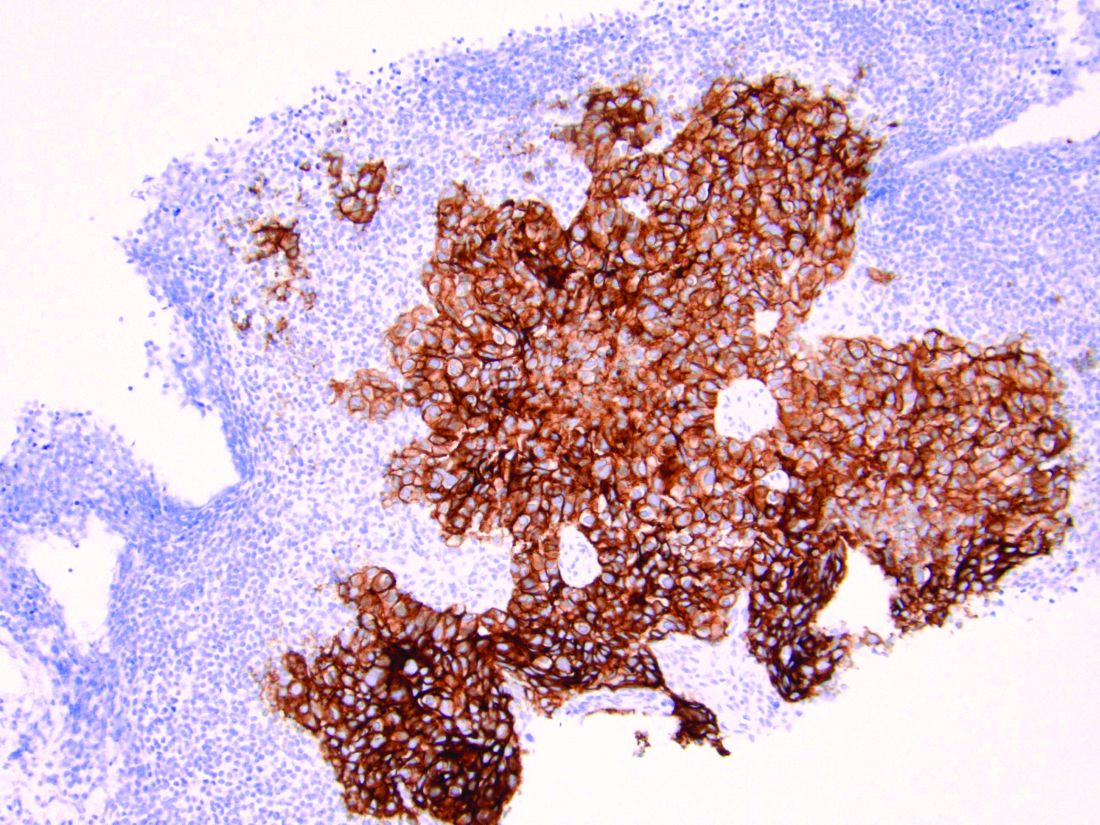
Endometrial cancers can be tested for MMR-d by performing immunohistochemistry to look for loss of expression in the four most common MMR genes. If there is loss of expression of MLH1, additional triage testing can be performed to determine if this loss is caused by the epigenetic phenomenon of hypermethylation. When present, this excludes Lynch syndrome and suggests a sporadic form origin of the disease. If there is loss of expression of the MMR genes (including loss of MLH1 and subsequent negative testing for promotor methylation), the patient should receive genetic testing for the presence of a germline mutation indicating Lynch syndrome. As an adjunct or alternative to immunohistochemistry, PCR studies or next-generation sequencing can be used to measure the presence of microsatellite instability in a process that identifies the expansion or reduction in repetitive DNA sequences of the tumor, compared with normal tumor.2
It is of the highest importance to identify endometrial cancers caused by Lynch syndrome because this enables providers to offer cascade testing of relatives, and to intensify screening or preventative measures for the many other cancers (such as colon, upper gastrointestinal, breast, and urothelial) for which these patients are at risk. Therefore, routine screening for MMR-d tumors is recommended in all cases of endometrial cancer, not simply those of a young age at diagnosis or for whom a strong family history exists.3 Using family history factors, primary tumor site, and age as a trigger for screening for Lynch syndrome, such as the Bethesda Guidelines, is associated with a 82% sensitivity in identifying Lynch syndrome. In a meta-analysis including testing results from 1,159 women with endometrial cancer, 43% of patients who were diagnosed with Lynch syndrome via molecular analysis would have been missed by clinical screening using Bethesda Guidelines.2
Discovering cases of Lynch syndrome is not the only benefit of routine testing for MMR-d in endometrial cancers. There is also significant value in the characterization of sporadic mismatch repair–deficient tumors because this information provides prognostic information and guides therapy. Tumors with a microsatellite-high phenotype/MMR-d were identified as one of the four distinct molecular subgroups of endometrial cancer by the Cancer Genome Atlas.4 Patients with this molecular profile exhibited “intermediate” prognostic outcomes, performing better than the “serous-like” cancers with p53 mutations, yet worse than patients with a POLE ultramutated group who rarely experience recurrences or death, even in the setting of unfavorable histology.
Beyond prognostication, the molecular profile of endometrial cancers also influence their responsiveness to therapeutics, highlighting the importance of splitting, not lumping endometrial cancers into relevant molecular subgroups when designing research and practicing clinical medicine. The PORTEC-3 trial studied 410 women with high-risk endometrial cancer, and randomized participants to receive either adjuvant radiation alone, or radiation with chemotherapy.5 There were no differences in progression-free survival between the two therapeutic strategies when analyzed in aggregate. However, when analyzed by Cancer Genome Atlas molecular subgroup, it was noted that there was a clear benefit from chemotherapy for patients with p53 mutations. For patients with MMR-d tumors, no such benefit was observed. Patients assigned this molecular subgroup did no better with the addition of platinum and taxane chemotherapy over radiation alone. Unfortunately, for patients with MMR-d tumors, recurrence rates remained high, suggesting that we can and need to discover more effective therapies for these tumors than what is available with conventional radiation or platinum and taxane chemotherapy. Targeted therapy may be the solution to this problem. Through microsatellite instability, MMR-d tumors create somatic mutations which result in neoantigens, an immunogenic environment. This state up-regulates checkpoint inhibitor proteins, which serve as an actionable target for anti-PD-L1 antibodies, such as the drug pembrolizumab which has been shown to be highly active against MMR-d endometrial cancer. In the landmark, KEYNOTE-158 trial, patients with advanced, recurrent solid tumors that exhibited MMR-d were treated with pembrolizumab.6 This included 49 patients with endometrial cancer, among whom there was a 79% response rate. Subsequently, pembrolizumab was granted Food and Drug Administration approval for use in advanced, recurrent MMR-d/MSI-high endometrial cancer. Trials are currently enrolling patients to explore the utility of this drug in the up-front setting in both early- and late-stage disease with a hope that this targeted therapy can do what conventional cytotoxic chemotherapy has failed to do.
Therefore, given the clinical significance of mismatch repair deficiency, all patients with endometrial cancer should be investigated for loss of expression in these proteins, and if present, considered for the possibility of Lynch syndrome. While most will not have an inherited cause, this information regarding their tumor biology remains critically important in both prognostication and decision-making surrounding other therapies and their eligibility for promising clinical trials.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Simpkins SB et al. Hum. Mol. Genet. 1999;8:661-6.
2. Kahn R et al. Cancer. 2019 Sep 15;125(18):2172-3183.
3. SGO Clinical Practice Statement: Screening for Lynch Syndrome in Endometrial Cancer. https://www.sgo.org/clinical-practice/guidelines/screening-for-lynch-syndrome-in-endometrial-cancer/
4. Kandoth et al. Nature. 2013;497(7447):67-73.
5. Leon-Castillo A et al. J Clin Oncol. 2020 Oct 10;38(29):3388-97.
6. Marabelle A et al. J Clin Oncol. 2020 Jan 1;38(1):1-10.
Women with Lynch syndrome are known to carry an approximately 60% lifetime risk of endometrial cancer. These cancers result from inherited deleterious mutations in genes that code for mismatch repair proteins. However, mismatch repair deficiency (MMR-d) is not exclusively found in the tumors of patients with Lynch syndrome, and much is being learned about this group of endometrial cancers, their behavior, and their vulnerability to targeted therapies.
During the processes of DNA replication, recombination, or chemical and physical damage, mismatches in base pairs frequently occurs. Mismatch repair proteins function to identify and repair such errors, and the loss of their function causes the accumulation of the insertions or deletions of short, repetitive sequences of DNA. This phenomenon can be measured using polymerase chain reaction (PCR) screening of known microsatellites to look for the accumulation of errors, a phenotype which is called microsatellite instability (MSI). The accumulation of errors in DNA sequences is thought to lead to mutations in cancer-related genes.
The four predominant mismatch repair genes include MLH1, MSH2, MSH 6, and PMS2. These genes may possess loss of function through a germline/inherited mechanism, such as Lynch syndrome, or can be sporadically acquired. Approximately 20%-30% of endometrial cancers exhibit MMR-d with acquired, sporadic losses in function being the majority of cases and only approximately 10% a result of Lynch syndrome. Mutations in PMS2 are the dominant genotype of Lynch syndrome, whereas loss of function in MLH1 is most frequent aberration in sporadic cases of MMR-d endometrial cancer.1
Endometrial cancers can be tested for MMR-d by performing immunohistochemistry to look for loss of expression in the four most common MMR genes. If there is loss of expression of MLH1, additional triage testing can be performed to determine if this loss is caused by the epigenetic phenomenon of hypermethylation. When present, this excludes Lynch syndrome and suggests a sporadic form origin of the disease. If there is loss of expression of the MMR genes (including loss of MLH1 and subsequent negative testing for promotor methylation), the patient should receive genetic testing for the presence of a germline mutation indicating Lynch syndrome. As an adjunct or alternative to immunohistochemistry, PCR studies or next-generation sequencing can be used to measure the presence of microsatellite instability in a process that identifies the expansion or reduction in repetitive DNA sequences of the tumor, compared with normal tumor.2
It is of the highest importance to identify endometrial cancers caused by Lynch syndrome because this enables providers to offer cascade testing of relatives, and to intensify screening or preventative measures for the many other cancers (such as colon, upper gastrointestinal, breast, and urothelial) for which these patients are at risk. Therefore, routine screening for MMR-d tumors is recommended in all cases of endometrial cancer, not simply those of a young age at diagnosis or for whom a strong family history exists.3 Using family history factors, primary tumor site, and age as a trigger for screening for Lynch syndrome, such as the Bethesda Guidelines, is associated with a 82% sensitivity in identifying Lynch syndrome. In a meta-analysis including testing results from 1,159 women with endometrial cancer, 43% of patients who were diagnosed with Lynch syndrome via molecular analysis would have been missed by clinical screening using Bethesda Guidelines.2
Discovering cases of Lynch syndrome is not the only benefit of routine testing for MMR-d in endometrial cancers. There is also significant value in the characterization of sporadic mismatch repair–deficient tumors because this information provides prognostic information and guides therapy. Tumors with a microsatellite-high phenotype/MMR-d were identified as one of the four distinct molecular subgroups of endometrial cancer by the Cancer Genome Atlas.4 Patients with this molecular profile exhibited “intermediate” prognostic outcomes, performing better than the “serous-like” cancers with p53 mutations, yet worse than patients with a POLE ultramutated group who rarely experience recurrences or death, even in the setting of unfavorable histology.
Beyond prognostication, the molecular profile of endometrial cancers also influence their responsiveness to therapeutics, highlighting the importance of splitting, not lumping endometrial cancers into relevant molecular subgroups when designing research and practicing clinical medicine. The PORTEC-3 trial studied 410 women with high-risk endometrial cancer, and randomized participants to receive either adjuvant radiation alone, or radiation with chemotherapy.5 There were no differences in progression-free survival between the two therapeutic strategies when analyzed in aggregate. However, when analyzed by Cancer Genome Atlas molecular subgroup, it was noted that there was a clear benefit from chemotherapy for patients with p53 mutations. For patients with MMR-d tumors, no such benefit was observed. Patients assigned this molecular subgroup did no better with the addition of platinum and taxane chemotherapy over radiation alone. Unfortunately, for patients with MMR-d tumors, recurrence rates remained high, suggesting that we can and need to discover more effective therapies for these tumors than what is available with conventional radiation or platinum and taxane chemotherapy. Targeted therapy may be the solution to this problem. Through microsatellite instability, MMR-d tumors create somatic mutations which result in neoantigens, an immunogenic environment. This state up-regulates checkpoint inhibitor proteins, which serve as an actionable target for anti-PD-L1 antibodies, such as the drug pembrolizumab which has been shown to be highly active against MMR-d endometrial cancer. In the landmark, KEYNOTE-158 trial, patients with advanced, recurrent solid tumors that exhibited MMR-d were treated with pembrolizumab.6 This included 49 patients with endometrial cancer, among whom there was a 79% response rate. Subsequently, pembrolizumab was granted Food and Drug Administration approval for use in advanced, recurrent MMR-d/MSI-high endometrial cancer. Trials are currently enrolling patients to explore the utility of this drug in the up-front setting in both early- and late-stage disease with a hope that this targeted therapy can do what conventional cytotoxic chemotherapy has failed to do.
Therefore, given the clinical significance of mismatch repair deficiency, all patients with endometrial cancer should be investigated for loss of expression in these proteins, and if present, considered for the possibility of Lynch syndrome. While most will not have an inherited cause, this information regarding their tumor biology remains critically important in both prognostication and decision-making surrounding other therapies and their eligibility for promising clinical trials.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Simpkins SB et al. Hum. Mol. Genet. 1999;8:661-6.
2. Kahn R et al. Cancer. 2019 Sep 15;125(18):2172-3183.
3. SGO Clinical Practice Statement: Screening for Lynch Syndrome in Endometrial Cancer. https://www.sgo.org/clinical-practice/guidelines/screening-for-lynch-syndrome-in-endometrial-cancer/
4. Kandoth et al. Nature. 2013;497(7447):67-73.
5. Leon-Castillo A et al. J Clin Oncol. 2020 Oct 10;38(29):3388-97.
6. Marabelle A et al. J Clin Oncol. 2020 Jan 1;38(1):1-10.
Women with Lynch syndrome are known to carry an approximately 60% lifetime risk of endometrial cancer. These cancers result from inherited deleterious mutations in genes that code for mismatch repair proteins. However, mismatch repair deficiency (MMR-d) is not exclusively found in the tumors of patients with Lynch syndrome, and much is being learned about this group of endometrial cancers, their behavior, and their vulnerability to targeted therapies.
During the processes of DNA replication, recombination, or chemical and physical damage, mismatches in base pairs frequently occurs. Mismatch repair proteins function to identify and repair such errors, and the loss of their function causes the accumulation of the insertions or deletions of short, repetitive sequences of DNA. This phenomenon can be measured using polymerase chain reaction (PCR) screening of known microsatellites to look for the accumulation of errors, a phenotype which is called microsatellite instability (MSI). The accumulation of errors in DNA sequences is thought to lead to mutations in cancer-related genes.
The four predominant mismatch repair genes include MLH1, MSH2, MSH 6, and PMS2. These genes may possess loss of function through a germline/inherited mechanism, such as Lynch syndrome, or can be sporadically acquired. Approximately 20%-30% of endometrial cancers exhibit MMR-d with acquired, sporadic losses in function being the majority of cases and only approximately 10% a result of Lynch syndrome. Mutations in PMS2 are the dominant genotype of Lynch syndrome, whereas loss of function in MLH1 is most frequent aberration in sporadic cases of MMR-d endometrial cancer.1
Endometrial cancers can be tested for MMR-d by performing immunohistochemistry to look for loss of expression in the four most common MMR genes. If there is loss of expression of MLH1, additional triage testing can be performed to determine if this loss is caused by the epigenetic phenomenon of hypermethylation. When present, this excludes Lynch syndrome and suggests a sporadic form origin of the disease. If there is loss of expression of the MMR genes (including loss of MLH1 and subsequent negative testing for promotor methylation), the patient should receive genetic testing for the presence of a germline mutation indicating Lynch syndrome. As an adjunct or alternative to immunohistochemistry, PCR studies or next-generation sequencing can be used to measure the presence of microsatellite instability in a process that identifies the expansion or reduction in repetitive DNA sequences of the tumor, compared with normal tumor.2
It is of the highest importance to identify endometrial cancers caused by Lynch syndrome because this enables providers to offer cascade testing of relatives, and to intensify screening or preventative measures for the many other cancers (such as colon, upper gastrointestinal, breast, and urothelial) for which these patients are at risk. Therefore, routine screening for MMR-d tumors is recommended in all cases of endometrial cancer, not simply those of a young age at diagnosis or for whom a strong family history exists.3 Using family history factors, primary tumor site, and age as a trigger for screening for Lynch syndrome, such as the Bethesda Guidelines, is associated with a 82% sensitivity in identifying Lynch syndrome. In a meta-analysis including testing results from 1,159 women with endometrial cancer, 43% of patients who were diagnosed with Lynch syndrome via molecular analysis would have been missed by clinical screening using Bethesda Guidelines.2
Discovering cases of Lynch syndrome is not the only benefit of routine testing for MMR-d in endometrial cancers. There is also significant value in the characterization of sporadic mismatch repair–deficient tumors because this information provides prognostic information and guides therapy. Tumors with a microsatellite-high phenotype/MMR-d were identified as one of the four distinct molecular subgroups of endometrial cancer by the Cancer Genome Atlas.4 Patients with this molecular profile exhibited “intermediate” prognostic outcomes, performing better than the “serous-like” cancers with p53 mutations, yet worse than patients with a POLE ultramutated group who rarely experience recurrences or death, even in the setting of unfavorable histology.
Beyond prognostication, the molecular profile of endometrial cancers also influence their responsiveness to therapeutics, highlighting the importance of splitting, not lumping endometrial cancers into relevant molecular subgroups when designing research and practicing clinical medicine. The PORTEC-3 trial studied 410 women with high-risk endometrial cancer, and randomized participants to receive either adjuvant radiation alone, or radiation with chemotherapy.5 There were no differences in progression-free survival between the two therapeutic strategies when analyzed in aggregate. However, when analyzed by Cancer Genome Atlas molecular subgroup, it was noted that there was a clear benefit from chemotherapy for patients with p53 mutations. For patients with MMR-d tumors, no such benefit was observed. Patients assigned this molecular subgroup did no better with the addition of platinum and taxane chemotherapy over radiation alone. Unfortunately, for patients with MMR-d tumors, recurrence rates remained high, suggesting that we can and need to discover more effective therapies for these tumors than what is available with conventional radiation or platinum and taxane chemotherapy. Targeted therapy may be the solution to this problem. Through microsatellite instability, MMR-d tumors create somatic mutations which result in neoantigens, an immunogenic environment. This state up-regulates checkpoint inhibitor proteins, which serve as an actionable target for anti-PD-L1 antibodies, such as the drug pembrolizumab which has been shown to be highly active against MMR-d endometrial cancer. In the landmark, KEYNOTE-158 trial, patients with advanced, recurrent solid tumors that exhibited MMR-d were treated with pembrolizumab.6 This included 49 patients with endometrial cancer, among whom there was a 79% response rate. Subsequently, pembrolizumab was granted Food and Drug Administration approval for use in advanced, recurrent MMR-d/MSI-high endometrial cancer. Trials are currently enrolling patients to explore the utility of this drug in the up-front setting in both early- and late-stage disease with a hope that this targeted therapy can do what conventional cytotoxic chemotherapy has failed to do.
Therefore, given the clinical significance of mismatch repair deficiency, all patients with endometrial cancer should be investigated for loss of expression in these proteins, and if present, considered for the possibility of Lynch syndrome. While most will not have an inherited cause, this information regarding their tumor biology remains critically important in both prognostication and decision-making surrounding other therapies and their eligibility for promising clinical trials.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Simpkins SB et al. Hum. Mol. Genet. 1999;8:661-6.
2. Kahn R et al. Cancer. 2019 Sep 15;125(18):2172-3183.
3. SGO Clinical Practice Statement: Screening for Lynch Syndrome in Endometrial Cancer. https://www.sgo.org/clinical-practice/guidelines/screening-for-lynch-syndrome-in-endometrial-cancer/
4. Kandoth et al. Nature. 2013;497(7447):67-73.
5. Leon-Castillo A et al. J Clin Oncol. 2020 Oct 10;38(29):3388-97.
6. Marabelle A et al. J Clin Oncol. 2020 Jan 1;38(1):1-10.
Endometriosis-associated ovarian cancer
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Intraoperative rupture of ovarian cancer: Does it worsen outcomes?
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.
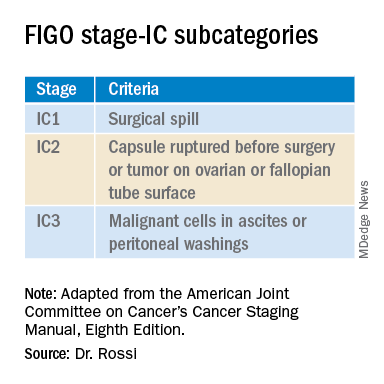
In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Neoadjuvant chemotherapy for advanced ovarian cancer
Historically the standard treatment approach for advanced ovarian cancer has been to perform up-front primary cytoreduction surgery or primary “debulking” surgery (PDS) followed by adjuvant chemotherapy. The goal of surgery was to establish cytoreduction of the tumor to optimal (<1 cm3 disease) or, ideally, complete (no gross residual disease). While PDS has long been considered the default treatment approach, neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery, typically after three or four cycles of chemotherapy, was the alternative strategy if it was anticipated or known that an “optimal” cytoreduction was not possible, feasible, or associated with acceptable morbidity. However, NACT was, and to some degree still is, widely considered the inferior strategy, reserved for patients with the worst prognosis. While mounting data challenges the inherent superiority of PDS, it still largely remains the default.
Why was PDS considered superior?
Why was PDS for advanced ovarian cancer considered a superior sequencing when it is so rarely considered appropriate for other disseminated cancers? This was born from the observation among retrospective data showing that survival was best when surgery was performed first, and when surgery was able to remove most or all visible disease (“complete” or “optimal” cytoreduction), NACT was performed.1 Several theories were proposed to explain the observations. These included the theory that bulky tumors contained avascular regions that would be less well accessed by chemotherapy, as well as the notion that chemotherapy exerts a constant fraction of kill on tumor cells, and if there is a lower burden of tumor cells to begin with, fewer cycles of chemotherapy will be necessary to eliminate all cells. Coupled with this was the notion that, if fewer cycles of chemotherapy are necessary, there would be less opportunity for development of drug resistance. Other theories such as the inflammatory effects of surgery impacting immune-mediated kill of malignant cells also are reported. These theories were largely found in the pages of textbooks, only supported by heavily biased observational series and not in the results of elegant translational studies. Of course, the observed superiority of PDS in these cohort studies was not surprising given that the patients who were historically selected for NACT had their treatment course chosen specifically for their poor prognostic factors (large volume, unresectable disease, poor performance status, and comorbidities). These “studies” were self-fulfilling prophecies.
Anecdotally I can attest that most patients are enthusiastic about a primary surgical approach to their advanced cancer. There is something concretely satisfying for patient and surgeon alike in the physical act of removing disease. As surgeons, if we believe that our added surgical effort will be rewarded with better outcomes for the patients, we will “try harder” in the operating room in order for them to do better. However, mounting data challenges whether it is our aggressive surgical effort as much as it is primary tumor biology that is the driver of prognosis in this disease. And aggressive primary surgery may add little other than perioperative morbidity.
Why that perspective may be changing
A culmination of many years of sophisticated translational research led by Anil Sood, MD, from the University of Texas MD Anderson Cancer Center, Houston, established there are fundamental biologic differences in the tumors of patients with ovarian cancer whose disease is amenable or not to a complete cytoreduction with PDS.2 In their work, the researchers sampled tumors from patients with advanced ovarian cancer who had been triaged either to PDS or NACT based on a standardized, validated laparoscopic algorithm that predicted a high probability of complete surgical resection. They performed pretreatment biopsies in both groups of patients and conducted a range of “omics” analyses to stratify these two subsets of patients – those who had a disease burden amenable to complete surgical resection versus those whose presenting disease burden exceeded an acceptable surgical effort). They identified several key molecular differences in the pretreatment biopsies of these two groups of patients, including alterations which might explain better or worse responses to therapy. These results suggest that the tumors of patients who go on to have successful PDS to no gross residual disease have different tumor biology to begin with. Otherwise said, perhaps it is favorable tumor biology that is associated with both a disease burden that is more amenable to both primary complete cytoreduction and better oncologic outcomes, rather than the surgical effort in and of itself.
This finding is supported by a study in which ovarian cancer survival outcomes were stratified by disease burden, surgical complexity scores, and postoperative residual disease among patients who were enrolled in GOG-182.3 Investigators led by Neil Horowitz, MD, created scores for surgical complexity, disease burden, and residual disease. They observed that the radicality of surgery (complexity score) was not an independent determinant of survival, but rather, patients who presented with a lower disease burden that required a less radical surgery had the best oncologic outcomes.
If the complexity of surgery does not influence outcomes as much as the predetermined, unmodifiable tumor biology, how should surgeons make decisions about the sequencing of treatment? Over the past 10 years, four randomized trials have been completed including more than 1,600 patients randomized to either PDS or NACT.4-7 All four have found no difference in the oncologic outcomes (progression-free or overall survival) between patients when randomized to PDS or NACT. While the statistical designs vary slightly, some being designed to look for noninferiority and others for superiority, they all showed that the sequence in which surgery and chemotherapy was performed mattered less than whether optimal cytoreduction was achieved when surgery was performed. As stated above, this phenomenon seems to be best determined by unmodifiable tumor biology. Unsurprisingly, these studies also have consistently found that perioperative outcomes (e.g., surgical complications, length of stay, death) were worse with PDS because of the higher surgical complexity that it demands. In the most recent SCORPION trial, rates of major postoperative complications in the PDS group were 25.9%, compared with only 7.6% in the NACT group (P < .0001) and all of the deaths from postoperative complications occurred in the PDS group at a rate of 8.3% (7 of 84 patients).7
Therefore, the wealth of data supports that oncologic outcomes are equivalent, and perioperative outcomes are improved for patients who undergo NACT for advanced, bulky ovarian cancer.
Why physicians still are questioning
Unfortunately, because ofthe nature of the disease, these prospective trials include heterogeneous populations of disease presentation, surgeon skill, and hospital settings. They have been criticized for achieving “low” rates of complete or optimal cytoreduction in the PDS arm. They also identified subgroups of patients who may do better with PDS (such as those with lower-volume stage IIIC disease) and those who have better outcomes with NACT (patients with stage IV disease). Therefore, not satisfied that we have definitively answered the question, a fifth randomized study, the TRUST trial, is underway.8 This study includes surgeons at high-volume institutions, purported to have the highest degree of skill and quality in executing radical debulking procedures. Perhaps this fifth trial will show that, if performed in the most skilled hands and quality settings, PDS is preferable to NACT. Perhaps. However, the generalizability of these results will be poor for all patients with advanced ovarian cancer, most of whom will have limited access to these highest-volume surgeons.
What can be agreed upon is that an individualized and nuanced approach is best for advanced ovarian cancer. There will be some patients who benefit from PDS (e.g., healthy, young patients with low-volume disease). However, for most patients, the bulk of prospective and translational research supports NACT as the default treatment course, associated with noninferior survival and superior perioperative outcomes (including postoperative death). While it may not be a one-size-fits-all approach, one could argue that NACT should be the default strategy, and surgeons should look for reasons to “opt in” to PDS in special circumstances guided by biomarkers such as imaging, tumor markers, clinical factors, and surgical findings.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2006 Dec. doi: 10.1016/j.ygyno.2006.06.025.
2. Cell Rep. 2020 Apr 14. doi: 10.1016/j.celrep.2020.03.066.
3. J Clin Oncol. 2015 Mar 10. doi: 10.1200/JCO.2014.56.3106.
4. N Engl J Med. 2010 Sep 2. doi: 10.1056/NEJMoa0908806.
5. Lancet. 2015 Jul 18. doi: 10.1016/S0140-6736(14)62223-6.
6. Eur J Cancer. 2020. doi: 10.1016/j.ejca.2020.02.020.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001640.
8. Int J Gynecol Cancer. 2019. doi: 10.1136/ijgc-2019-000682.
Historically the standard treatment approach for advanced ovarian cancer has been to perform up-front primary cytoreduction surgery or primary “debulking” surgery (PDS) followed by adjuvant chemotherapy. The goal of surgery was to establish cytoreduction of the tumor to optimal (<1 cm3 disease) or, ideally, complete (no gross residual disease). While PDS has long been considered the default treatment approach, neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery, typically after three or four cycles of chemotherapy, was the alternative strategy if it was anticipated or known that an “optimal” cytoreduction was not possible, feasible, or associated with acceptable morbidity. However, NACT was, and to some degree still is, widely considered the inferior strategy, reserved for patients with the worst prognosis. While mounting data challenges the inherent superiority of PDS, it still largely remains the default.
Why was PDS considered superior?
Why was PDS for advanced ovarian cancer considered a superior sequencing when it is so rarely considered appropriate for other disseminated cancers? This was born from the observation among retrospective data showing that survival was best when surgery was performed first, and when surgery was able to remove most or all visible disease (“complete” or “optimal” cytoreduction), NACT was performed.1 Several theories were proposed to explain the observations. These included the theory that bulky tumors contained avascular regions that would be less well accessed by chemotherapy, as well as the notion that chemotherapy exerts a constant fraction of kill on tumor cells, and if there is a lower burden of tumor cells to begin with, fewer cycles of chemotherapy will be necessary to eliminate all cells. Coupled with this was the notion that, if fewer cycles of chemotherapy are necessary, there would be less opportunity for development of drug resistance. Other theories such as the inflammatory effects of surgery impacting immune-mediated kill of malignant cells also are reported. These theories were largely found in the pages of textbooks, only supported by heavily biased observational series and not in the results of elegant translational studies. Of course, the observed superiority of PDS in these cohort studies was not surprising given that the patients who were historically selected for NACT had their treatment course chosen specifically for their poor prognostic factors (large volume, unresectable disease, poor performance status, and comorbidities). These “studies” were self-fulfilling prophecies.
Anecdotally I can attest that most patients are enthusiastic about a primary surgical approach to their advanced cancer. There is something concretely satisfying for patient and surgeon alike in the physical act of removing disease. As surgeons, if we believe that our added surgical effort will be rewarded with better outcomes for the patients, we will “try harder” in the operating room in order for them to do better. However, mounting data challenges whether it is our aggressive surgical effort as much as it is primary tumor biology that is the driver of prognosis in this disease. And aggressive primary surgery may add little other than perioperative morbidity.
Why that perspective may be changing
A culmination of many years of sophisticated translational research led by Anil Sood, MD, from the University of Texas MD Anderson Cancer Center, Houston, established there are fundamental biologic differences in the tumors of patients with ovarian cancer whose disease is amenable or not to a complete cytoreduction with PDS.2 In their work, the researchers sampled tumors from patients with advanced ovarian cancer who had been triaged either to PDS or NACT based on a standardized, validated laparoscopic algorithm that predicted a high probability of complete surgical resection. They performed pretreatment biopsies in both groups of patients and conducted a range of “omics” analyses to stratify these two subsets of patients – those who had a disease burden amenable to complete surgical resection versus those whose presenting disease burden exceeded an acceptable surgical effort). They identified several key molecular differences in the pretreatment biopsies of these two groups of patients, including alterations which might explain better or worse responses to therapy. These results suggest that the tumors of patients who go on to have successful PDS to no gross residual disease have different tumor biology to begin with. Otherwise said, perhaps it is favorable tumor biology that is associated with both a disease burden that is more amenable to both primary complete cytoreduction and better oncologic outcomes, rather than the surgical effort in and of itself.
This finding is supported by a study in which ovarian cancer survival outcomes were stratified by disease burden, surgical complexity scores, and postoperative residual disease among patients who were enrolled in GOG-182.3 Investigators led by Neil Horowitz, MD, created scores for surgical complexity, disease burden, and residual disease. They observed that the radicality of surgery (complexity score) was not an independent determinant of survival, but rather, patients who presented with a lower disease burden that required a less radical surgery had the best oncologic outcomes.
If the complexity of surgery does not influence outcomes as much as the predetermined, unmodifiable tumor biology, how should surgeons make decisions about the sequencing of treatment? Over the past 10 years, four randomized trials have been completed including more than 1,600 patients randomized to either PDS or NACT.4-7 All four have found no difference in the oncologic outcomes (progression-free or overall survival) between patients when randomized to PDS or NACT. While the statistical designs vary slightly, some being designed to look for noninferiority and others for superiority, they all showed that the sequence in which surgery and chemotherapy was performed mattered less than whether optimal cytoreduction was achieved when surgery was performed. As stated above, this phenomenon seems to be best determined by unmodifiable tumor biology. Unsurprisingly, these studies also have consistently found that perioperative outcomes (e.g., surgical complications, length of stay, death) were worse with PDS because of the higher surgical complexity that it demands. In the most recent SCORPION trial, rates of major postoperative complications in the PDS group were 25.9%, compared with only 7.6% in the NACT group (P < .0001) and all of the deaths from postoperative complications occurred in the PDS group at a rate of 8.3% (7 of 84 patients).7
Therefore, the wealth of data supports that oncologic outcomes are equivalent, and perioperative outcomes are improved for patients who undergo NACT for advanced, bulky ovarian cancer.
Why physicians still are questioning
Unfortunately, because ofthe nature of the disease, these prospective trials include heterogeneous populations of disease presentation, surgeon skill, and hospital settings. They have been criticized for achieving “low” rates of complete or optimal cytoreduction in the PDS arm. They also identified subgroups of patients who may do better with PDS (such as those with lower-volume stage IIIC disease) and those who have better outcomes with NACT (patients with stage IV disease). Therefore, not satisfied that we have definitively answered the question, a fifth randomized study, the TRUST trial, is underway.8 This study includes surgeons at high-volume institutions, purported to have the highest degree of skill and quality in executing radical debulking procedures. Perhaps this fifth trial will show that, if performed in the most skilled hands and quality settings, PDS is preferable to NACT. Perhaps. However, the generalizability of these results will be poor for all patients with advanced ovarian cancer, most of whom will have limited access to these highest-volume surgeons.
What can be agreed upon is that an individualized and nuanced approach is best for advanced ovarian cancer. There will be some patients who benefit from PDS (e.g., healthy, young patients with low-volume disease). However, for most patients, the bulk of prospective and translational research supports NACT as the default treatment course, associated with noninferior survival and superior perioperative outcomes (including postoperative death). While it may not be a one-size-fits-all approach, one could argue that NACT should be the default strategy, and surgeons should look for reasons to “opt in” to PDS in special circumstances guided by biomarkers such as imaging, tumor markers, clinical factors, and surgical findings.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2006 Dec. doi: 10.1016/j.ygyno.2006.06.025.
2. Cell Rep. 2020 Apr 14. doi: 10.1016/j.celrep.2020.03.066.
3. J Clin Oncol. 2015 Mar 10. doi: 10.1200/JCO.2014.56.3106.
4. N Engl J Med. 2010 Sep 2. doi: 10.1056/NEJMoa0908806.
5. Lancet. 2015 Jul 18. doi: 10.1016/S0140-6736(14)62223-6.
6. Eur J Cancer. 2020. doi: 10.1016/j.ejca.2020.02.020.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001640.
8. Int J Gynecol Cancer. 2019. doi: 10.1136/ijgc-2019-000682.
Historically the standard treatment approach for advanced ovarian cancer has been to perform up-front primary cytoreduction surgery or primary “debulking” surgery (PDS) followed by adjuvant chemotherapy. The goal of surgery was to establish cytoreduction of the tumor to optimal (<1 cm3 disease) or, ideally, complete (no gross residual disease). While PDS has long been considered the default treatment approach, neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery, typically after three or four cycles of chemotherapy, was the alternative strategy if it was anticipated or known that an “optimal” cytoreduction was not possible, feasible, or associated with acceptable morbidity. However, NACT was, and to some degree still is, widely considered the inferior strategy, reserved for patients with the worst prognosis. While mounting data challenges the inherent superiority of PDS, it still largely remains the default.
Why was PDS considered superior?
Why was PDS for advanced ovarian cancer considered a superior sequencing when it is so rarely considered appropriate for other disseminated cancers? This was born from the observation among retrospective data showing that survival was best when surgery was performed first, and when surgery was able to remove most or all visible disease (“complete” or “optimal” cytoreduction), NACT was performed.1 Several theories were proposed to explain the observations. These included the theory that bulky tumors contained avascular regions that would be less well accessed by chemotherapy, as well as the notion that chemotherapy exerts a constant fraction of kill on tumor cells, and if there is a lower burden of tumor cells to begin with, fewer cycles of chemotherapy will be necessary to eliminate all cells. Coupled with this was the notion that, if fewer cycles of chemotherapy are necessary, there would be less opportunity for development of drug resistance. Other theories such as the inflammatory effects of surgery impacting immune-mediated kill of malignant cells also are reported. These theories were largely found in the pages of textbooks, only supported by heavily biased observational series and not in the results of elegant translational studies. Of course, the observed superiority of PDS in these cohort studies was not surprising given that the patients who were historically selected for NACT had their treatment course chosen specifically for their poor prognostic factors (large volume, unresectable disease, poor performance status, and comorbidities). These “studies” were self-fulfilling prophecies.
Anecdotally I can attest that most patients are enthusiastic about a primary surgical approach to their advanced cancer. There is something concretely satisfying for patient and surgeon alike in the physical act of removing disease. As surgeons, if we believe that our added surgical effort will be rewarded with better outcomes for the patients, we will “try harder” in the operating room in order for them to do better. However, mounting data challenges whether it is our aggressive surgical effort as much as it is primary tumor biology that is the driver of prognosis in this disease. And aggressive primary surgery may add little other than perioperative morbidity.
Why that perspective may be changing
A culmination of many years of sophisticated translational research led by Anil Sood, MD, from the University of Texas MD Anderson Cancer Center, Houston, established there are fundamental biologic differences in the tumors of patients with ovarian cancer whose disease is amenable or not to a complete cytoreduction with PDS.2 In their work, the researchers sampled tumors from patients with advanced ovarian cancer who had been triaged either to PDS or NACT based on a standardized, validated laparoscopic algorithm that predicted a high probability of complete surgical resection. They performed pretreatment biopsies in both groups of patients and conducted a range of “omics” analyses to stratify these two subsets of patients – those who had a disease burden amenable to complete surgical resection versus those whose presenting disease burden exceeded an acceptable surgical effort). They identified several key molecular differences in the pretreatment biopsies of these two groups of patients, including alterations which might explain better or worse responses to therapy. These results suggest that the tumors of patients who go on to have successful PDS to no gross residual disease have different tumor biology to begin with. Otherwise said, perhaps it is favorable tumor biology that is associated with both a disease burden that is more amenable to both primary complete cytoreduction and better oncologic outcomes, rather than the surgical effort in and of itself.
This finding is supported by a study in which ovarian cancer survival outcomes were stratified by disease burden, surgical complexity scores, and postoperative residual disease among patients who were enrolled in GOG-182.3 Investigators led by Neil Horowitz, MD, created scores for surgical complexity, disease burden, and residual disease. They observed that the radicality of surgery (complexity score) was not an independent determinant of survival, but rather, patients who presented with a lower disease burden that required a less radical surgery had the best oncologic outcomes.
If the complexity of surgery does not influence outcomes as much as the predetermined, unmodifiable tumor biology, how should surgeons make decisions about the sequencing of treatment? Over the past 10 years, four randomized trials have been completed including more than 1,600 patients randomized to either PDS or NACT.4-7 All four have found no difference in the oncologic outcomes (progression-free or overall survival) between patients when randomized to PDS or NACT. While the statistical designs vary slightly, some being designed to look for noninferiority and others for superiority, they all showed that the sequence in which surgery and chemotherapy was performed mattered less than whether optimal cytoreduction was achieved when surgery was performed. As stated above, this phenomenon seems to be best determined by unmodifiable tumor biology. Unsurprisingly, these studies also have consistently found that perioperative outcomes (e.g., surgical complications, length of stay, death) were worse with PDS because of the higher surgical complexity that it demands. In the most recent SCORPION trial, rates of major postoperative complications in the PDS group were 25.9%, compared with only 7.6% in the NACT group (P < .0001) and all of the deaths from postoperative complications occurred in the PDS group at a rate of 8.3% (7 of 84 patients).7
Therefore, the wealth of data supports that oncologic outcomes are equivalent, and perioperative outcomes are improved for patients who undergo NACT for advanced, bulky ovarian cancer.
Why physicians still are questioning
Unfortunately, because ofthe nature of the disease, these prospective trials include heterogeneous populations of disease presentation, surgeon skill, and hospital settings. They have been criticized for achieving “low” rates of complete or optimal cytoreduction in the PDS arm. They also identified subgroups of patients who may do better with PDS (such as those with lower-volume stage IIIC disease) and those who have better outcomes with NACT (patients with stage IV disease). Therefore, not satisfied that we have definitively answered the question, a fifth randomized study, the TRUST trial, is underway.8 This study includes surgeons at high-volume institutions, purported to have the highest degree of skill and quality in executing radical debulking procedures. Perhaps this fifth trial will show that, if performed in the most skilled hands and quality settings, PDS is preferable to NACT. Perhaps. However, the generalizability of these results will be poor for all patients with advanced ovarian cancer, most of whom will have limited access to these highest-volume surgeons.
What can be agreed upon is that an individualized and nuanced approach is best for advanced ovarian cancer. There will be some patients who benefit from PDS (e.g., healthy, young patients with low-volume disease). However, for most patients, the bulk of prospective and translational research supports NACT as the default treatment course, associated with noninferior survival and superior perioperative outcomes (including postoperative death). While it may not be a one-size-fits-all approach, one could argue that NACT should be the default strategy, and surgeons should look for reasons to “opt in” to PDS in special circumstances guided by biomarkers such as imaging, tumor markers, clinical factors, and surgical findings.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Gynecol Oncol. 2006 Dec. doi: 10.1016/j.ygyno.2006.06.025.
2. Cell Rep. 2020 Apr 14. doi: 10.1016/j.celrep.2020.03.066.
3. J Clin Oncol. 2015 Mar 10. doi: 10.1200/JCO.2014.56.3106.
4. N Engl J Med. 2010 Sep 2. doi: 10.1056/NEJMoa0908806.
5. Lancet. 2015 Jul 18. doi: 10.1016/S0140-6736(14)62223-6.
6. Eur J Cancer. 2020. doi: 10.1016/j.ejca.2020.02.020.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001640.
8. Int J Gynecol Cancer. 2019. doi: 10.1136/ijgc-2019-000682.
Are uterine manipulators safe for gynecologic cancer surgery?
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.
Over the past 4 decades there has been increasing use of minimally invasive surgery (MIS) for gynecologic cancer, particularly endometrial and cervical cancers. Uterine manipulators are a device inserted into the uterine cavity during MIS approaches to aid in directing the uterus within the pelvis, facilitating access to the uterine blood supply, defining the cardinal ligaments, lateralizing the ureters, and delineating the cervicovaginal junction. However, concerns have been raised regarding whether these devices are safe to use when the uterine corpus or cervix contains cancer.
In 2018, the LACC trial was published and demonstrated decreased survival for patients with cervical cancer who had undergone radical hysterectomy via a minimally invasive route.1 Several hypotheses were proposed to explain this finding including possible tumor disruption from use of a uterine manipulator. Regrettably, this study did not document manipulator use, and therefore its influence on outcomes could not be measured. However, since that time there has been honed interest into the potential negative influence of uterine manipulators on endometrial and cervical cancer surgery.
Uterine manipulators typically are inserted through the uterine cervix and reside in the endometrial cavity. It is often an inflated balloon which stabilizes the device within the cavity. Hypotheses for how they may contribute to the spread of malignancy include the massage of endometrial tumor from the pressure of the inflated balloon, facilitation of tumor dissemination through cervical lymphatics or vasculature as the manipulator traverses or punctures a cervical cancer, and possibly perforation of the uterine cavity during placement of the manipulator, and in doing so, contaminating the peritoneal cavity with endometrial or cervical cancer cells that have been dragged through with the device.
Interestingly, uterine manipulator placement is not the only time during which endometrial or cervical cancers may be disturbed prior to resection. Many diagnostic procedures such as cervical excisional procedures (loop electrosurgical excision procedure and conizations) or hysteroscopic resections cause significant intentional disruption of tumor. In the case of hysteroscopy for endometrial cancer, endometrial cancer cells have been detected in the peritoneal washings of endometrial cancer patients who have undergone this procedure, however, no worse outcomes have been associated when hysteroscopy was included as part of the diagnostic work-up, suggesting that more than simply efflux into the peritoneal cavity is necessary for those tumor cells to have metastatic potential.2
Indeed the data is mixed regarding oncologic outcomes with uterine manipulator use, especially for endometrial cancer. In one recent study the outcomes of 951 patients with endometrial cancer from seven Italian centers were evaluated.3 There was no difference in recurrence rates or disease-specific survival between the 579 patients in whom manipulators were used and the 372 patients in which surgery was performed without manipulators. More recently a Spanish study reported retrospectively on 2,661 patients at 15 centers and determined that use of a uterine manipulator (two-thirds of the cohort) was associated with a hazard ratio of 1.74 (95% confidence interval, 1.07-2.83) for risk of death.4 Unfortunately, in this study there were substantial differences between sites that used manipulators and those that did not. Additionally, while one would expect different patterns of recurrence if the manipulator was introducing a unique mechanism for metastasis, this was not observed between the manipulator and nonmanipulator arms. Finally, the groups were intrinsically different with respect to important risk factors such as lymphovascular space invasion, which might have contributed to the observed outcomes. It is important to recognize that, in both the LAP-2 and LACE trials, minimally invasive hysterectomy for endometrial cancer had been shown to have noninferior survival outcomes, compared with open hysterectomy.5,6 While these large randomized, controlled trials did not capture uterine manipulator usage, presumably it was utilized in at least some or most cases, and without apparent significant negative effect.
In cervical cancer, there is more competing data raising concern regarding manipulator use. The SUCCOR study was completed in 2020 and included a retrospective evaluation of 1,272 patients who had undergone open or MIS radical hysterectomy for early stage cervical cancer across 126 European centers during 2013-2014.7 They were able to evaluate for variables, such as uterine manipulator use. While they found that recurrence was higher for patients who had MIS hysterectomy, the HR (2.07) was similar to the HR for recurrence (2.76) among those who had uterine manipulator use. Conversely, the hazard ratio for recurrence following MIS radical hysterectomy without a manipulator was comparable with the superior rates seen with open surgery. This study was retrospective and therefore is largely hypothesis generating, however it does raise the question of whether the technique of MIS radical hysterectomy can be performed safely if particular steps, such as avoidance of a uterine manipulator, are followed. We await definitive results from prospective trials to determine this.
As mentioned earlier, the uterine manipulator is an important safety and feasibility tool for MIS hysterectomy. When not utilized, surgeons may need to add additional ports and instrumentation to maneuver the uterus and may have difficulty completing hysterectomy via a MIS approach for obese patients. There are additional urologic safety concerns when uterine elevation and cervicovaginal delineation is missing. Therefore, While the wealth of prospective data suggests that manipulators are most likely safe in hysterectomy for endometrial cancer, they should be avoided if a minimally invasive approach to cervical cancer is employed.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to report. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2018 Nov 15. doi: 10.1056/NEJMoa1806395.
2. Fertil Steril. 2011 Oct. doi: 10.1016/j.fertnstert.2011.07.1146.
3. Am J Obstet Gynecol. 2017 Jun. doi: 10.1016/j.ajog.2017.01.027.
4. Am J Obstet Gynecol. 2020 Jul 18. doi: 10.1016/j.ajog.2020.07.025.
5. J Clin Oncol. 2009 Nov 10. doi: 10.1200/JCO.2009.22.3248.
6. JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.2068.
7. Int J Gynecol Cancer. 2020. doi: 10.1136/ijgc-2020-001506.
Molecular developments in treatment of UPSC
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Treating VIN while preventing recurrence
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
Should all patients with advanced ovarian cancer receive frontline maintenance therapy?
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
What is the significance of isolated tumor cells in endometrial cancer?
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.
When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.