User login
Cannabis use in pregnancy and lactation: A changing landscape
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
AED exposure from breastfeeding appears to be low
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
FROM JAMA NEUROLOGY
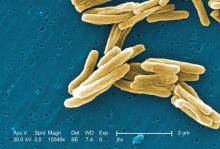
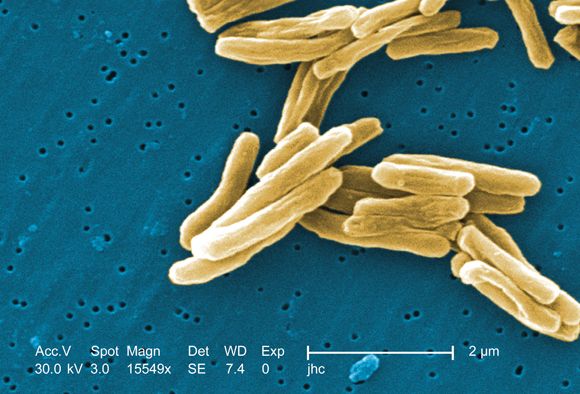
Antituberculosis drugs in pregnancy and lactation
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at obnews@mdedge.com.
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at obnews@mdedge.com.
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at obnews@mdedge.com.
Zulresso: Hope and lingering questions
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at admin@womensmentalhealth.org.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at admin@womensmentalhealth.org.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at admin@womensmentalhealth.org.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Safety of ondansetron for nausea and vomiting of pregnancy
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Antimalarials in pregnancy and lactation
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Bipolar disorder during pregnancy: Lessons learned
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
2018 FDA-approved new drugs
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at obnews@mdedge.com.
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at obnews@mdedge.com.
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at obnews@mdedge.com.
Preventing postpartum depression: Start with women at greatest risk
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
Antimigraine agents in pregnancy and lactation
Migraine is a common neurovascular disorder with episodic attacks of throbbing headache, nausea/vomiting, photophobia, and phonophobia. Migraine symptoms will lessen in up to 70% of pregnant women, usually during the second and third trimesters, but will worsen in 4%-8%, and new-onset migraine may account for as many as 16% of all cases of the disorder in pregnancy.
Prevention of migraines
A 2002 review by Goadsby PJ et al. (N Engl J Med. 2002 Jan 24;346[4]:257-70) identified 11 drugs or drug classes for the prevention of migraine attacks. Four agents that are available in the United States were thought to have proven efficacy or were well accepted: metoprolol, propranolol, amitriptyline, and valproate. Valproate should not be used during pregnancy because it is a known teratogen and can cause other fetal problems throughout gestation. Metoprolol, a cardioselective beta-blocker, is a risk for intrauterine growth restriction (IUGR) in the second/third trimesters.
Three other agents are not available in the United States: flunarizine (an agent with calcium channel blocking activity) and two serotonin antagonists, pizotifen and methysergide (a semisynthetic ergot alkaloid). Based on the drug class, flunarizine probably is compatible with pregnancy. Ergot alkaloids, including methysergide, are contraindicated in pregnancy.
Verapamil (Calan, Isoptin) and the selective serotonin reuptake inhibitors (SSRIs) were widely used, but the reviewers concluded that there was poor evidence of benefit.
Use of the SSRI antidepressants in the third trimester may cause newborn toxicity.
Only amitriptyline, verapamil, and low-dose propranolol (30-40 mg/day) have sufficient data to classify as compatible throughout pregnancy, but higher doses of propranolol may cause IUGR and other fetal/neonatal toxicity.
Two agents, gabapentin (Neurontin) and topiramate (Topamax), were considered promising agents for migraine prophylaxis. However, topiramate is best avoided in pregnancy. There are inadequate human data to assess the risk of gabapentin and topiramate, therefore both agents should be avoided in the first trimester.
Breastfeeding: Prevention therapy
Based on their potential for harm, topiramate and valproate should not be used during breastfeeding. All of the other drugs probably are compatible with breastfeeding, but the nursing infant should be monitored for adverse effects common in nonpregnant adults.
Acute treatment of migraines
There are 11 single-agent drugs that are indicated for the acute treatment of migraine.
Almotriptan (Axert) is available as an oral tablet. There are no human pregnancy data and the animal data suggest low risk. However, there is a possible risk of preterm birth.
Dihydroergotamine (Migranal, D.H.E. 45) is available for injection and nasal spray. It is contraindicated, especially near term (it has oxytocic properties), and the animal data suggest risk of IUGR.
Eletriptan (Relpax) is an oral tablet. There are no human data and the animal data suggest low risk. There is a possible risk of preterm birth.
Ergotamine (Ergomar), an oral tablet, is contraindicated in pregnancy and breastfeeding (see above and below).
Methysergide (Sansert) is contraindicated but is not available in United States.
Frovatriptan (Frova) has no human pregnancy data. The animal data suggests low risk but there is the possibility of preterm birth.
There are 93 cases in pregnancy for naratriptan (Amerge) but none during breastfeeding. The drug did not cause major defects in animals but did produce dose-related embryo and fetal development toxicity. In addition, the data did suggest a possible increase in preterm births.
For the rizatriptan (Maxalt, Maxalt-MLT) tablet, animal data suggest low risk. There was exposure to the drug in more than 81 human pregnancy cases. Although there was no evidence of birth defects, the data raised the concern of preterm birth.
There is no evidence that sumatriptan (Alsuma, Imitrex, Imitrex Statdose, Sumavel DosePro, Zecuity) is a human teratogen but there is a possible increase in preterm birth. The drug was teratogenic in one animal species. This agent is available as a tablet and for injection.
There is no human pregnancy data for zolmitriptan (Zomig; Zomig-ZMT) but, as with all triptans, there is a possible risk of preterm birth. The animal data suggest low risk. It is available as an oral tablet.
There are three multiagent products that can be used to treat migraines. There are no published human pregnancy data for these combination products. The combination oral drug sumatriptan and naproxen (Treximet) is best avoided in pregnancy because first trimester exposure to the NSAID naproxen may cause embryo-fetal harm (structural anomalies and abortion) or close to term (premature closure of the ductus arteriosus) and other newborn toxicities.
Caffeine and ergotamine (Cafergot, Migergot) tablets should be considered contraindicated in pregnancy and breastfeeding. Although small, infrequent doses of this product do not appear to be fetotoxic or teratogenic, idiosyncratic responses may occur with ergotamine that endanger the fetus.
The third product, acetaminophen, dichloralphenazone, and isometheptene (Epidrin, LarkaDrin, Migragesic IDA) is an over-the-counter tablet. It does not appear to be related to embryo-fetal harm, but its effectiveness is unknown.
Breastfeeding: Acute treatment
Consistent with their relatively low molecular weights, all of the acute treatment agents most likely are excreted into breast milk. With the exception of the two ergotamine products, both of which should be considered contraindicated, all of the other agents probably are compatible during breastfeeding.
Summary
Migraine headaches are common but can be prevented and/or treated safely in pregnancy and when nursing. In addition to the review mentioned above, three other reviews are well worth reading: Becker WJ. Continuum (Minneap Minn). 2015 Aug;21(4 Headache):953-72; Becker WJ. Headache. 2015 Jun;55(6):778-93; Hutchinson S et al. Headache. 2013 Apr;53(4):614-27.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Migraine is a common neurovascular disorder with episodic attacks of throbbing headache, nausea/vomiting, photophobia, and phonophobia. Migraine symptoms will lessen in up to 70% of pregnant women, usually during the second and third trimesters, but will worsen in 4%-8%, and new-onset migraine may account for as many as 16% of all cases of the disorder in pregnancy.
Prevention of migraines
A 2002 review by Goadsby PJ et al. (N Engl J Med. 2002 Jan 24;346[4]:257-70) identified 11 drugs or drug classes for the prevention of migraine attacks. Four agents that are available in the United States were thought to have proven efficacy or were well accepted: metoprolol, propranolol, amitriptyline, and valproate. Valproate should not be used during pregnancy because it is a known teratogen and can cause other fetal problems throughout gestation. Metoprolol, a cardioselective beta-blocker, is a risk for intrauterine growth restriction (IUGR) in the second/third trimesters.
Three other agents are not available in the United States: flunarizine (an agent with calcium channel blocking activity) and two serotonin antagonists, pizotifen and methysergide (a semisynthetic ergot alkaloid). Based on the drug class, flunarizine probably is compatible with pregnancy. Ergot alkaloids, including methysergide, are contraindicated in pregnancy.
Verapamil (Calan, Isoptin) and the selective serotonin reuptake inhibitors (SSRIs) were widely used, but the reviewers concluded that there was poor evidence of benefit.
Use of the SSRI antidepressants in the third trimester may cause newborn toxicity.
Only amitriptyline, verapamil, and low-dose propranolol (30-40 mg/day) have sufficient data to classify as compatible throughout pregnancy, but higher doses of propranolol may cause IUGR and other fetal/neonatal toxicity.
Two agents, gabapentin (Neurontin) and topiramate (Topamax), were considered promising agents for migraine prophylaxis. However, topiramate is best avoided in pregnancy. There are inadequate human data to assess the risk of gabapentin and topiramate, therefore both agents should be avoided in the first trimester.
Breastfeeding: Prevention therapy
Based on their potential for harm, topiramate and valproate should not be used during breastfeeding. All of the other drugs probably are compatible with breastfeeding, but the nursing infant should be monitored for adverse effects common in nonpregnant adults.
Acute treatment of migraines
There are 11 single-agent drugs that are indicated for the acute treatment of migraine.
Almotriptan (Axert) is available as an oral tablet. There are no human pregnancy data and the animal data suggest low risk. However, there is a possible risk of preterm birth.
Dihydroergotamine (Migranal, D.H.E. 45) is available for injection and nasal spray. It is contraindicated, especially near term (it has oxytocic properties), and the animal data suggest risk of IUGR.
Eletriptan (Relpax) is an oral tablet. There are no human data and the animal data suggest low risk. There is a possible risk of preterm birth.
Ergotamine (Ergomar), an oral tablet, is contraindicated in pregnancy and breastfeeding (see above and below).
Methysergide (Sansert) is contraindicated but is not available in United States.
Frovatriptan (Frova) has no human pregnancy data. The animal data suggests low risk but there is the possibility of preterm birth.
There are 93 cases in pregnancy for naratriptan (Amerge) but none during breastfeeding. The drug did not cause major defects in animals but did produce dose-related embryo and fetal development toxicity. In addition, the data did suggest a possible increase in preterm births.
For the rizatriptan (Maxalt, Maxalt-MLT) tablet, animal data suggest low risk. There was exposure to the drug in more than 81 human pregnancy cases. Although there was no evidence of birth defects, the data raised the concern of preterm birth.
There is no evidence that sumatriptan (Alsuma, Imitrex, Imitrex Statdose, Sumavel DosePro, Zecuity) is a human teratogen but there is a possible increase in preterm birth. The drug was teratogenic in one animal species. This agent is available as a tablet and for injection.
There is no human pregnancy data for zolmitriptan (Zomig; Zomig-ZMT) but, as with all triptans, there is a possible risk of preterm birth. The animal data suggest low risk. It is available as an oral tablet.
There are three multiagent products that can be used to treat migraines. There are no published human pregnancy data for these combination products. The combination oral drug sumatriptan and naproxen (Treximet) is best avoided in pregnancy because first trimester exposure to the NSAID naproxen may cause embryo-fetal harm (structural anomalies and abortion) or close to term (premature closure of the ductus arteriosus) and other newborn toxicities.
Caffeine and ergotamine (Cafergot, Migergot) tablets should be considered contraindicated in pregnancy and breastfeeding. Although small, infrequent doses of this product do not appear to be fetotoxic or teratogenic, idiosyncratic responses may occur with ergotamine that endanger the fetus.
The third product, acetaminophen, dichloralphenazone, and isometheptene (Epidrin, LarkaDrin, Migragesic IDA) is an over-the-counter tablet. It does not appear to be related to embryo-fetal harm, but its effectiveness is unknown.
Breastfeeding: Acute treatment
Consistent with their relatively low molecular weights, all of the acute treatment agents most likely are excreted into breast milk. With the exception of the two ergotamine products, both of which should be considered contraindicated, all of the other agents probably are compatible during breastfeeding.
Summary
Migraine headaches are common but can be prevented and/or treated safely in pregnancy and when nursing. In addition to the review mentioned above, three other reviews are well worth reading: Becker WJ. Continuum (Minneap Minn). 2015 Aug;21(4 Headache):953-72; Becker WJ. Headache. 2015 Jun;55(6):778-93; Hutchinson S et al. Headache. 2013 Apr;53(4):614-27.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Migraine is a common neurovascular disorder with episodic attacks of throbbing headache, nausea/vomiting, photophobia, and phonophobia. Migraine symptoms will lessen in up to 70% of pregnant women, usually during the second and third trimesters, but will worsen in 4%-8%, and new-onset migraine may account for as many as 16% of all cases of the disorder in pregnancy.
Prevention of migraines
A 2002 review by Goadsby PJ et al. (N Engl J Med. 2002 Jan 24;346[4]:257-70) identified 11 drugs or drug classes for the prevention of migraine attacks. Four agents that are available in the United States were thought to have proven efficacy or were well accepted: metoprolol, propranolol, amitriptyline, and valproate. Valproate should not be used during pregnancy because it is a known teratogen and can cause other fetal problems throughout gestation. Metoprolol, a cardioselective beta-blocker, is a risk for intrauterine growth restriction (IUGR) in the second/third trimesters.
Three other agents are not available in the United States: flunarizine (an agent with calcium channel blocking activity) and two serotonin antagonists, pizotifen and methysergide (a semisynthetic ergot alkaloid). Based on the drug class, flunarizine probably is compatible with pregnancy. Ergot alkaloids, including methysergide, are contraindicated in pregnancy.
Verapamil (Calan, Isoptin) and the selective serotonin reuptake inhibitors (SSRIs) were widely used, but the reviewers concluded that there was poor evidence of benefit.
Use of the SSRI antidepressants in the third trimester may cause newborn toxicity.
Only amitriptyline, verapamil, and low-dose propranolol (30-40 mg/day) have sufficient data to classify as compatible throughout pregnancy, but higher doses of propranolol may cause IUGR and other fetal/neonatal toxicity.
Two agents, gabapentin (Neurontin) and topiramate (Topamax), were considered promising agents for migraine prophylaxis. However, topiramate is best avoided in pregnancy. There are inadequate human data to assess the risk of gabapentin and topiramate, therefore both agents should be avoided in the first trimester.
Breastfeeding: Prevention therapy
Based on their potential for harm, topiramate and valproate should not be used during breastfeeding. All of the other drugs probably are compatible with breastfeeding, but the nursing infant should be monitored for adverse effects common in nonpregnant adults.
Acute treatment of migraines
There are 11 single-agent drugs that are indicated for the acute treatment of migraine.
Almotriptan (Axert) is available as an oral tablet. There are no human pregnancy data and the animal data suggest low risk. However, there is a possible risk of preterm birth.
Dihydroergotamine (Migranal, D.H.E. 45) is available for injection and nasal spray. It is contraindicated, especially near term (it has oxytocic properties), and the animal data suggest risk of IUGR.
Eletriptan (Relpax) is an oral tablet. There are no human data and the animal data suggest low risk. There is a possible risk of preterm birth.
Ergotamine (Ergomar), an oral tablet, is contraindicated in pregnancy and breastfeeding (see above and below).
Methysergide (Sansert) is contraindicated but is not available in United States.
Frovatriptan (Frova) has no human pregnancy data. The animal data suggests low risk but there is the possibility of preterm birth.
There are 93 cases in pregnancy for naratriptan (Amerge) but none during breastfeeding. The drug did not cause major defects in animals but did produce dose-related embryo and fetal development toxicity. In addition, the data did suggest a possible increase in preterm births.
For the rizatriptan (Maxalt, Maxalt-MLT) tablet, animal data suggest low risk. There was exposure to the drug in more than 81 human pregnancy cases. Although there was no evidence of birth defects, the data raised the concern of preterm birth.
There is no evidence that sumatriptan (Alsuma, Imitrex, Imitrex Statdose, Sumavel DosePro, Zecuity) is a human teratogen but there is a possible increase in preterm birth. The drug was teratogenic in one animal species. This agent is available as a tablet and for injection.
There is no human pregnancy data for zolmitriptan (Zomig; Zomig-ZMT) but, as with all triptans, there is a possible risk of preterm birth. The animal data suggest low risk. It is available as an oral tablet.
There are three multiagent products that can be used to treat migraines. There are no published human pregnancy data for these combination products. The combination oral drug sumatriptan and naproxen (Treximet) is best avoided in pregnancy because first trimester exposure to the NSAID naproxen may cause embryo-fetal harm (structural anomalies and abortion) or close to term (premature closure of the ductus arteriosus) and other newborn toxicities.
Caffeine and ergotamine (Cafergot, Migergot) tablets should be considered contraindicated in pregnancy and breastfeeding. Although small, infrequent doses of this product do not appear to be fetotoxic or teratogenic, idiosyncratic responses may occur with ergotamine that endanger the fetus.
The third product, acetaminophen, dichloralphenazone, and isometheptene (Epidrin, LarkaDrin, Migragesic IDA) is an over-the-counter tablet. It does not appear to be related to embryo-fetal harm, but its effectiveness is unknown.
Breastfeeding: Acute treatment
Consistent with their relatively low molecular weights, all of the acute treatment agents most likely are excreted into breast milk. With the exception of the two ergotamine products, both of which should be considered contraindicated, all of the other agents probably are compatible during breastfeeding.
Summary
Migraine headaches are common but can be prevented and/or treated safely in pregnancy and when nursing. In addition to the review mentioned above, three other reviews are well worth reading: Becker WJ. Continuum (Minneap Minn). 2015 Aug;21(4 Headache):953-72; Becker WJ. Headache. 2015 Jun;55(6):778-93; Hutchinson S et al. Headache. 2013 Apr;53(4):614-27.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.