User login
2021 Update on menopause
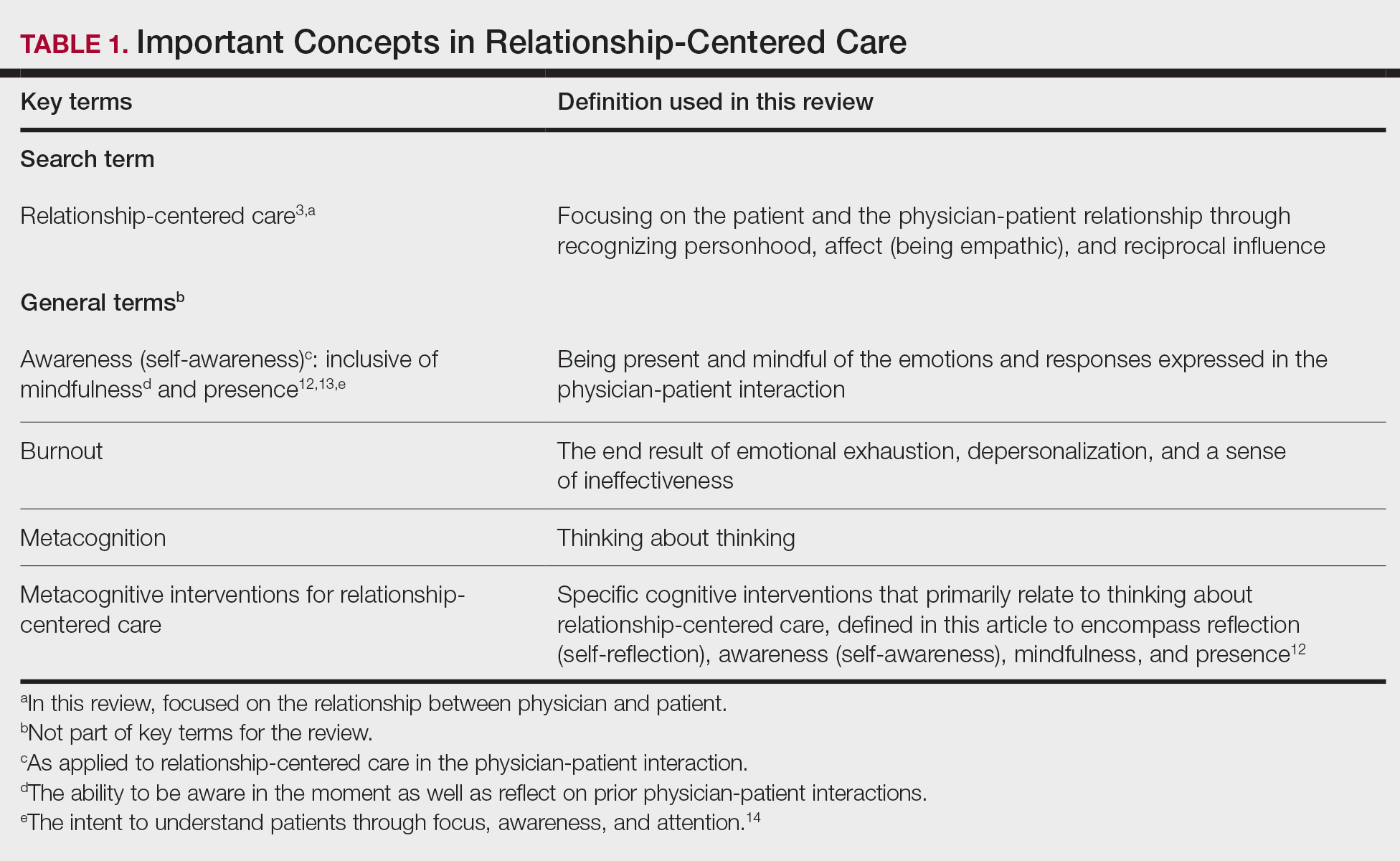
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
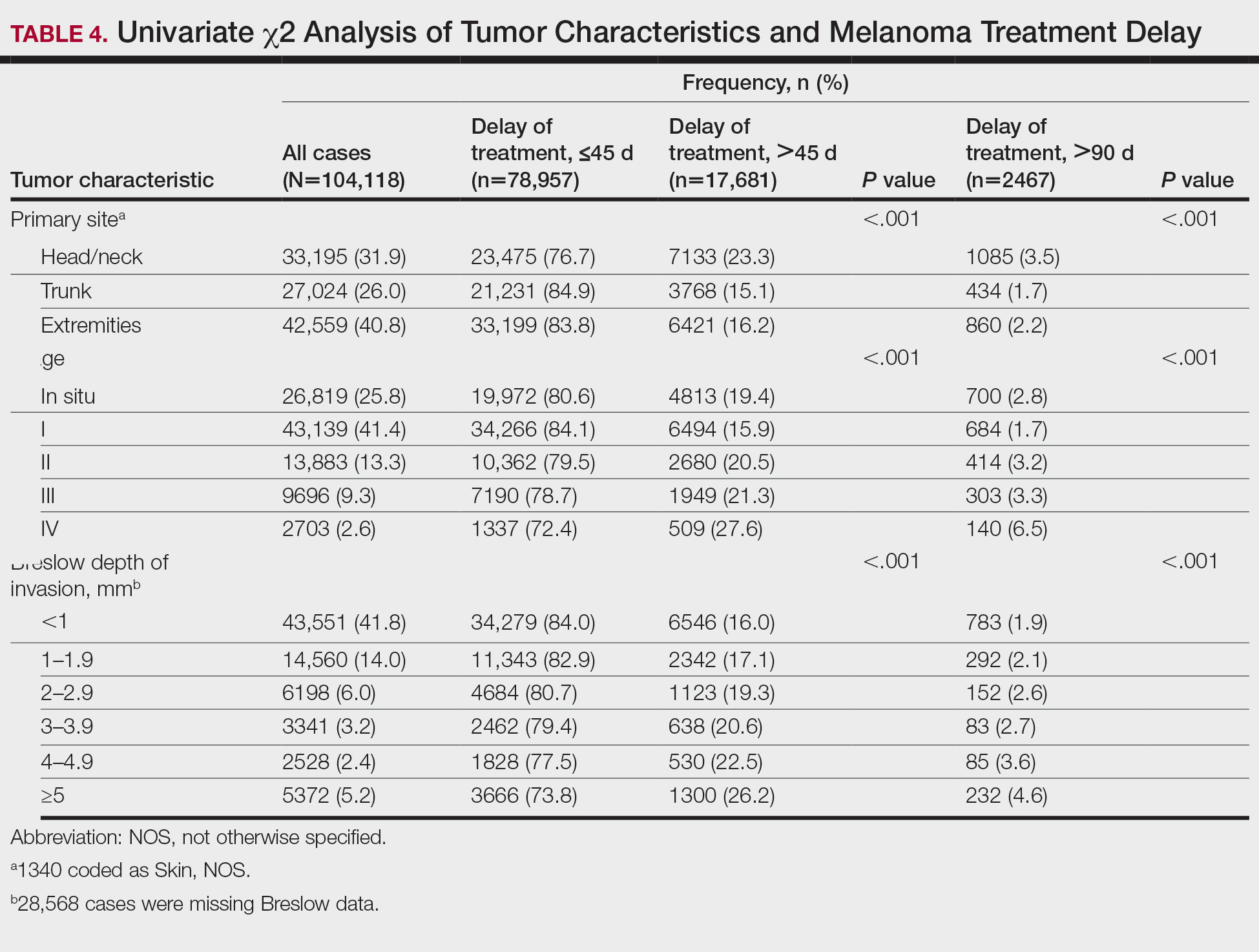
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
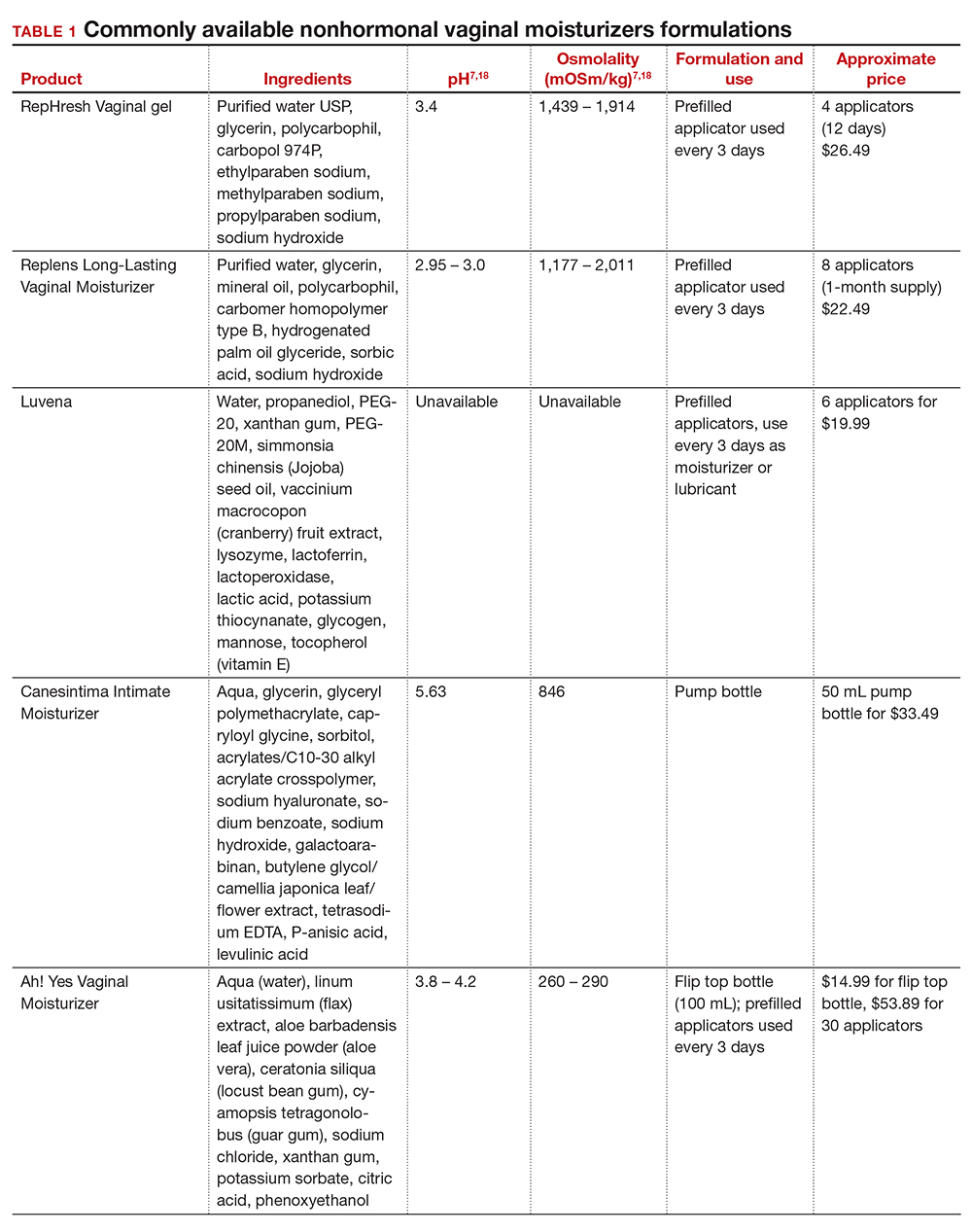
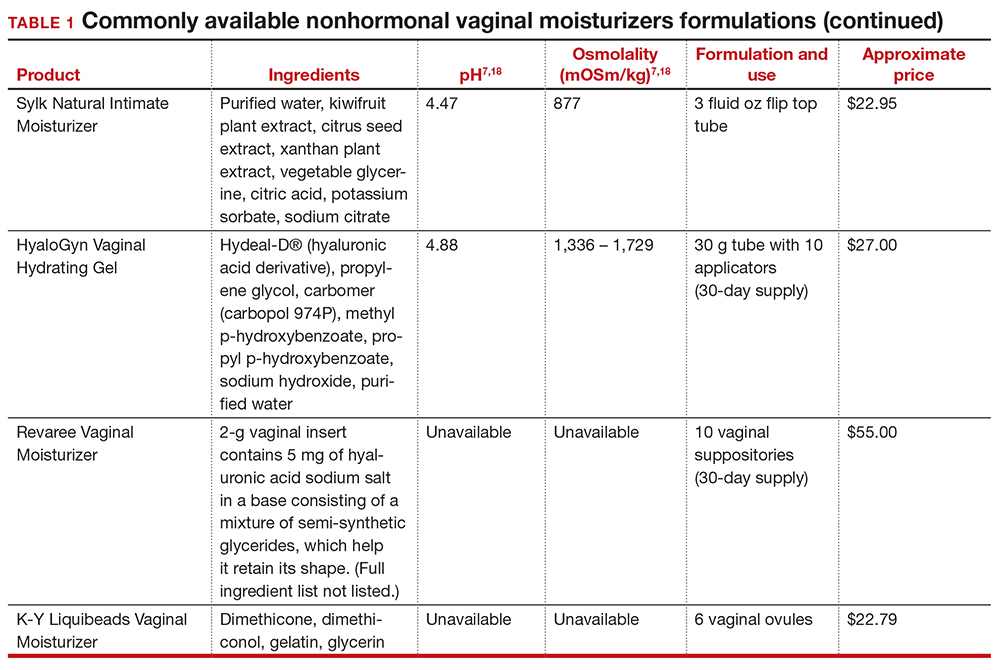
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
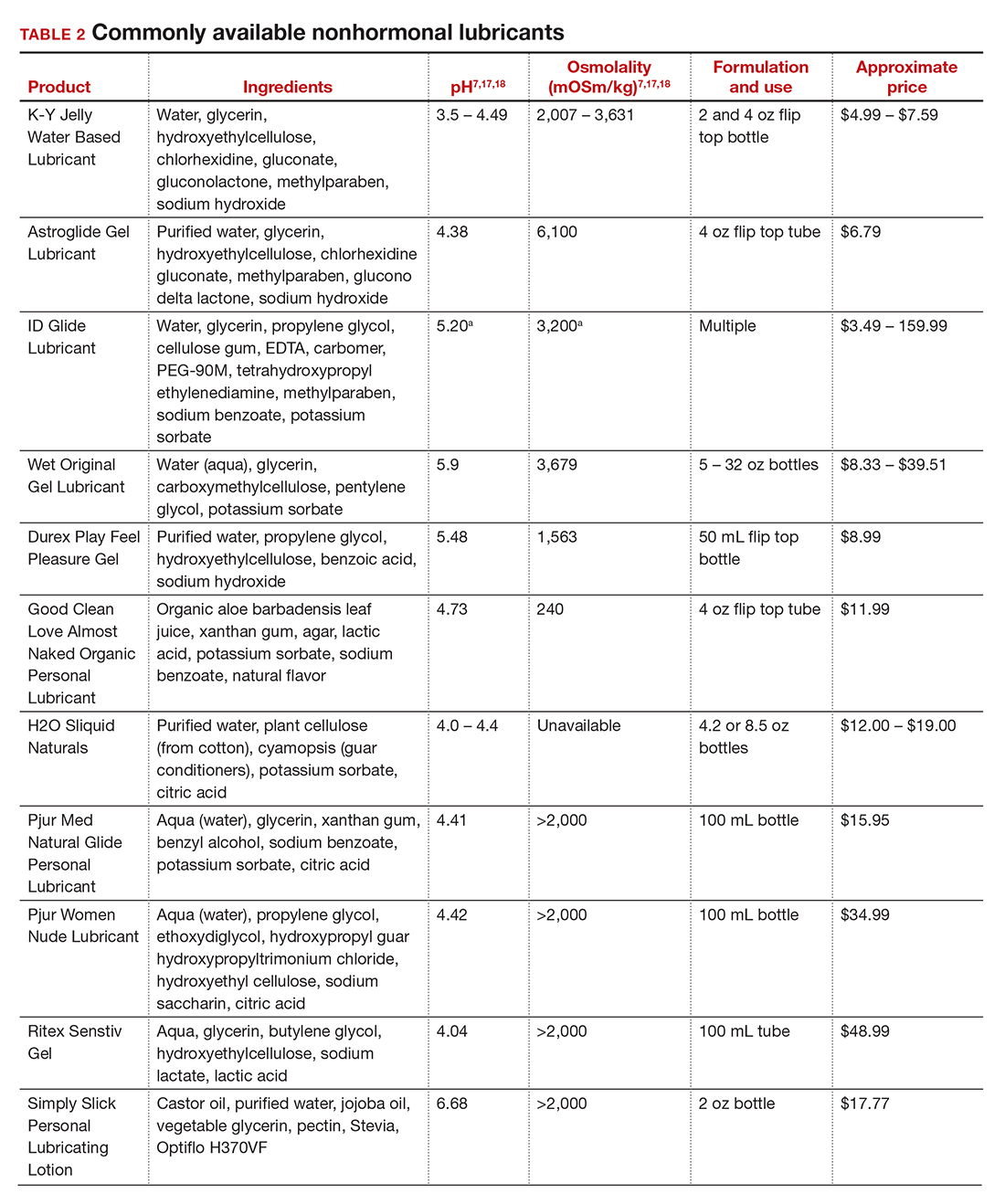
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
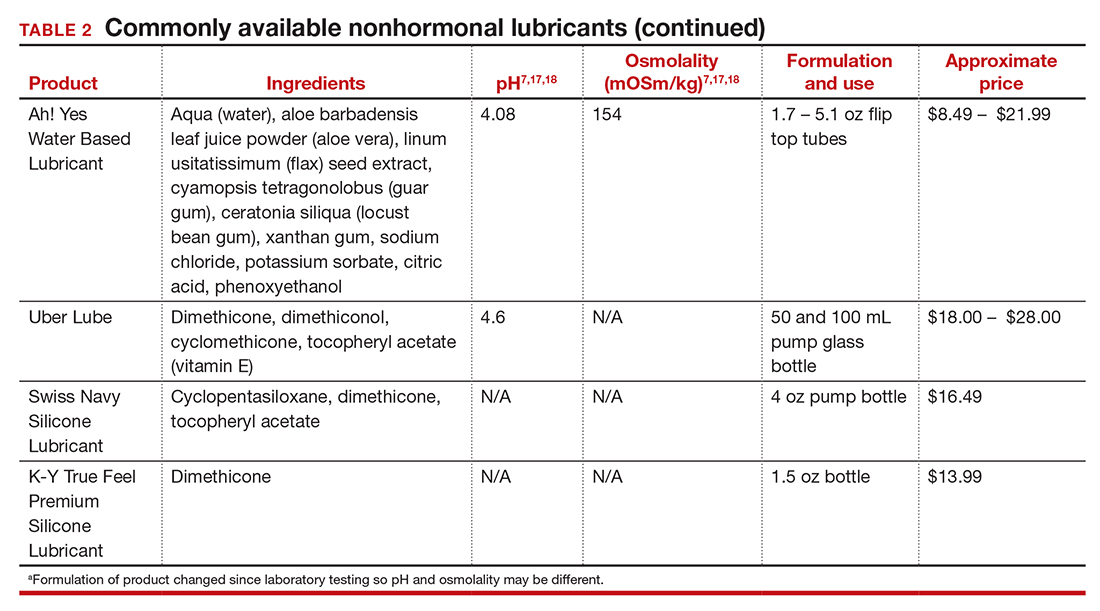
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
COVID-19 vaccination during pregnancy: Expert guidance on counseling your patients
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Relationship-Centered Care in the Physician-Patient Interaction: Improving Your Understanding of Metacognitive Interventions
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Practice Points
- Relationship-centered care emphasizes that all relationships in health care are important, including not only relationships between physicians and patients but also among physicians and colleagues, staff, students, community, and self.
- The physician-patient relationship can be complex, and metacognition can lead to habitual practice of simple techniques to optimize the interaction
Dynamic ultrasonography: An idea whose time has come (videos)
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--
VIDEO 3A Video clip of a classic endometrioma
--
VIDEO 3B Classic endometrioma showing no Doppler flow internally
--
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
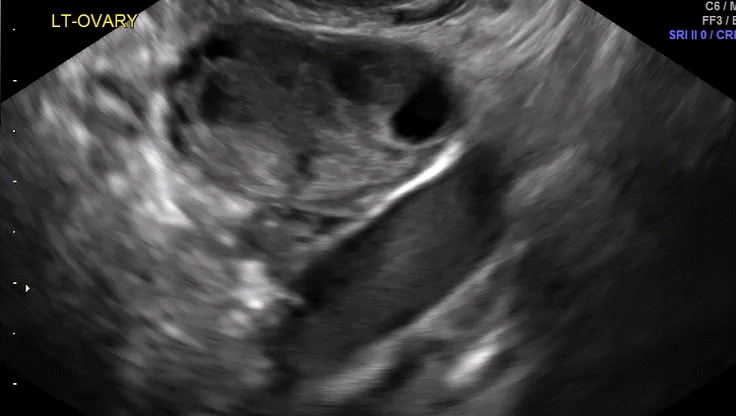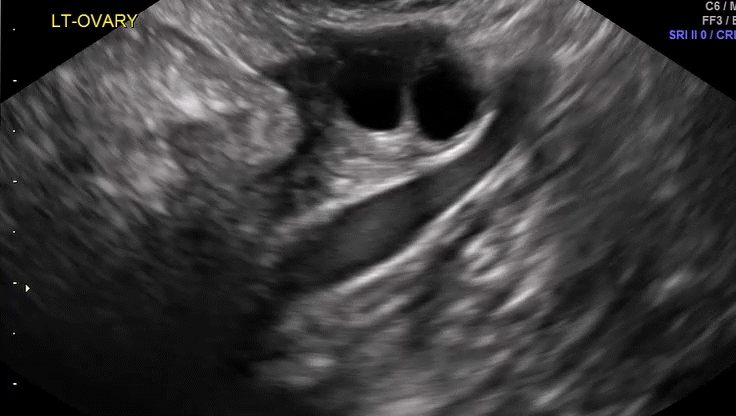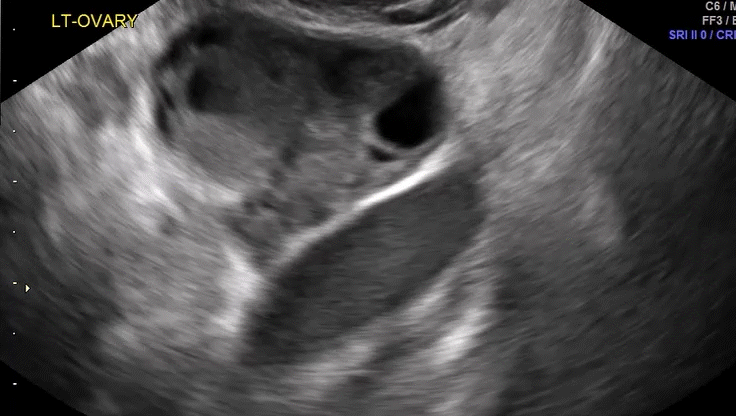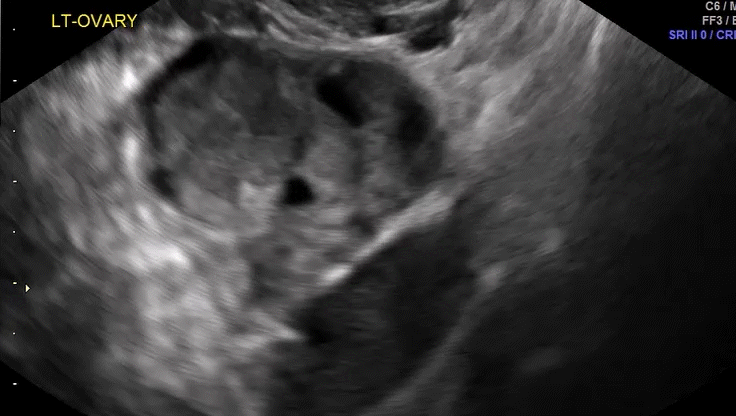
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
Use of Comprehensive Geriatric Assessment in Oncology Patients to Guide Treatment Decisions and Predict Chemotherapy Toxicity
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
Treatment Delay in Melanoma: A Risk Factor Analysis of an Impending Crisis
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Practice Points
- Melanoma treatment delays (MTDs) have been linked to poor outcomes.
- Based on the National Cancer Database, the mean MTD has increased significantly from 2004 to 2015 (P11<.001).
- More delays are seen in patients who are older than 50 years, female, nonwhite, not privately insured, and treated at an academic facility and who have more advanced tumor stage and head/neck primaries.
Female genital cutting: Caring for patients through the lens of health care equity
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13
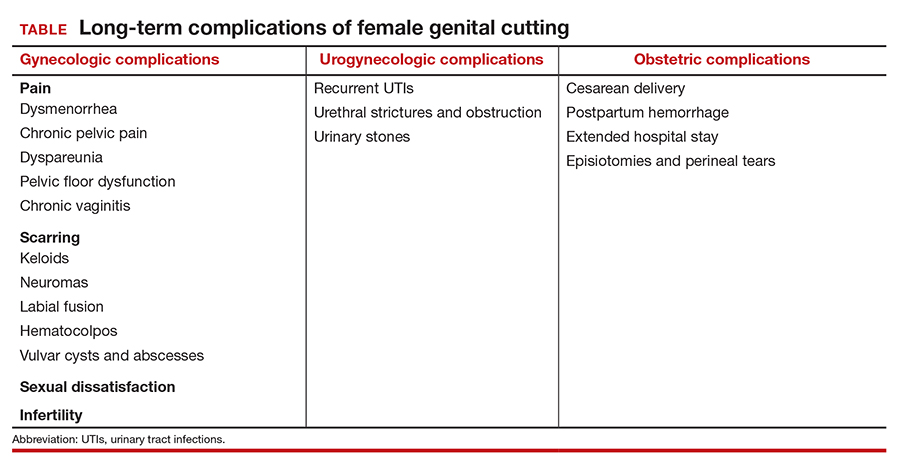
Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.